Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.1, pp.13-23, 2010 jmmce.org Printed in the USA. All rights reserved 13 Sensitisation Study of Normalized 316L Stainless Steel P. Atanda1 , A. Fatudimu1 and O. Oluwole2* 1Materials Science and Engineering Department, Obafemi Awolowo University,Ile-ife,Nigeria. 2Mechanical Engineering Dept, University of Ibadan *Corresponding author: oluwoleo2@asme.org ABSTRACT Austenitic stainless steels with excellent corrosion resistance and good weldability have wide applications in industry. These iron-based alloys contain a high level of chromium which form protective oxide film on the surface hence resisting corrosion. The oxide film regenerates when damaged, making the steel 'stainless'. However, carbide precipitation due to a welding process or heat treatment can cause the occurrence of chromium-depleted zones at the boundaries, leading to a phenomenon known as sensitisation, in which the depleted zones become the focus of the intense corrosion. The present work was concerned with the study of the sensitization and desensitisation of 316L steel at the normalizing temperatures of 750- 9500 C and soaking times of 0.5, 1, 2 and 8 hrs. 316L stainless steel was observed to be sensitized when heated to 750- 8500 C and held for short soaking times of 0.5 – 2hrs before normalizing. Increasing soaking times at these temperatures to 8 hrs triggered the desensitization process which was fully accomplished at 7500 C but ongoing at 800 and 8500 C. At 9000 C, sensitization did not occur at 30 mins soaking time but observed at soaking times of 1 and 2hrs. At a longer soaking time of 8 hrs, there was full desensitization. At 9500 C, sensitization was already observed at 30 mins. Soaking time and desensitization was observed to be in progress at 1 and 2 hrs soaking time. By 8 hrs there was full desensitization. Thus it was observed that at 9500 C, diffusion of Cr was thermally aided making desensitization fast. The hardness of normalized 316L stainless steel was also observed to decrease with soaking time and normalization temperature. Key words: Sensitisation; Normalization