Paper Menu >>
Journal Menu >>
 Journal of Mi nerals & Materials Characterization & Engineering, Vol. 8, No.7, pp 563-568, 2009 jmmce.org Printed in the USA. All rights reserved 563 Preparation of Al-5Ti Master Alloys for the In-Situ Processing of Al-TiC Metal Matrix Composites Satish Babu Boppana1, K .Chennakeshavalu2 Department of Mech. Engg., K.S.I.T, Bangalore-560062, India 1satishbabu3@yahoo.co.in, 2kcl_ksit@yahoo.com ABSTRACT The present paper reports on the preparation of various Al-5Ti master alloys by the reaction of halide salt (K2TiF6) with molten Al in an induction furnace. The master alloys are supposed to be used for the in-situ processing of Al-TiC Metal Matrix Composites. During the preparation of these master alloys the reaction temperature was varied from 800-10000C with the intervals of 1000C, while the reaction time was varied at 45, 60 and 75 min. The binary master alloys prepared were characterized using image analyzer and particle size was measured. It was found that the master alloy Al-5Ti prepared at 8000C, and 45 minutes had a minimum particle size of 9.0 micrometer. Also it is observed that the population of particles less than 10 micrometer decreases at higher reaction temperature. KEYWORDS: Master-alloy, Al-TiC Metal Matrix Composites, Characterization 1. INTRODUCTION Applications of composite materials are among the most important developments in materials engineering in recent years. Metal Matrix Composites have emerged as an important class of materials and are increasingly utilized in various engineering applications, such as aerospace, marine, automobile and turbine-compressor engineering, which require materials offering a combination of light weight with considerably accelerated mechanical and physical properties such as strength, toughness, stiffness and resistance to high temperature. Titanium is a normal impurity of aluminum, originating from the few percent TiO2 present in bauxites. It is also a common addition to many alloys because it is a very potent grain refiner. At the aluminum end of diagram there is a peritectic reaction, liq.+TiAl3→Alss, at 938K, in which  564 Satish Babu Boppana, K .Chennakeshavalu Vol.8, No.7 the liquid contains 0.12-0.15%Ti and the Alss 1.2-1.4% Ti solubility decreases to 0.2-0.3% Ti at 800K and liquid solubility increases regularly to 1% Ti at 1200K. By quenching from the liquid up to 5% Ti can be held in solution. TiAl3 (37.2%Ti) has a range of existence from 36.5 to 37.5%, and is formed by peritectic reaction at 1610K from