Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 8, No.7, pp 531-539, 2009 jmmce.org Printed in the USA. All rights reserved 531 Model for Predictive Analysis of Heat Absorbed by Oxalic Acid Solution Relative to the Solution Temperature during Leaching of Iron Oxide Ore C. I. Nwoye*1, C. S. Nwobodo1, C. Nlebedim2, U.C. Nwoye3, R. A. Umana4 and G. C.Obasi5 1Department of Materials and Metallurgical Engineering, Federal University of Technology, Owerri, Nigeria. 2Department of Material Science, University of Cadiff, Wales, United Kingdom 3Data Processing, Modelling and Simulation Unit, Weatherford Nig. Ltd. Port-Harcourt Nigeria. 4Department of Mathematics and Computer Science, Federal University of Technology, Owerri, Nigeria. 5Department of Material Science, Aveiro University, Portugal. *Corresponding Author: chikeyn@yahoo.com Abstract Model for predictive analysis of the quantity of heat absorbed by oxalic acid solution during leaching of iron oxide ore has been derived. It was observed that the validity of the model is rooted in the expression (lnQ)/N = √T where both sides of the relationship are correspondingly almost equal. The model was found to depend on the value of the final solution temperature measured during the experiment. The respective deviation of the model-predicted Q values from the corresponding experimental values was found to be less than 21% which is quite within the acceptable range of deviation limit of experimental results. The positive values of heat absorbed as obtained from experiment and model indicate and agree that the leaching process is endothermic in nature. Keywords: Model, Predictive Analysis, Heat Absorbed, Solution Temperature, Oxalic Acid, Iron Oxide Ore, Leaching. 1. INTRODUCTION The use of different organic and inorganic acids has been evaluated in several studies. Sidhu et al. [1] evaluated the dissolution of iron oxides and oxyhydroxides in hydrochloric and perchloric acids. Lim-Nunez and Gilkes [2] used synthetic metal-containing goethite and haematite in their  532 C. I. Nwoye, et al. Vol.8, No.7 evaluation while Borghi et al. [3] studied the effect of EDTA and Fe(II) during the dissolution of magnetite. The industrial use of sulphuric acid and other inorganic acids to dissolve iron oxide has not fared too well. Chiarizia and Hotwitz [4] studied the dissolution of goethite in several inorganic acids belonging