treatment; 316L Stainless steel  14 P. Atanda , A. Fatudimu and O. Oluwole Vol.9, No.1 1. INTRODUCTION The basic 18Cr8Ni (18/8) austenitic stainless steel is so widely used that it accounts for about 50% of all stainless steel production. The stainless character occurs when the concentration of chromium exceeds about 12wt%, whereas higher chro mium concentrations and the judicious us e of other solutes such as molybdenum, nickel and nitrogen is then needed to ensure a robust mater i al1-3 . The “L” grades are used to provide extra corrosion resistance after welding. The letter L after a stainless steel type indicates low carbon (as in 316L). The carbon content is kept to 0.03% or less to avoid grain boundary precipitation of chromium carbide in the critical range (430 to 900oC). Grain boundary precipitation deprives the steel of the chromium in solution and promotes corrosion adjacent to grain boundaries. By controlling the amount of carbon, this is minimized4-6. Chromium is, of course, the primary element for forming the passive film (i.e. high-temperature, corrosion-resistance chromium oxide). Other elements can influence the effectiveness of chromium in forming or maintaining the film, but no other element can, by itself, create the stainless steel. Nickel in sufficient quantities, is used to stabilize the austenitic phase and to produce austenitic stainless steels3. A corrosion benefit is obtained as well, especially in reducing environments. Nickel is particularly useful in promoting increased resistance to mineral acids. When nickel is increased to about 8 to 10% this is a level required to ensure austenitic structures in a stainless steel that has about 18% chromium. Molybdenum in moderate amounts in combination with chromium is very effective in terms of stabilizing the passive film in the presence of chlorides. Molybdenum is especially effective in enhancing the resistance to pitting and crevi c e cor rosion4. Nitrogen is beneficial to austenitic stainless in that it enhances pitting resistance, retards formation of sigma phase. The change in the microstructure of an alloy can be achieved by heat treatment. 