a -phase, which is a solid solution of aluminium in TiAl. TiAl3 is tetragonal; space group I4/mm3; 8 atoms to the unit cell; parameters a=3.85110-10m; density 3.370kg/m3. Accordingly at temperature below 850K the lattice parameter are a = 3.87510- 10m, c=33.87310-10m, and the unit cell contains 32 atoms. The hardness of TiAl3 is 4,000- 7,000MN/m2. Heat of formation is 35 kJ/gm-mole. In an attempt to understand the mechanism of the reaction between the molten aluminium and the salts during the production of Al-Ti-B master alloys, detailed experiments have been carried out by Kori et al. [1] in the laboratory with different mixtures of salts and aluminium using techniques such as differential thermal analysis (DTA), thermo gravimetric analysis (TGA), infrared (IR) spectroscopy, X ray diffraction (XRD), chemical analysis and microscopy. A number of patents exist on the preparation of Al-Ti, Al-B and Al-Ti-B master alloys. Most of the past work was confined to grain refinement studies carried out using commercial grain refinements. A number of methods of production of grain refiners have been reported in the literature, such as the reaction of fluoride salts with aluminium, reaction of molten aluminium with oxides, thermal reduction and electrolysis, melting of elemental blends, mechanical alloying, and rapid solidification processing [2]. Sivaramakrishnan [4, 5] successfully prepared Al-Ti and Al-Ti-B master alloys in both wire and pellet form by the reaction of B2O3 and TiO3 with molten aluminium. They found that these alloys were better than grain refiners compared to some of the commercial grain refiners used in their studies. Zhuxian [6] have prepared Al-Ti-B master alloys by the thermal reduction and electrolysis of titanium dioxide and diboron trioxide in cryolite alumina melts in the presence of aluminium at 1000oC. They were successful in preparing master alloy containing up to 4% Ti and close to 2% B. The Al-Ti-B master alloy produced by electrolysis has higher purity, though the rate of production is very low by this method. Venkateswarlu [7] have prepared Al-5Ti master alloy by melting aluminium and titanium sponge together and suggested that master alloys prepared by using titanium sponge show better grain refining efficiency in the as cast condition.This has been attributed to higher titanium recovery in the case of the sponge route and hence a larger volume fraction of TiAl3 particles. Krishnan [8] has used a combination of the melting route and the salt route for making Al-Ti master