to the families of the carboxylic and diphosphoric acids in the presence of reducing agents. Ambikadevi and Lalithambika [5] evaluated the effectiveness of several organic acids (such as acetic, formic, citric, ascorbic acids etc.) used for dissolving iron from iron compounds. Oxalic acid was found to be the most promising because of its acid strength, good comlexing characteristics and high reducing power, compared to other organic acids. Using oxalic acid, the dissolved iron can be precipitated from the leach solution as ferrous oxalate, which can be re-processed to form pure haematite by calcinations [6]. Many researchers have studied the use of oxalic acid to dissolve iron oxide on a laboratory scale [7-13]. Lee et al [14] used 0.19-0.48M oxalic acid to dissolve hydrated iron oxide. Iron dissolution was found [14] to reach 90% for a 20% slurry within 60mins. using 0.19M oxalic for the finer fraction (< 150μm) containing 0.56% Fe2O3.The coarser fraction (>150μm) containing 1.06% Fe2O3 achieved a lower iron removal, reaching a steady state of only 78% after 1 h of leaching. Although the pH was not measured or controlled, it was expected that the liquor pH is < pH1 at the oxalic acid concentration range studied (0.19-0.48). Taxiarchou et al. [6] found that the maximum iron dissolution of only 40% is within 3 h at temperatures in the range 90-1000C. At 0.5M oxalate and all temperatures (25, 60 and 800C) the dissolution of iron was faster at a lower pH in the range pH 1-5 studied. Biological processes for iron dissolution have been evaluated by several researchers based on the use of several micro organisms that were easily sourced and isolated. Mandal and Banerjee [15] recently presented their findings on the study of the use of Aspergillus niger and their cultural filtrates for dissolving iron present in iron compounds. Nwoye [16] derived a model for evaluating the final pH of the leaching solution during leaching of iron oxide ore in oxalic acid solution. The model evaluates the pH value as the sum of two parts, involving the % concentrations of Fe and Fe2O3 dissolved. The model can be expressed as; = 0.5 K1 + K2 %Fe % Fe2O3 (1) Where K1 and K2 = dissolution constants of Fe and Fe2O3 respectively. = final pH of leaching solution (after time t). It was also found that the model [16] could predict the concentration of Fe or Fe2O3 dissolved in the