2. MATERIALS AND METHOD 2.1 Materials 316L stainless steel was obtained from the market for the purpose of this experimentation. 2.2 Methods  Vol.9, No.1 Sensitisation Study of Normalized 316L Stainless Steel 15 2.2.1 Heat treatment Normalization heat treatments were performed on the samples by varying the temperature and the soaking period. The following temperatures were used: 750, 800, 850, 900 and 950oC. The different soaking times at these temperatures were 30 mins, 1 hour, 2 hours and 8 hours. 2.2.2 Mounting and grinding First, each specimen was mounted on thermosetting plastic for ease of grinding and polishing. The grinding of the specimens was done on silicon carbide paper7-12. The surface of the papers was flushed by a current of water, which serves not only as a lubricant in grinding, but also carries away coarse emery particles, which might oth erwise scratch the surface of the specimen. The silicon carbide papers used in achieving the proper grinding of the specimen were in the following grades 120, 240, 320 and 400 grits, grinding respectively in that order of grades. 2.2.3 Polishing The polishing was done on polishing cloth using alumina powder dissolved in water at a reasonable proportion. The alumina powder was of the grade 1 micron and 0.3 micron respectively. Light pressure was applied until the surfaces were free of scratches7-12. The samples were cleaned, dr ied and then examined und er the microscope, using a magnification between 50 and 100 so as to check whether the samples were free of scratches. 2.2.4 Etching After polishing, the samples were etched with 5gm of FeCl3 + 10ml of HCl + 50ml of H2O7-12. To etch these specimens, they were washed free of any adhering polishing compound and plunged into the etching solution, agitated vigorously for 3 minutes. The specimens were then very quickly transferred to running water, in order to wash away the etchant as rapidly as possible. It was then exa mined with the naked eye, to see to w hat extent et ching has taken place. The successfully etched surf ace appeared dull. After this, the specimens were observed under optical microscope and photomicrographs taken. 