Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 8, No.10, pp.803-811, 2009 jmmce.org Printed in the USA. All rights reserved 803 Corrosion Behavior of 18/8 Stainless Steel and Nickel-Plated Low Carbon Steel in Cassava Fluid O.O. Oluwole1*, P.O. Atanda2, O.A. Odekunbi2 and E. Odegbaju2 1Mechanical Engineering Department,University of Ibadan, Nigeria 2Materials Science and Engineering Department,Obafemi Awolowo University, Nigeria *Corresponding author: leke_oluwole@yahoo.co.uk ABSTRACT This research work investigated the corrosion resistance of nickel- plated medium carbon steel and 18/8 stainless steel in cassava fluid (i.e. containing hydrogen cyanide). It simulated the effect of continuous use of the materials in a cyanide environment where corrosion products are left in place. Low carbon steel sample was nickel electroplated at 4V for 35 minutes. The plated sample, the unplated and the 18/8 stainless steel were then subjected to a cassava fluid environment for thirty days. The electrode potentials, in mV (SCE), were measured every day. Weight loss was determined at intervals of 5 days for duration of the exposure period. The result showed little corrosion attack on the nickel-plated steel on the fifth and tenth days which quickly dropped to zero by the 15th day and remained at the passive state till the 20th day when corrosion picked up again increasing steadily, linearly until the end of the test day. Corrosion of the 18/8 stainless steel was very low as well decreasing till the last day of the test. The pH of the cassava solution which initially was acidic because of the cyanide content in the cassava was observed to progress to neutrality within five days and to alkalinity at the end of the thirty days test (because of corrosion product contamination of the cyanide). Un-plated steel was found to be unsuitable for the fabrication of cassava processing machinery because of the very high corrosion rate. 18/8 stainless steel was found suitable for use in this environment. The renewed corrosion activity on nickel plated steel after the 20th day (pH=12) of continuous use in cyanide