oxalic acid solution at a particular final solution pH by taking Fe or Fe2O3 as the subject formular. The prevailing process conditions under which the model works include: leaching time of 30mins., constant leaching temperature of 30oC, average ore grain size; 150µm and 0.1M oxalic acid. Nwoye [17] has reported that the heat absorbed by oxalic acid solution during leaching of iron oxide ore can be predicted using the model he derived which works under the process condition; initial pH 6.9, average ore grain size; 150µm and leaching temperature; 300C. The model [17] can be stated as  Vol.8, No.7 Model for Predictive Analysis of Heat Absorbed 533 Q = KN (2) %Fe2O3 Where Q = Quantity of heat absorbed by oxalic acid solution during the leaching process. (J) = Final pH of the leaching solution (at time t). %Fe2O3= Concentration of haematite dissolved in oxalic acid solution during the leaching process. KN = 4.57(Haematite dissolution constant in oxalic acid solution) determined in the experiment [17]. Nwoye [17] carried out further work on the model using the same process conditions and observed that on re-arranging the model as; %Fe2O3 = KN (3) Q the concentrations of haematite predicted deviated very insignificantly from the corresponding experimental values. In this case, the value of Q was calculated by considering the specific heat capacity of oxalic acid. Values of heat absorbed by the oxalic acid solution during the leaching of iron oxide ore as predicted by the model [17] agree with the experimental values that the leaching process is endothermic. This is because all the predicted values of the heat absorbed by the oxalic acid solution were positive. The model shows that the quantity of heat absorbed by oxalic acid solution during the leaching process is directly proportional to the final pH of the solution and inversely proportional to the concentration of haematite dissolved. The aim of this work is to derive a model for predictive analysis of the heat absorbed by oxalic acid solution relative to the solution temperature during leaching of Itakpe (Nigeria) iron oxide ore in oxalic acid solution. 2. MODEL During the leaching process, the iron ore (being in solid phase) was assumed to be stationary. The leaching occurs as a result of the attack on the ore by hydrogen ions from the oxalic acid within the liquid phase (in the presence of oxygen). 