3. RESULTS AND DISCUSSIONS 3.1 Results The photomicrographs of the normalized specimens are shown in Figs. 1 – 5. Figs 1a-d show the photomicrographs of the normalized specimens from 7500 C each held at the soaking 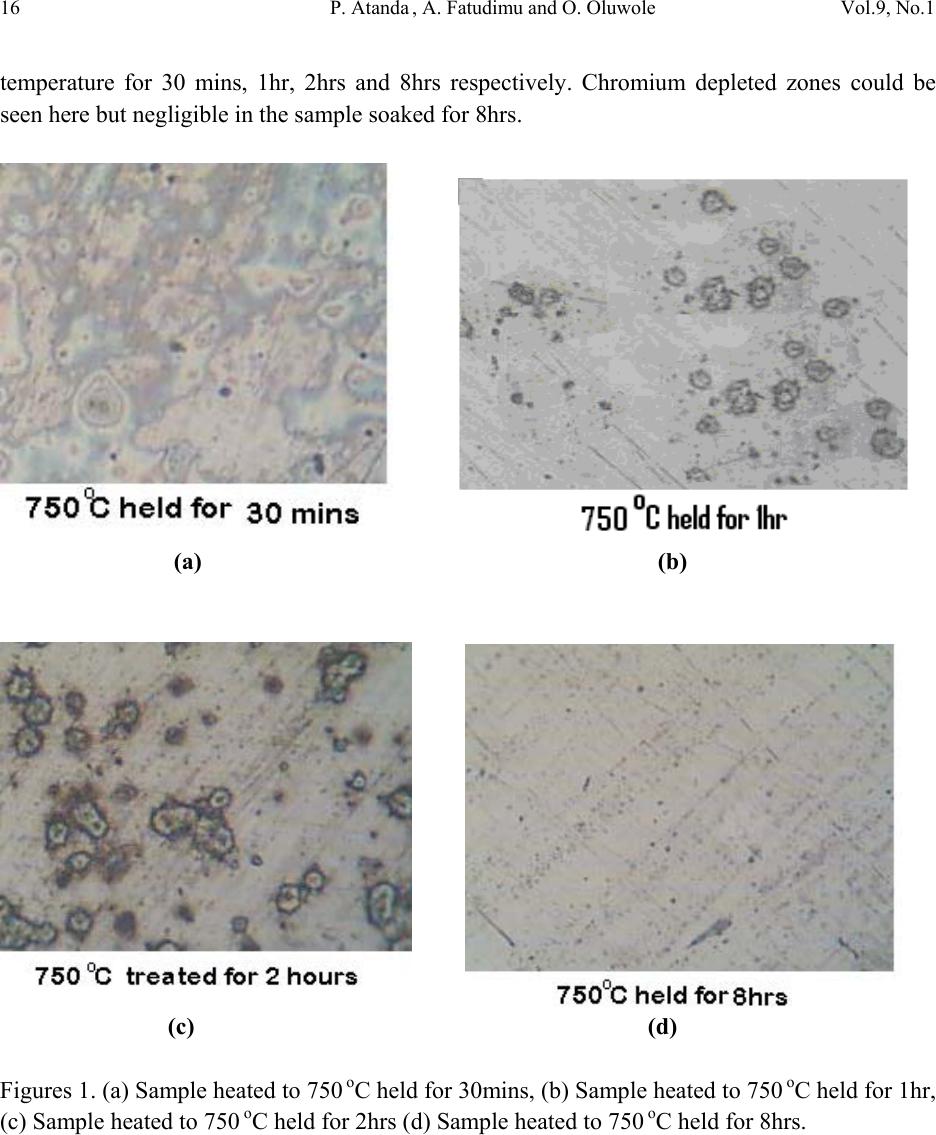 16 P. Atanda , A. Fatudimu and O. Oluwole Vol.9, No.1 temperature for 30 mins, 1hr, 2hrs and 8hrs respectively. Chromium depleted zones could be seen here but negligible in the sample soaked fo r 8hrs. (a) (b) (c) (d) Figures 1. (a) Sample heated to 750 oC held for 30mins, (b) Sample heated to 750 oC held for 1hr, (c) Sample heated to 750 oC held for 2hrs (d) Sample heated to 750 oC held for 8hrs. 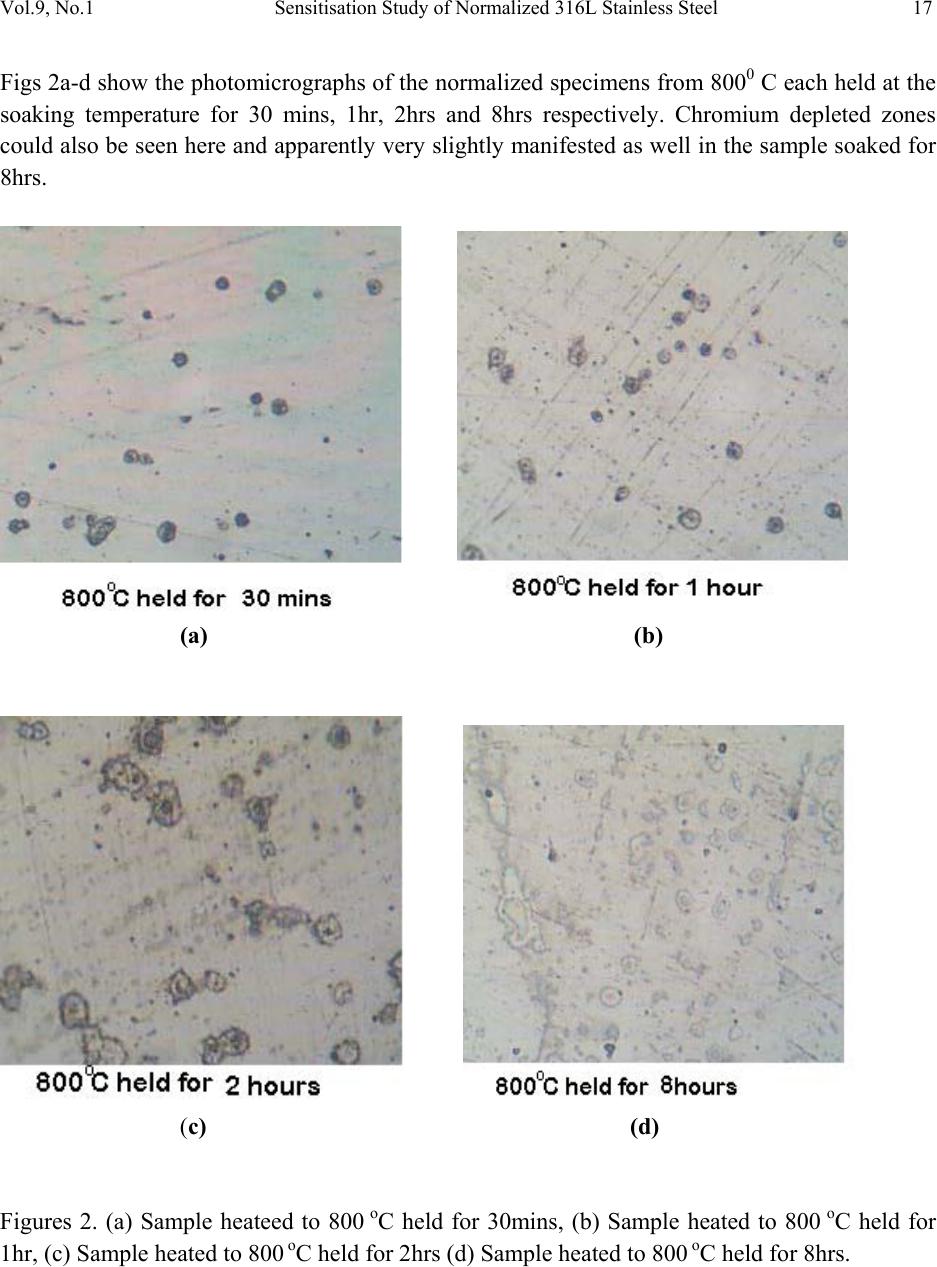 Vol.9, No.1 Sensitisation Study of Normalized 316L Stainless Steel 17 Figs 2a-d show the photomicrographs of the normalized specimens from 8000 C each held at the soaking temperature for 30 mins, 1hr, 2hrs and 8hrs respectively. Chromium depleted zones could also be seen here and apparently very slightly manifested as well i n the sample soaked for 8hrs. (a) (b) (c) (d) Figures 2. (a) Sample heateed to 800 oC held for 30mins, (b) Sample heated to 800 oC held for 1hr, (c) Sample heated to 800 oC held fo r 2hrs (d) Sample h e ated to 800 oC held for 8hrs. 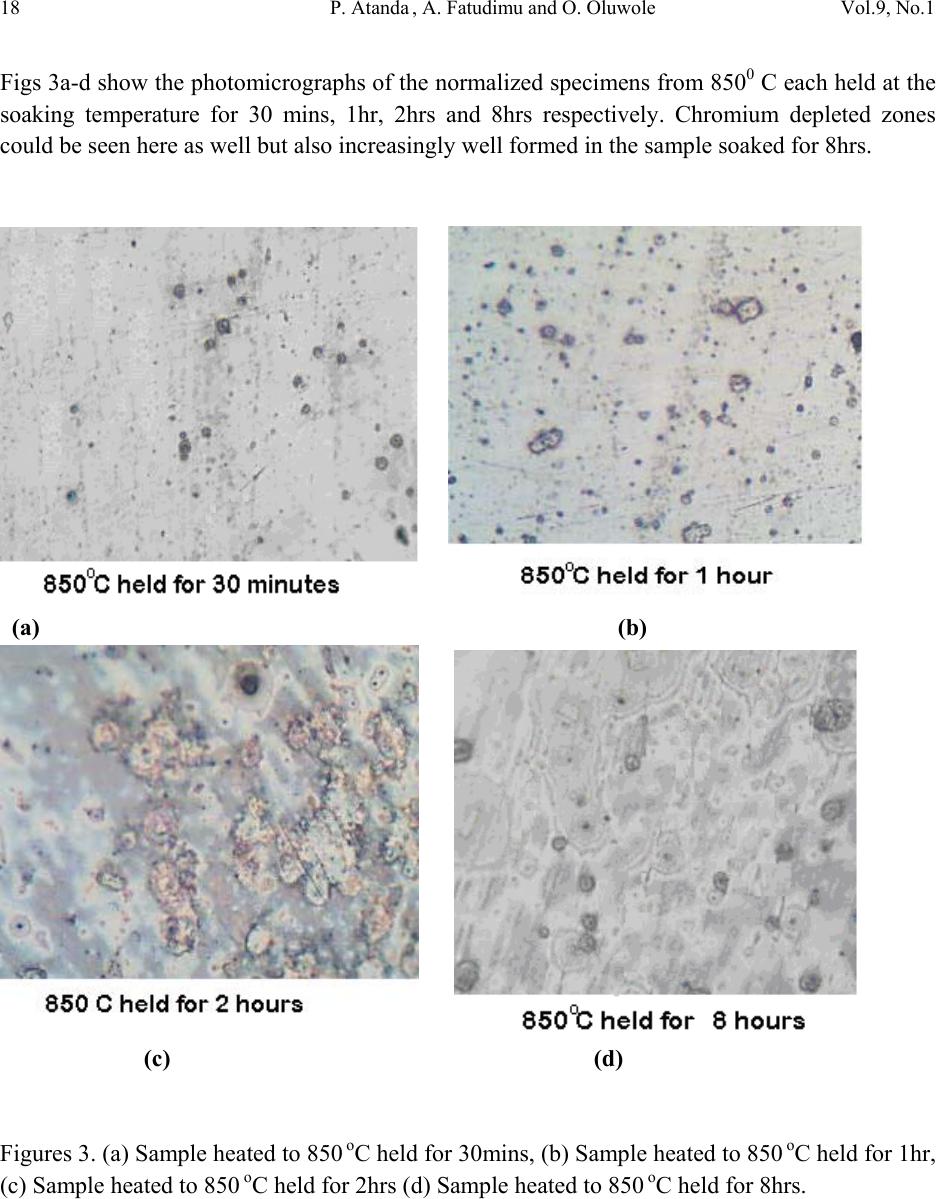 18 P. Atanda , A. Fatudimu and O. Oluwole Vol.9, No.1 Figs 3a-d show the photomicrographs of the normalized specimens from 8500 C each held at the soaking temperature for 30 mins, 1hr, 2hrs and 8hrs respectively. Chromium depleted zones could be seen here as well but also increasingly well formed in the sample soaked for 8hrs. (a) (b) (c) (d) Figures 3. (a) Sample heated to 850 oC held for 30mins, (b) Sample heated to 850 oC held for 1hr, (c) Sample heated to 850 oC held for 2hrs (d) Sample heated to 850 oC held for 8hrs. 