environment makes it unsuitable for use. Keywords: Corrosion resistance,18/8 stainless steel, nickel-plated low carbon steel, Cassava fluid 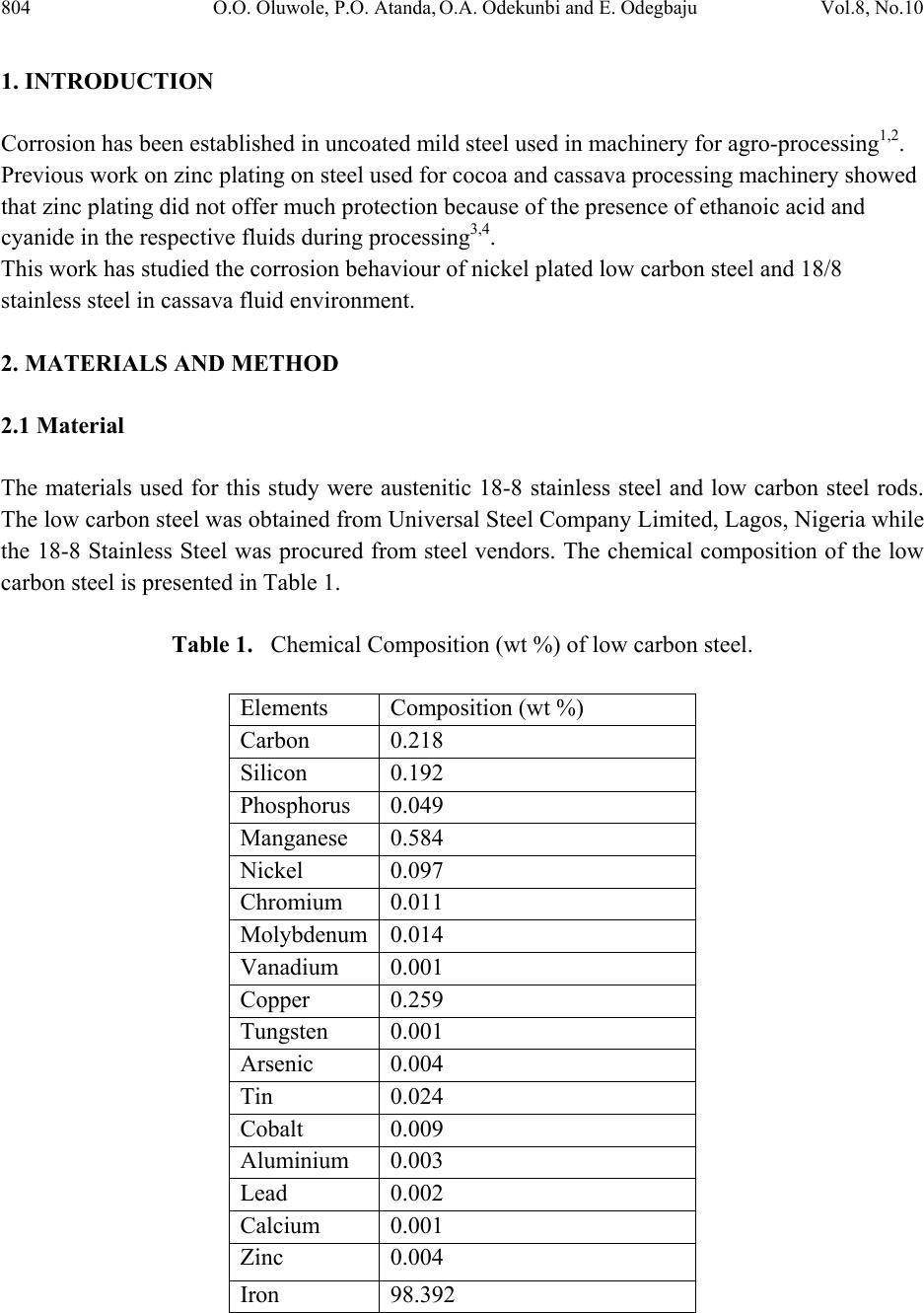 804 O.O. Oluwole, P.O. Atanda, O.A. Odekunbi and E. Odegbaju Vol.8, No.10 1. INTRODUCTION Corrosion has been established in uncoated mild steel used in machinery for agro-processing1,2. Previous work on zinc plating on steel used for cocoa and cassava processing machinery showed that zinc plating did not offer much protection because of the presence of ethanoic acid and cyanide in the respective fluids during processing3,4. This work has studied the corrosion behaviour of nickel plated low carbon steel and 18/8 stainless steel in cassava fluid environment. 2. MATERIALS AND METHOD 2.1 Material The materials used for this study were austenitic 18-8 stainless steel and low carbon steel rods. The low carbon steel was obtained from Universal Steel Company Limited, Lagos, Nigeria while the 18-8 Stainless Steel was procured from steel vendors. The chemical composition of the low carbon steel is presented in Table 1. Table 1. Chemical Composition (wt %) of low carbon steel. Elements Composition (wt %) Carbon 0.218 Silicon 0.192 Phosphorus 0.049 Manganese 0.584 Nickel 0.097 Chromium 0.011 Molybdenum0.014 Vanadium 0.001 Copper 0.259 Tungsten 0.001 Arsenic 0.004 Tin 0.024 Cobalt 0.009 Aluminium 0.003 Lead 0.002 Calcium 0.001 Zinc 0.004 Iron 98.392 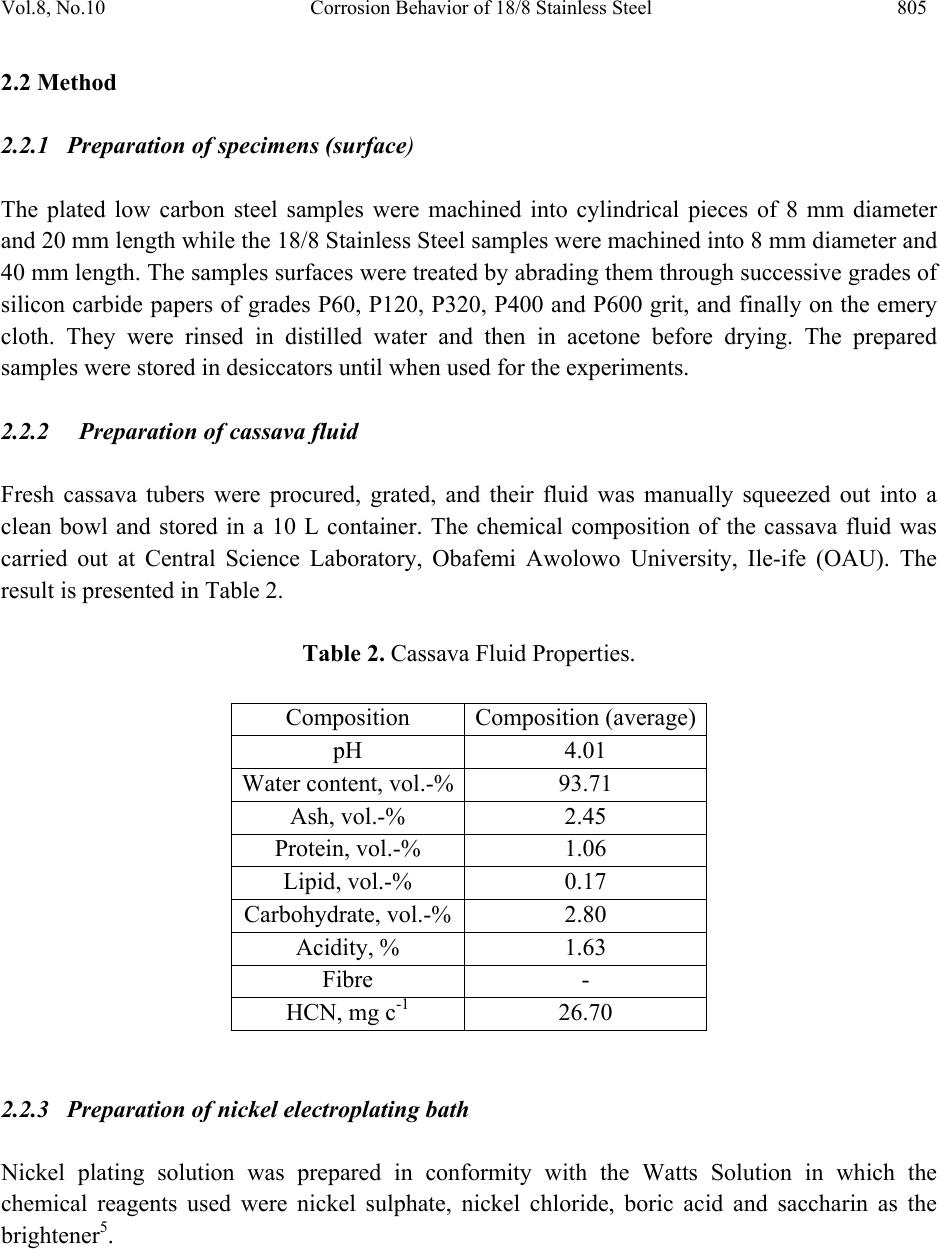 Vol.8, No.10 Corrosion Behavior of 18/8 Stainless Steel 805 2.2 Method 2.2.1 Preparation of specimens (surface) The plated low carbon steel samples were machined into cylindrical pieces of 8 mm diameter and 20 mm length while the 18/8 Stainless Steel samples were machined into 8 mm diameter and 40 mm length. The samples surfaces were treated by abrading them through successive grades of silicon carbide papers of grades P60, P120, P320, P400 and P600 grit, and finally on the emery cloth. They were rinsed in distilled water and then in acetone before drying. The prepared samples were stored in desiccators until when used for the experiments. 