2.1 Model Formulation Results of previous research work [18] carried out were used for this work. Statistical and computational analysis of these results [18] presented in Table 1, gave rise to Table 2 which indicate that; (lnQ) = √T (approximately) (4) N lnQ = N(√T) (5) 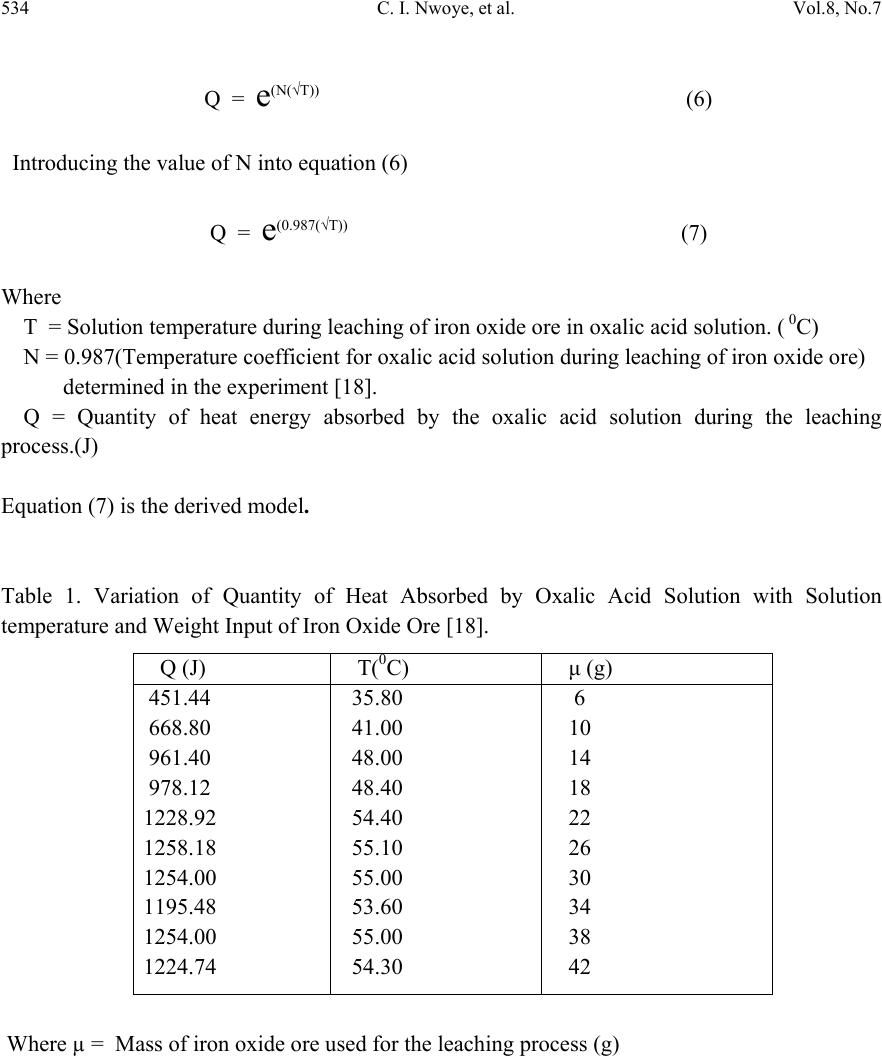 534 C. I. Nwoye, et al. Vol.8, No.7 Q = e(N(√T)) (6) Introducing the value of N into equation (6) Q = e(0.987(√T)) (7) Where T = Solution temperature during leaching of iron oxide ore in oxalic acid solution. ( 0C) N = 0.987(Temperature coefficient for oxalic acid solution during leaching of iron oxide ore) determined in the experiment [18]. Q = Quantity of heat energy absorbed by the oxalic acid solution during the leaching process.(J) Equation (7) is the derived model. Table 1. Variation of Quantity of Heat Absorbed by Oxalic Acid Solution with Solution temperature and Weight Input of Iron Oxide Ore [18]. Where µ = Mass of iron oxide ore used for the leaching process (g) Q (J) T(0C) μ (g) 451.44 668.80 961.40 978.12 1228.92 1258.18 1254.00 1195.48 1254.00 1224.74 35.80 41.00 48.00 48.40 54.40 55.10 55.00 53.60 55.00 54.30 6 10 14 18 22 26 30 34 38 42 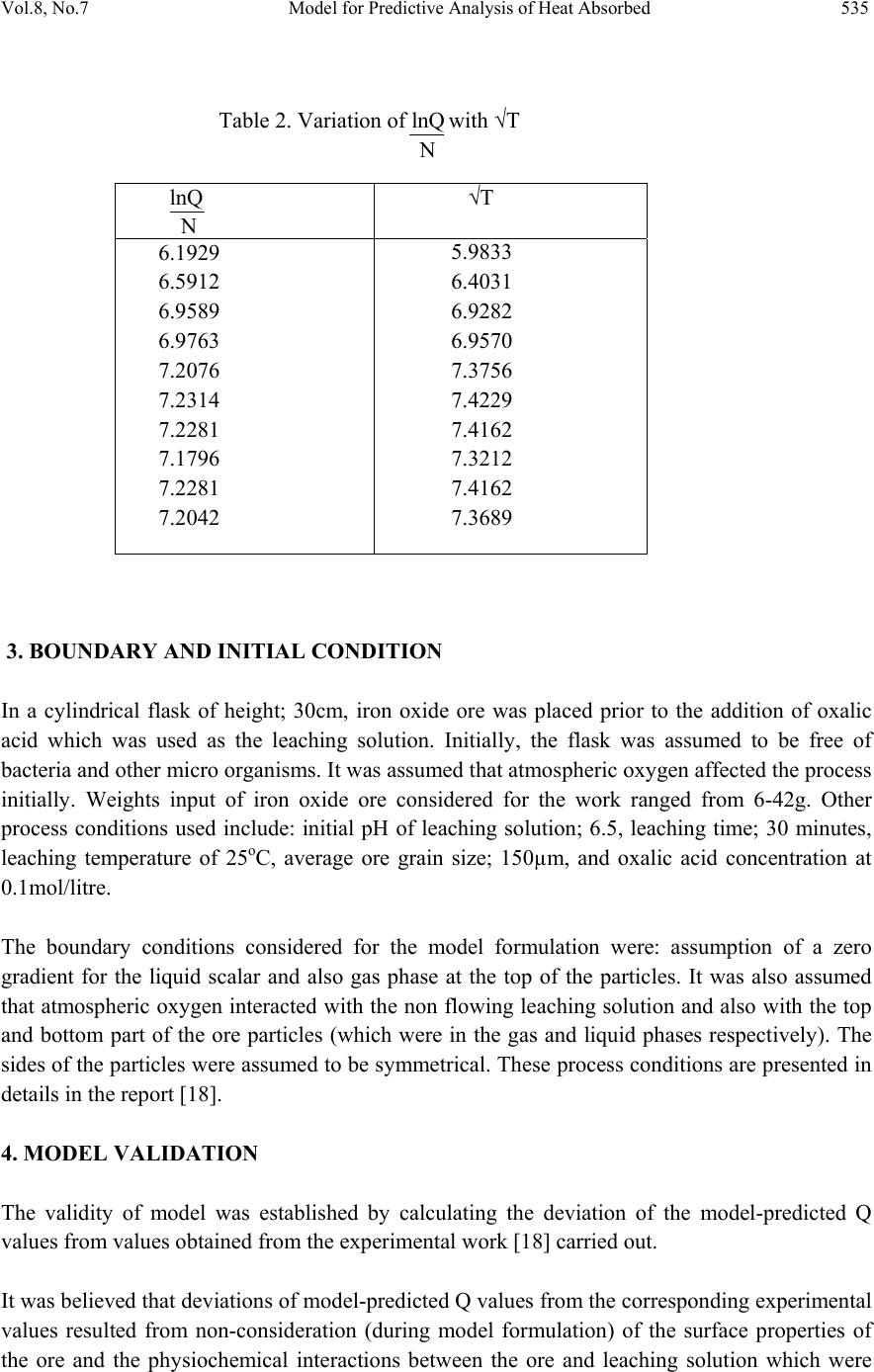 Vol.8, No.7 Model for Predictive Analysis of Heat Absorbed 535 Table 2. Variation of lnQ with √T N 3. BOUNDARY AND INITIAL CONDITION In a cylindrical flask of height; 30cm, iron oxide ore was placed prior to the addition of oxalic acid which was used as the leaching solution. Initially, the flask was assumed to be free of bacteria and other micro organisms. It was assumed that atmospheric oxygen affected the process initially. Weights input of iron oxide ore considered for the work ranged from 6-42g. Other process conditions used include: initial pH of leaching solution; 6.5, leaching time; 30 minutes, leaching temperature of 25oC, average ore grain size; 150µm, and oxalic acid concentration at 0.1mol/litre. The boundary conditions considered for the model formulation were: assumption of a zero gradient for the liquid scalar and also gas phase at the top of the particles. It was also assumed that atmospheric oxygen interacted with the non flowing leaching solution and also with the top and bottom part of the ore particles (which were in the gas and liquid phases respectively). The sides of the particles were assumed to be symmetrical. These process conditions are presented in details in the report [18]. 