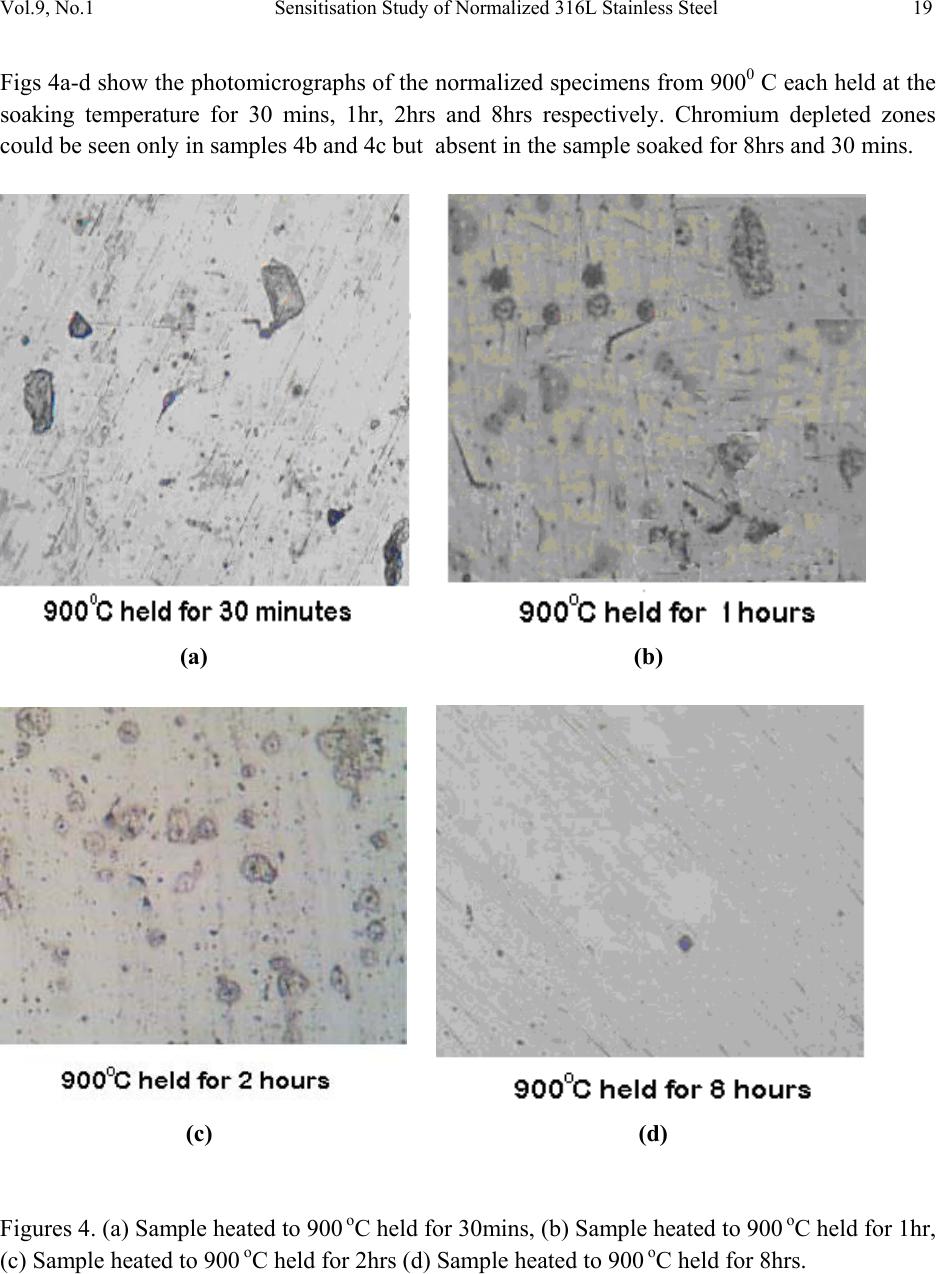 Vol.9, No.1 Sensitisation Study of Normalized 316L Stainless Steel 19 Figs 4a-d show the photomicrographs of the normalized specimens from 9000 C each held at the soaking temperature for 30 mins, 1hr, 2hrs and 8hrs respectively. Chromium depleted zones could be seen only in samples 4b and 4c but absent in the sample soaked for 8hrs and 30 mins. (a) (b) (c) (d) Figures 4. (a) Sample heated to 900 oC held for 30mins, (b) Sample heated to 900 oC held for 1hr, (c) Sample heated to 900 oC held for 2hrs (d) Sample heated to 900 oC held for 8hrs.  20 P. Atanda , A. Fatudimu and O. Oluwole Vol.9, No.1 Figs 5a-d show the photomicrographs of the normalized specimens from 9500 C each held at the soaking temperature for 30 mins, 1hr, 2hrs and 8hrs respectively. Chromium depleted zones are negligible in all the specimens here and could be seen to be observed faintly only in 4b and c which correspond to short soaking times of 1and 2 hrs respectively. (a) (b) (c) (d) Figures 5. (a) Sample heated to 950 oC held for 30mins, (b) Sample heated to 950 oC held for 1hr, (c) Sample heated to 950 oC held for 2hrs (d) Sample heated to 950 oC held for 8hrs. Figure 6 shows the micrograph of the untr eated sample.  Vol.9, No.1 Sensitisation Study of Normalized 316L Stainless Steel 21 Figure 6. Untreated Sample. The photomicrostructures of these heat treated austenitic steel samples have been show a clear cut difference between the treatment using long time (8 hours) and short times (0.5hr, 1hr and 2hrs). The chromium depleted zones increased considerably up to the 2 hours treatment but reduced on the samples soaked for 8 hrs. The Rockwell hardness of some of the samples is presented in Table 1 and Fig. 7. Table 1. Brinnel Hardness of some Samples. S/N SAMPLES HARDNESS, HB 1 Untreated Sample 187 2 750 oC held for 30mins 169 3 750 oC held for 1hr 167 4 750 oC held for 2hrs 167 5 750 oC held for 8hrs 166 6 800 oC held for 30mins 164 7 800 oC held for 1hr 163 8 800 oC held for 2hrs 163 9 800 oC held for 8hrs 161 10 850 oC held for 30mins 156 11 850 oC held for 1hr 152 12 850 oC held for 2hrs 149 13 850 oC held for 8hrs 143 14 900 oC held for 30mins 131 15 900 oC held for 1hr 115 16 900 oC held for 2hrs 111 17 900 oC held for 8hrs 110 18 950 oC held for 30mins 109 19 950 oC held for 1hr 107 20 950 oC held for 2hrs 99  22 P. Atanda , A. Fatudimu and O. Oluwole Vol.9, No.1 Fig. 7. Comparative Brinnel Hardness chart of samples in Table1. 