2.2.2 Preparation of cassava fluid Fresh cassava tubers were procured, grated, and their fluid was manually squeezed out into a clean bowl and stored in a 10 L container. The chemical composition of the cassava fluid was carried out at Central Science Laboratory, Obafemi Awolowo University, Ile-ife (OAU). The result is presented in Table 2. Table 2. Cassava Fluid Properties. Composition Composition (average) pH 4.01 Water content, vol.-%93.71 Ash, vol.-% 2.45 Protein, vol.-% 1.06 Lipid, vol.-% 0.17 Carbohydrate, vol.-% 2.80 Acidity, % 1.63 Fibre - HCN, mg c-1 26.70 2.2.3 Preparation of nickel electroplating bath Nickel plating solution was prepared in conformity with the Watts Solution in which the chemical reagents used were nickel sulphate, nickel chloride, boric acid and saccharin as the brightener5.  806 O.O. Oluwole, P.O. Atanda, O.A. Odekunbi and E. Odegbaju Vol.8, No.10 2.2.4 Samples pretreatment before electroplating operations The low carbon steel sample was removed from the desiccators in turn and pickled in 0.5M H2SO4 for 2 min and rinsed in distilled water in order to remove rust. The pickled samples were degreased in a 100 liter electrolytic degreasing tank containing 200g KOH and 100g NaOH in distilled water for 2 minutes, after which the samples were rinsed in distilled water. The samples were weighed using a digital weighing balance model Mettler Toledo Pb153 of ± 0 .001g accuracy and the weight was recorded as the initial weight. 2.2.5 Electroplating operation The laboratory nickel electroplating bath was stirred with the aid of a stirrer for 1 min. The sample already attached to the flexible copper wire was then hanged on the cathode arm of the nickel electroplating bath after which the electroplating rectifier was switched on. The electroplating rectifier was regulated to 4 V. Earlier work done on nickel electroplating of manganese steel6 for 35minutes gave good plating thickness. The steel sample was electroplated for 35mins. The unplated sample was kept as control sample. 