alloys. In their method they melted aluminium and titanium sponge together and allowed the melt to react with KBF4 in order to prepare Al-Ti-B master alloy. This method appears to yield master alloys that are more efficient than those produced by the salt route. Master alloys produced by mechanical alloying were found to be much more effective compared to conventional Al-Ti and Al-Ti-B master alloy. This is probably due to the possibility of formation of nanocrystalline TiAl3 and TiB2 particles, which may enhance the grain refining  Vol.8, No.7 Preparation of Al-5Ti Master Alloys 565 efficiency of master alloy. However, mechanical alloying has the disadvantage of high production cost, low production rate and the possibility of contamination from the milling media. 2. EXPERIMENTAL DETAILS 2.1 Preparation of Binary Al-Ti Master alloys A number of binary Al-Ti master alloys as shown in Table 1 have been prepared by the salt route, by reaction of K2TiF6 salt with liquid Al in an induction furnace. The process parameter such as reaction temperature was varied from 8000C-10000C with intervals of 1000C, while reaction time was varied from 45-75 min with intervals of 15min. For this, commercial purity aluminium (CPAl) was super heated to 8000C in an induction furnace. The furnace temperature was controlled to an accuracy of 5 0C by a digital temperature controller. Once the required temperature is attained, preheated (2000C) K2TiF6 salt was added to the melt. The reaction between molten Al and the halide salts is generally vigorous and highly exothermic. The entire melting operation was carried out under cover flux (45% NaCl+ 45%KCl+10%NaF). After the completion of the reaction, the spent salt was decanted from the surface of the molten alloy and the alloy is cast into a cylindrical graphite mould. These above prepared master alloys have been characterized for their particle size analysis using Clemex Image Analyzer(Nikon Microscope LV150). 2.2 Characterization of Master alloys A specimen of 5-7mm height was taken from the bottom of the casting. One surface of the specimen is initially polished using belt grinder and then on a series of emery papers with increasing fineness, to remove any of the scratches present. Final polishing was carried out on a disc polisher using 400-mesh alumina powder, until a mirror finish surface is obtained. Polished samples were cleaned with soap solution and distilled water. The samples so prepared were etched using Keller’s reagent (2.5%HNO3, 1.5%HCl, 1%HF and 95%H2O by