3.2 Discussions From the microstructures observed in Figs. 1a-c, 2a-c, 3a-c, 4a-c and 5a-c, the black patches form the depleted – zone. It could be observed that the width of the depleted zone increased with time, leading to a flatter chromium profile near the grain boundaries at the soaking temperatures of 750-8500 C and for short soaking times of 30 mins to 2 hrs. This heat treatment procedure had significant effect on the chromium concentration profile. The process of short time heat treatment brought about sensitization (the breakdown in corrosion resistance which occurs when austenitic st ainless st eels are r eheated in the te mperature r ange 550 oC to 850 oC). Sensitisation is associated with the preci pitat ion of the chr o mium- rich carbide su ch as Cr23C6 or Cr7C3 along the grain boundaries. During the carbide precipitation, interstitial carbon diffuses rapidly to the grain boundaries. However, chromium diffusion which is much more slower, resulted in the chro mium – depleted zones at the grain boundaries. In this state the steel is susceptible to intergranular corrosion. Figs 4 and 5 show a self – healing processs (desensitization) already taken place, refering to the return of corrosion resistance of the stainless steels after prolonged heat treatment in the temperature range which initially caused sensitization. Self – healing began when the chromium content at the carbide –matrix interface increased due to chromium diffusion from the matrix further away from the grain boundary . Thus, prolonged soaking at 900 and 9500 C did not have a deleterious effect on 316L stainless steel. The hardness of the samples was also observed to decrease with increase in temperature (Fig. 7).  