2.2.6 Corrosion resistance in cassava fluid The nickel electroplated low carbon steel and the 18/8 stainless steel samples were immersed in cassava fluid for durations up to 30 days, including an unplated sample as control. Electrodepotential (mV) measurements between the sample surface and the corrosive environment were carried out at regular interval of 24 h using a DT8300D digital multimeter with a zinc electrode used as a reference electrode. The reference electrode was not left in the cell for the duration of the experiment but used only at time of measurement of potential then afterwards removed. Values obtained were converted to saturated calomel electrode (SCE) values with the use of the formula; Ezn-1030mV = S.C.E values7. The corrosion samples were removed from the corrosion environment with the aid of a tong after which the samples were properly cleaned in distilled water and dried with cotton wool. The dried samples were weighed with the digital chemical weighing balance and recorded and this continued at regular intervals of 5 days. Corrosion rates in mm/yr were obtained from weight loss analysis8 using the expression CPR=87.6W/ρAT (mm/yr); where W is the weight loss in mg; ρ is the density of the specimen in g/cm3; A is the total exposed surface area of the specimen in cm2; and T is the exposure time in hours; mm/yr is the corrosion penetration rate expressed in millimeters penetration per year. 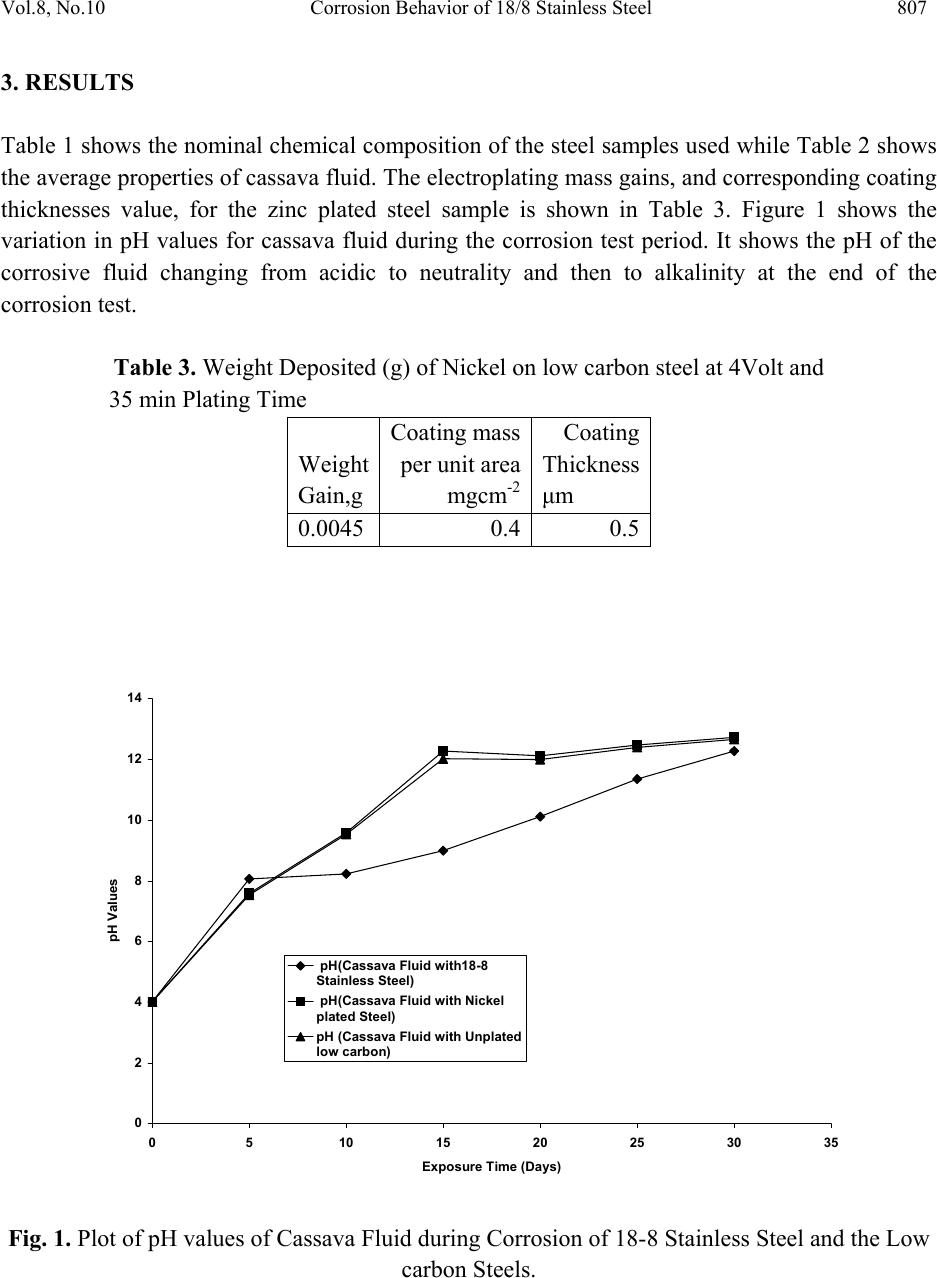 Vol.8, No.10 Corrosion Behavior of 18/8 Stainless Steel 807 3. RESULTS Table 1 shows the nominal chemical composition of the steel samples used while Table 2 shows the average properties of cassava fluid. The electroplating mass gains, and corresponding coating thicknesses value, for the zinc plated steel sample is shown in Table 3. Figure 1 shows the variation in pH values for cassava fluid during the corrosion test period. It shows the pH of the corrosive fluid changing from acidic to neutrality and then to alkalinity at the end of the corrosion test. Table 3. Weight Deposited (g) of Nickel on low carbon steel at 4Volt and 35 min Plating Time Weight Gain,g Coating mass per unit area mgcm-2 Coating Thickness μm 0.0045 0.4 0.5 0 2 4 6 8 10 12 14 0510 1520 2530 35 Exposure Time (Days) pH Values pH(Cassava Fluid with18-8 Stainless Steel) pH(Cassava Fluid with Nickel plated Steel) pH (Cassava Fluid with Unplated low carbon) Fig. 1. Plot of pH values of Cassava Fluid during Corrosion of 18-8 Stainless Steel and the Low carbon Steels. 