volume) and then they were taken for microstructural studies using Image Analyzer 3. RESULTS AND DISCUSSION The particle sizes of the various binary Al-5Ti master alloys prepared were studied by using Image analyzer via quantitative metallographic technique. This technique was used to get an idea of the size of the Al3Ti particles in the master alloys prepared in an induction furnace under different processing conditions. Statistical data obtained from particle size analysis carried out using Image Analyzer is shown in Table 2 Table 1 shows various binary master alloys prepared at different reaction temperatures of 8000C, 9000C and 10000C in an induction furnace with a reaction time of 45, 60 and 75 min. Master alloys prepared at a reaction temperature of 8000C, and with a reaction time of 45 min. were taken for SEM studies.Fig-1(a) and (b) show microphotographs of master alloys prepared at 8000C, 45 min and 9000C, 45 min obtained through SEM and Image Analyzer respectively. It 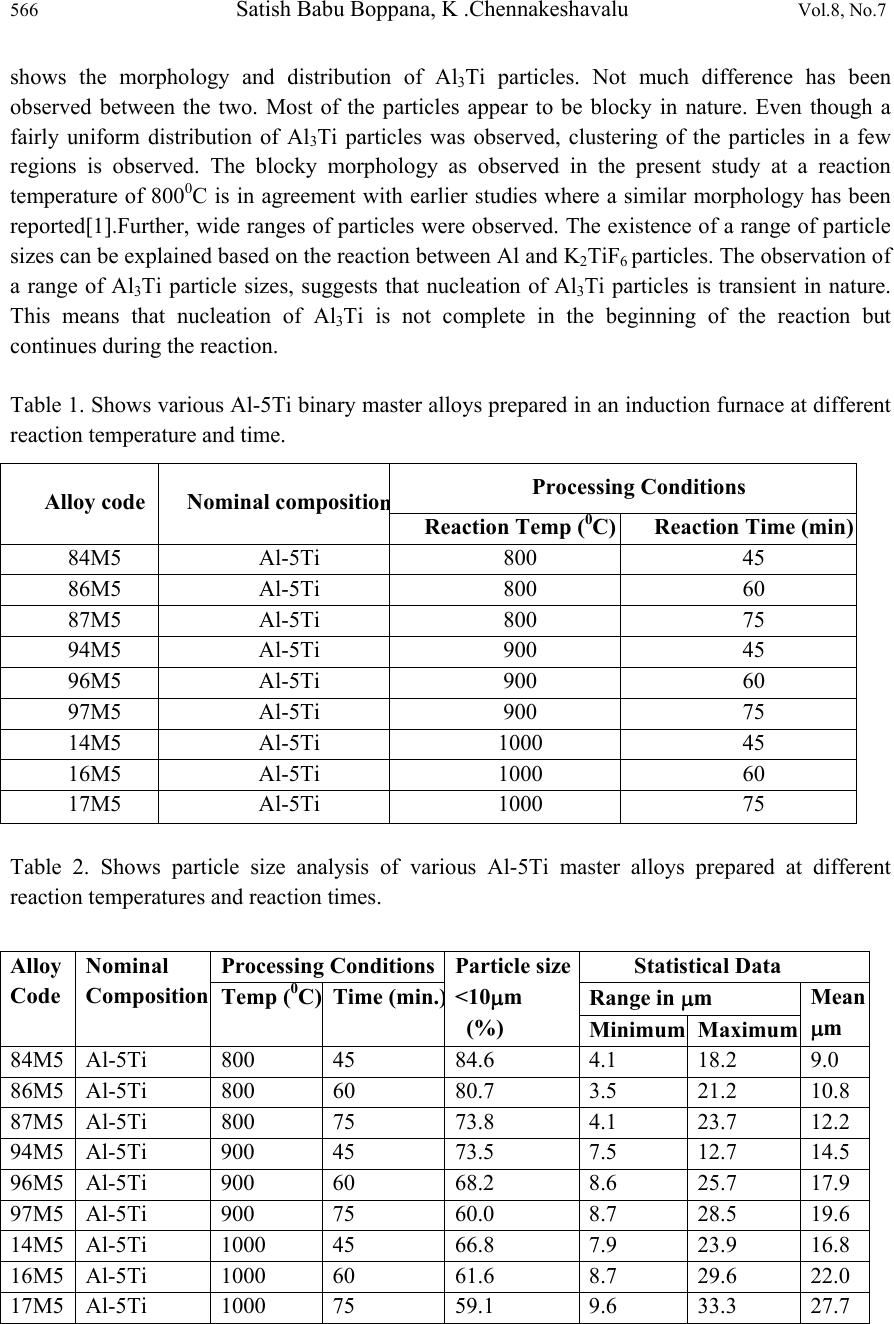 566 Satish Babu Boppana, K .Chennakeshavalu Vol.8, No.7 shows the morphology and distribution of Al3Ti particles. Not much difference has been observed between the two. Most of the particles appear to be blocky in nature. Even though a fairly uniform distribution of Al3Ti particles was observed, clustering of the particles in a few regions is observed. The blocky morphology as observed in the present study at a reaction temperature of 8000C is in agreement with earlier studies where a similar morphology has been reported[1].Further, wide ranges of particles were observed. The existence of a range of particle sizes can be explained based on the reaction between Al and K2TiF6 particles. The observation of a range of Al3Ti particle sizes, suggests that nucleation of Al3Ti particles is transient in nature. This means that nucleation of Al3Ti is not complete in the beginning of the reaction but continues during the reaction. Table 1. Shows various Al-5Ti binary master alloys prepared in an induction furnace at different reaction temperature and time. Table 2. Shows particle size analysis of various Al-5Ti master alloys prepared at different reaction temperatures and reaction times. Alloy code Nominal compositio n Processing Conditions Reaction Temp (0C)Reaction Time (min) 84M5 Al-5Ti 800 45 86M5 Al-5Ti 800 60 87M5 Al-5Ti 800 75 94M5 Al-5Ti 900 45 96M5 Al-5Ti 900 60 97M5 Al-5Ti 900 75 14M5 Al-5Ti 1000 45 16M5 Al-5Ti 1000 60 17M5 Al-5Ti 1000 75 Alloy Code Nominal Composition Processing