Vol.9, No.1 Sensitisation Study of Normalized 316L Stainless Steel 23 4. CONCLUSION 316L stainless steel was observed to go into sensitization when heated to 750- 8500 C and held for short soaking times of 0.5 – 2 hrs before normalizing. Incresing the soaking times at this temperatures triggered desensitization process which was fully accomplished at 7500 C but ongoing at 800 and 8500 C. At 9000 C, sensitization was observed at soaking times of 1 and 2 hrs before normalizing. At a longer soaking time of 8 hrs before normalization, there was full desensitization. At 9500 C, sensitization was already observed at 30 mins soaking time and desensitization was observed to be in progress at 1 and 2 hrs soaking time. By 8 hrs there was full desensitization of 316L stainless steel. Thus it was observed that at 9500 C, the diffusion of Cr was thermally aided making it very fast and initial sensitisation was cancelled out. The hardness of normalized 316L stainless steel was also observed to decrease with soaking time and normalization temperature. REFERENCES [1] Howard, O.T. and Leonard, W.A. (1963). ‘An Introduction to Stainless Steel’ New York. [2] Lacombe P., Baroux B., and Beranger G., editors. (1993) ‘Stainless steel’ The Journal of Physics. [3] Parr J.G. and Hanson A.(1965) ‘An Introduction to Stainless Steel’ American Society For Metals. [4] Gooch T. G and Willingham D.C.(1975) ‘Weld Decay in Austenitic Stainless Steel’, Welding Institute, Cambridge, United Kingdom. [5] Honeycombe R. W. K. and Bhadeshia H. K. D. H.(1995) ‘Steels-microstructure and properties’. Edward Arnold, 2nd edition. [6] Kirkaldy J. S and Young D.J.(1987) ‘Diffusion in the Condensed State’. Institute of Metals, London. [7] Brandon, D. G.(1966) ‘Modern Techniques in Metallography’. Butterworths, London. [8] Greaves, R. H. & H. Wrighton Practical Microscopical Metallography (4th Edition). Chapman and Hall, London. 1960 [9] Fawole, M.O. and Oso, B.A (2001). The Principles of Metallographic Laboratory Practice. Spectrum Books Ltd, Ibadan, Nigeria. [10] Fujita N. and Bhadeshia H. K. D. H. Mater. Sci. Tech., 15: 627 – 634, 1999. [11] Hughes, K.V.(1994) Practical Microscopical Metallograp hy. University of Missouri Extension, Columbia Publication. [12] Kehl, G. L. (1949) ‘The Principles of Metallographic Laboratory Practice’. (3rd edition). McGraw-Hill, New York, Toronto, London. |