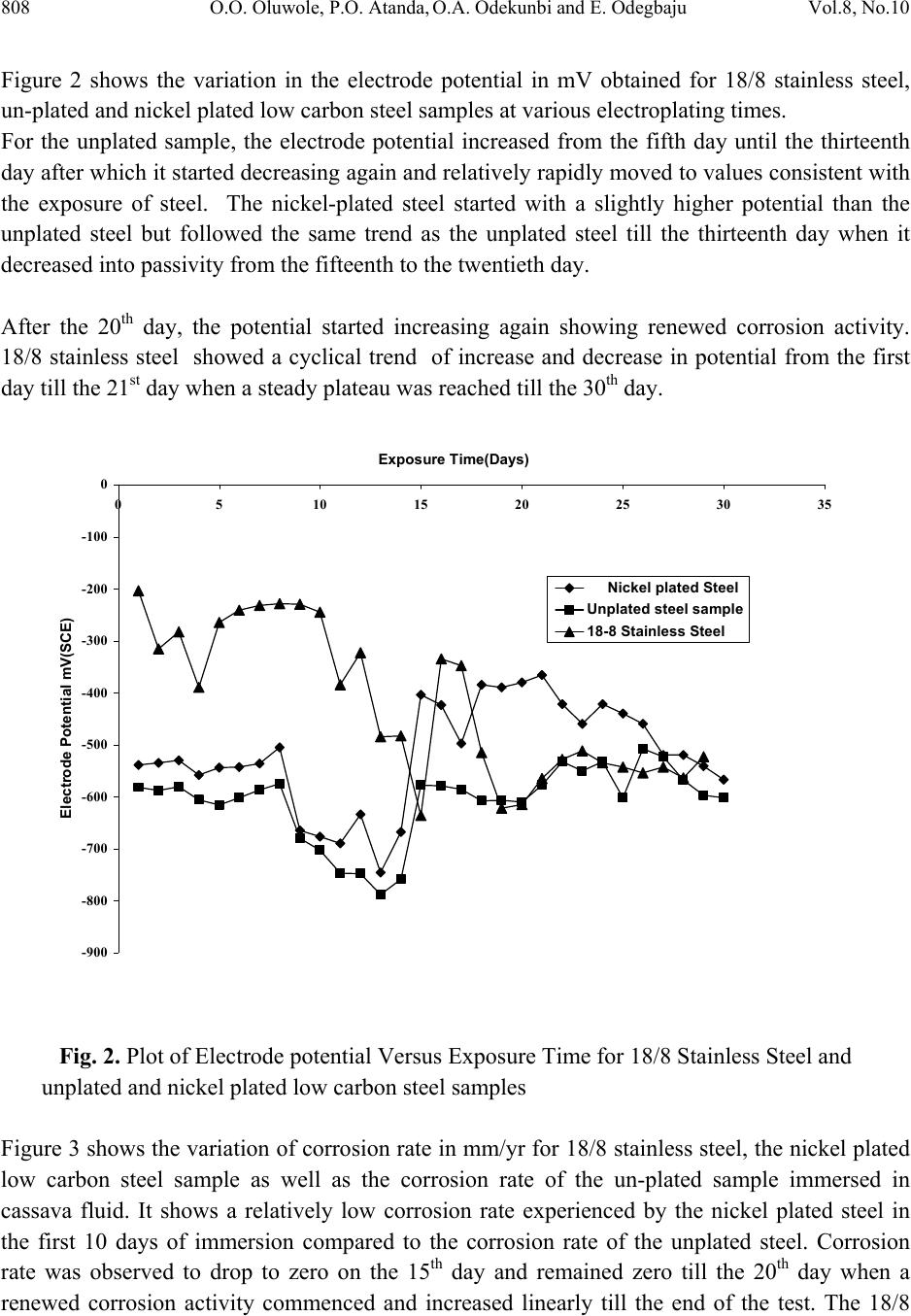 808 O.O. Oluwole, P.O. Atanda, O.A. Odekunbi and E. Odegbaju Vol.8, No.10 Figure 2 shows the variation in the electrode potential in mV obtained for 18/8 stainless steel, un-plated and nickel plated low carbon steel samples at various electroplating times. For the unplated sample, the electrode potential increased from the fifth day until the thirteenth day after which it started decreasing again and relatively rapidly moved to values consistent with the exposure of steel. The nickel-plated steel started with a slightly higher potential than the unplated steel but followed the same trend as the unplated steel till the thirteenth day when it decreased into passivity from the fifteenth to the twentieth day. After the 20th day, the potential started increasing again showing renewed corrosion activity. 18/8 stainless steel showed a cyclical trend of increase and decrease in potential from the first day till the 21st day when a steady plateau was reached till the 30th day. -900 -800 -700 -600 -500 -400 -300 -200 -100 0 0510 15 20 25 30 35 Exposure Time(Days) Elec trod e Potential mV(SCE) Nickel plated Steel Unplated steel sample 18-8 Stainless Steel Fig. 2. Plot of Electrode potential Versus Exposure Time for 18/8 Stainless Steel and unplated and nickel plated low carbon steel samples Figure 3 shows the variation of corrosion rate in mm/yr for 18/8 stainless steel, the nickel plated low carbon steel sample as well as the corrosion rate of the un-plated sample immersed in cassava fluid. It shows a relatively low corrosion rate experienced by the nickel plated steel in the first 10 days of immersion compared to the corrosion rate of the unplated steel. Corrosion rate was observed to drop to zero on the 15th day and remained zero till the 20th day when a renewed corrosion activity commenced