ConditionsParticle size <10m (%) Statistical Data Temp (0C) Time (min. ) Range in m Mean m Minimum Maximum 84M5 Al-5Ti 800 45 84.6 4.1 18.2 9.0 86M5 Al-5Ti 800 60 80.7 3.5 21.2 10.8 87M5 Al-5Ti 800 75 73.8 4.1 23.7 12.2 94M5 Al-5Ti 900 45 73.5 7.5 12.7 14.5 96M5 Al-5Ti 900 60 68.2 8.6 25.7 17.9 97M5 Al-5Ti 900 75 60.0 8.7 28.5 19.6 14M5 Al-5Ti 1000 45 66.8 7.9 23.9 16.8 16M5 Al-5Ti 1000 60 61.6 8.7 29.6 22.0 17M5 Al-5Ti 1000 75 59.1 9.6 33.3 27.7 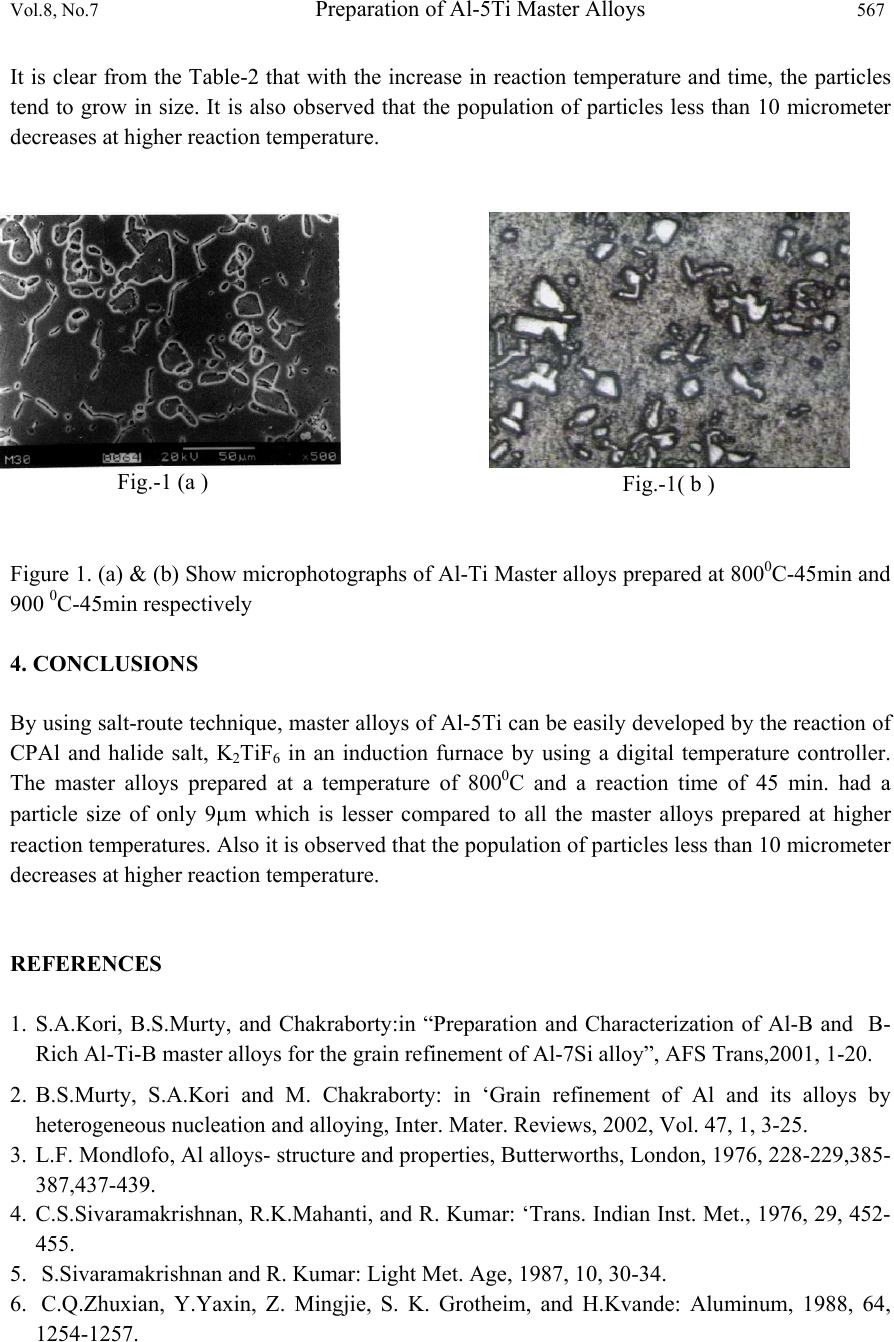 Vol.8, No.7 Preparation of Al-5Ti Master Alloys 567 It is clear from the Table-2 that with the increase in reaction temperature and time, the particles tend to grow in size. It is also observed that the population of particles less than 10 micrometer decreases at higher reaction temperature. Fig.-1 (a ) Fig.-1( b ) Figure 1. (a) & (b) Show microphotographs of Al-Ti Master alloys prepared at 8000C-45min and 900 0C-45min respectively 4. CONCLUSIONS By using salt-route technique, master alloys of Al-5Ti can be easily developed by the reaction of CPAl and halide salt, K2TiF6 in an induction furnace by using a digital temperature controller. The master alloys prepared at a temperature of 8000C and a reaction time of 45 min. had a particle size of only 9m which is lesser compared to all the master alloys prepared at higher reaction temperatures. Also it is observed that the population of particles less than 10 micrometer decreases at higher reaction temperature. REFERENCES 1. S.A.Kori, B.S.Murty, and Chakraborty:in “Preparation and Characterization of Al-B and B- Rich Al-Ti-B master alloys for the grain refinement of Al-7Si alloy”, AFS Trans,2001, 1-20. 2. B.S.Murty, S.A.Kori and M. Chakraborty: in ‘Grain refinement of Al and its alloys by heterogeneous nucleation and alloying, Inter. Mater. Reviews, 2002, Vol. 47, 1, 3-25. 3. L.F. Mondlofo, Al alloys- structure and properties, Butterworths, London, 1976, 228-229,385- 387,437-439. 4. C.S.Sivaramakrishnan, R.K.Mahanti, and R. Kumar: ‘Trans. Indian Inst. Met., 1976, 29, 452- 455. 5. S.Sivaramakrishnan and R. Kumar: Light Met. Age, 1987, 10, 30-34. 6. C.Q.Zhuxian, Y.Yaxin, Z. Mingjie, S. K. Grotheim, and H.Kvande: Aluminum, 1988, 64, 1254-1257.  568 Satish Babu Boppana, K .Chennakeshavalu Vol.8, No.7 7. K.Venkateswarlu, B.S.Murty, P.RamChandra Rao, and M. Chakraborty: Proc. Int. Seminar on ‘Non ferrous metals and materials’, Jan.2000, Jamshedpur, India, 193-198. 8. T.S.Krishnan, P.K.Rajagopalan.R.R Gund, J.Krishnan, and D.K.Bose: J.Alloy. Compd., 1998, 269, 138-140. |