and increased linearly till the end of the test. The 18/8 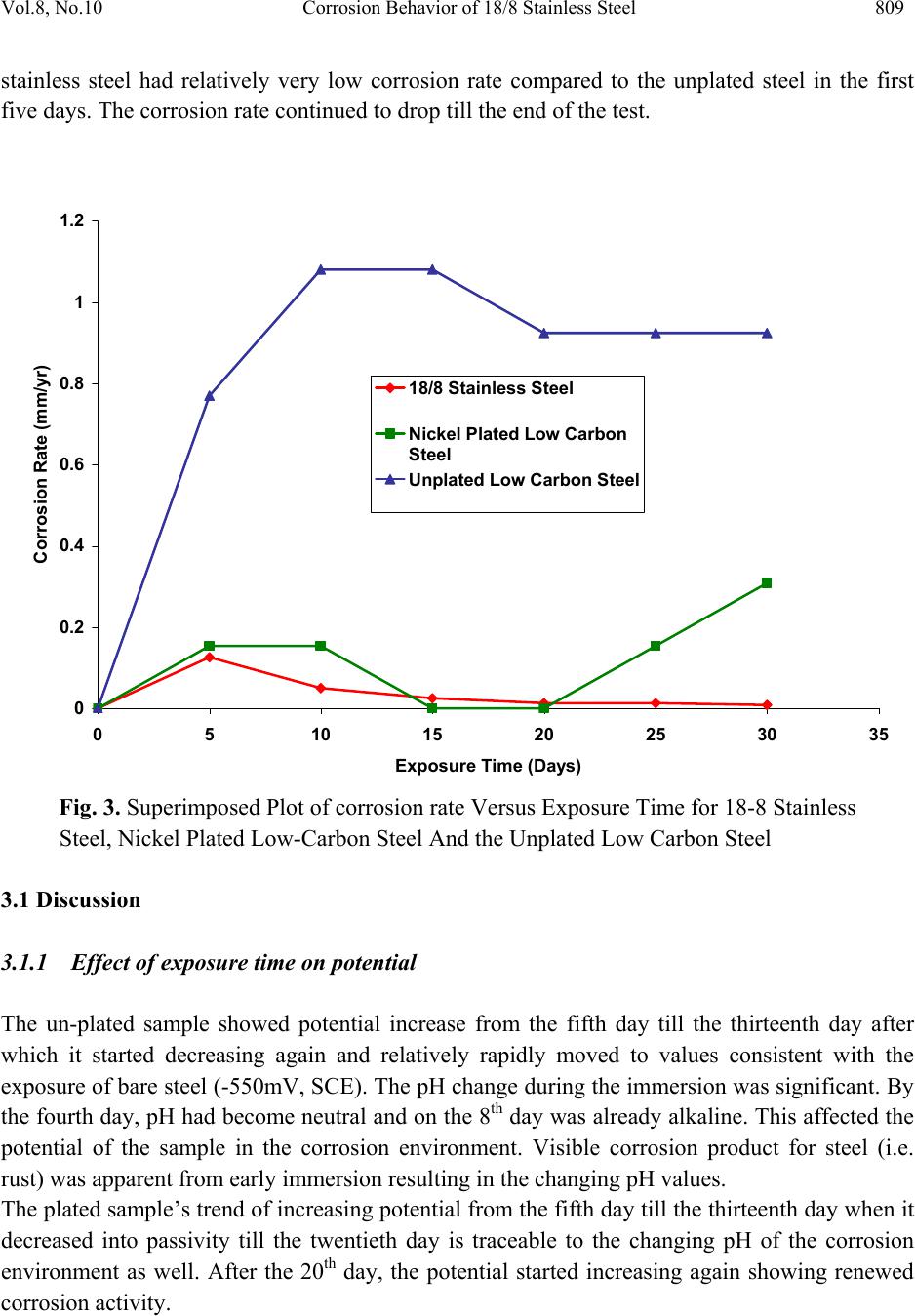 Vol.8, No.10 Corrosion Behavior of 18/8 Stainless Steel 809 stainless steel had relatively very low corrosion rate compared to the unplated steel in the first five days. The corrosion rate continued to drop till the end of the test. 0 0.2 0.4 0.6 0.8 1 1.2 0510 15 20 25 30 35 Exposure Time (Days) Corrosion Rate (mm/yr) 18/8 Stainless Steel Nickel Plated Low Carbon Steel Unplated Low Carbon Steel Fig. 3. Superimposed Plot of corrosion rate Versus Exposure Time for 18-8 Stainless Steel, Nickel Plated Low-Carbon Steel And the Unplated Low Carbon Steel 3.1 Discussion 3.1.1 Effect of exposure time on potential The un-plated sample showed potential increase from the fifth day till the thirteenth day after which it started decreasing again and relatively rapidly moved to values consistent with the exposure of bare steel (-550mV, SCE). The pH change during the immersion was significant. By the fourth day, pH had become neutral and on the 8th day was already alkaline. This affected the potential of the sample in the corrosion environment. Visible corrosion product for steel (i.e. rust) was apparent from early immersion resulting in the changing pH values. The plated sample’s trend of increasing potential from the fifth day till the thirteenth day when it decreased into passivity till the twentieth day is traceable to the changing pH of the corrosion environment as well. After the 20th day, the potential started increasing again showing renewed corrosion activity.  810 O.O. Oluwole, P.O. Atanda, O.A. Odekunbi and E. Odegbaju Vol.8, No.10 18/8 stainless steel showed a cyclical trend of increase/decrease in potential from the first day from a potential of -200mV(SCE) until the 23rd day when it settled in the potential characteristic of bare steel(-550mV(SCE)) till the last day of test. The pH of the corrosive environment was seen to move from acidic to neutral and to alkalinity within 5 days. This significant trend affected the potential as well as the corrosion activity. 3.1.2 Effect of exposure time on corrosion rate The extent of susceptibility to corrosion in natural fluids depends on the aggressiveness of chemical reactivities, transport properties of environment, concentration of corrosion species in the medium (pH), the metallurgy of the alloy sample and temperature of the corrosion medium9. In the cassava fluid, the corrosion rates of all the samples were observed to be influenced extremely by the changing pH of the corrosion environment. Corrosion rate of the 18/8 stainless steel decreased steadily after the fifth day because the pH had changed from acidity to alkalinity within five days. This decreasing trend continued till the last day of the test. For the nickel plated sample, there was active corrosion activity up to a pH of 12 which was the 15th day of exposure in the corrosion environment. Passivation was observed at a pH of 12 which was the 15th day and moved out of passivation on the 20th day with a pH of 12.5 .Thus, the corrosion rate for the nickel plated sample went into passivity with no corrosion occurring on the 15th to 20th days when the pH hit 12 and at a pH of 12.5 renewed corrosion activity began. This is showing that E-pH diagram for Ni-CN- prevalence diagram has a thin passive segment between pH of 12 and 12.5 after which corrosion species become prevalent. The unplated steel was experiencing increased high level corrosion activity up till the fifteenth day rising from a pH of 4.0 to 12 after which there was a decrease in corrosion rate on the 20th day remaining constant till the end of the test. This decrease corresponds to the decreased potential from -770Mv(SCE) to -550Mv(SCE) which is characteristic of bare steel and this potential remained steady till the end of the test. 3.1.3 Suitability of nickel coatings in a Cassava environment It is evident that uncoated steel is unsuitable for a material of construction for cassava processing equipment and this work tested the use of 18/8 stainless steel and nickel plated coatings as a potential protective coating. Unfortunately, nickel appears to have renewed rapid corrosion activity at a pH above 12.5 after a thin segment of passivity at pH of 12 to 12.5. The reason for the relatively rapid corrosion of nickel is undoubtedly due to the easy complexing of nickel as a cyanide species and the consequently more rapid corrosion after a pH of 12.5. Unfortunately, results in nickel being an unsuitable material for the protection of steel in cassava fluid. 18/8  Vol.8, No.10 Corrosion Behavior of 18/8 Stainless Steel 811 stainless steel is suitable for protection in cassava environment. REFERENCES [1] Adebiyi, K.A, Hammed, K.A, and Ajayi, E. O. (2003). Predictive model for evaluating corrosion rate of mild steel in six environments, LAUTECH Journal of Engineering and Technology. In Jekayinfa, S.O.(Eds),Effect of cassava fluid on corrosion performance of mild steel,Vol 52, No.5,pp.286-292. [2] Jekayinfa S.O., Waheed M.A., Adebiyi K.A. and Adebiyi F.T. (2005); Effect of cassava fluid on corrosion performance of mild steel. Anti-Corrosion Methods and Materials, Volume 52, No. 5,pp.286-292. [3] O.O. Oluwole, D.T. Oloruntoba and O. Awheme(2008)"Effect of zinc plating of low carbon steel on corrosion resistance in cocoa fluid environment" Materials and Design, Elsevier,29(6),1266-1274. [4] O.O. Oluwole, D.T. Oloruntoba and O. Awheme(2008)"Effect of zinc plating of low carbon steel on corrosion resistance in cassava fluid environment” Corrosion Engineering Science and Technology, Institute of Materials, Minerals and Mining, UK,43(4),320-323. [5] George Di Bari (1994): Nickel Plating, Surface Engineering, ASM International, Materials Park ASM Handbook, OH 44073 Volume 5, p 201. [6] Oluwole.O.O , Oloruntoba D.T,and Ibironke.D.E(2007) ‘Effectiveness Of Nickel Coating On Manganese Steel In Seawater Environment’ Continuing Research [7] Hibbert D.B. and JamesA.M.(1984); Dictionary of Electrochemistry, Macmillan Press, London. [8] Kakani.S.L and Kakani.A(2004) ‘Materials Science’ New Age International Ltd., New Delhi., p. 381-383. [9] Loto C.A. and Adesomo M.A. (1988); The corrosion of mild steel in maize juice, acidified maize juice and acetic acid environment, African Journal of Science and Technology Series A, Vol.7 No.1. |