4. MODEL VALIDATION The validity of model was established by calculating the deviation of the model-predicted Q values from values obtained from the experimental work [18] carried out. It was believed that deviations of model-predicted Q values from the corresponding experimental values resulted from non-consideration (during model formulation) of the surface properties of the ore and the physiochemical interactions between the ore and leaching solution which were lnQ N √T 6.1929 6.5912 6.9589 6.9763 7.2076 7.2314 7.2281 7.1796 7.2281 7.2042 5.9833 6.4031 6.9282 6.9570 7.3756 7.4229 7.4162 7.3212 7.4162 7.3689 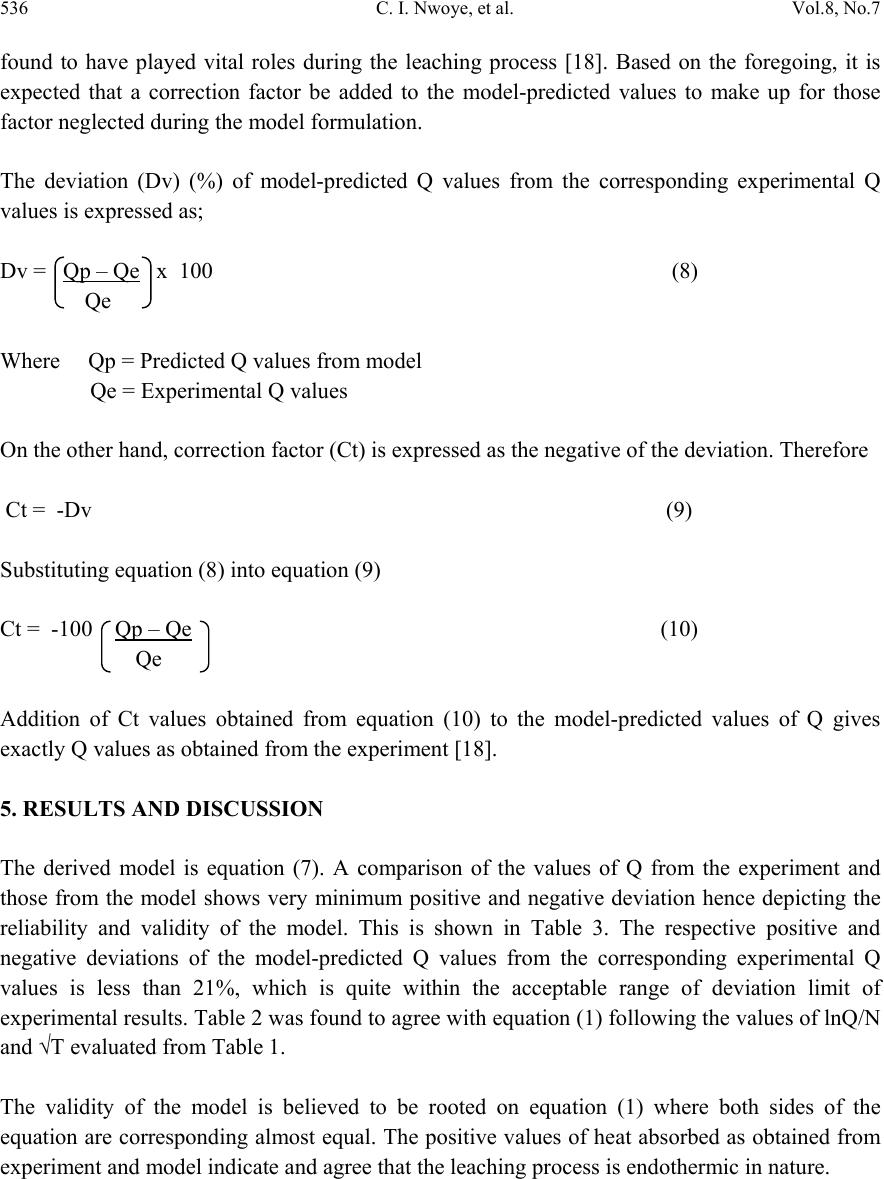 536 C. I. Nwoye, et al. Vol.8, No.7 found to have played vital roles during the leaching process [18]. Based on the foregoing, it is expected that a correction factor be added to the model-predicted values to make up for those factor neglected during the model formulation. The deviation (Dv) (%) of model-predicted Q values from the corresponding experimental Q values is expressed as; Dv = Qp – Qe x 100 (8) Qe Where Qp = Predicted Q values from model Qe = Experimental Q values On the other hand, correction factor (Ct) is expressed as the negative of the deviation. Therefore Ct = -Dv (9) Substituting equation (8) into equation (9) Ct = -100 Qp – Qe (10) Qe Addition of Ct values obtained from equation (10) to the model-predicted values of Q gives exactly Q values as obtained from the experiment [18]. 5. RESULTS AND DISCUSSION The derived model is equation (7). A comparison of the values of Q from the experiment and those from the model shows very minimum positive and negative deviation hence depicting the reliability and validity of the model. This is shown in Table 3. The respective positive and negative deviations of the model-predicted Q values from the corresponding experimental Q values is less than 21%, which is quite within the acceptable range of deviation limit of experimental results. Table 2 was found to agree with equation (1) following the values of lnQ/N and √T evaluated from Table 1. The validity of the model is believed to be rooted on equation (1) where both sides of the equation are corresponding almost equal. The positive values of heat absorbed as obtained from experiment and model indicate and agree that the leaching process is endothermic in nature. 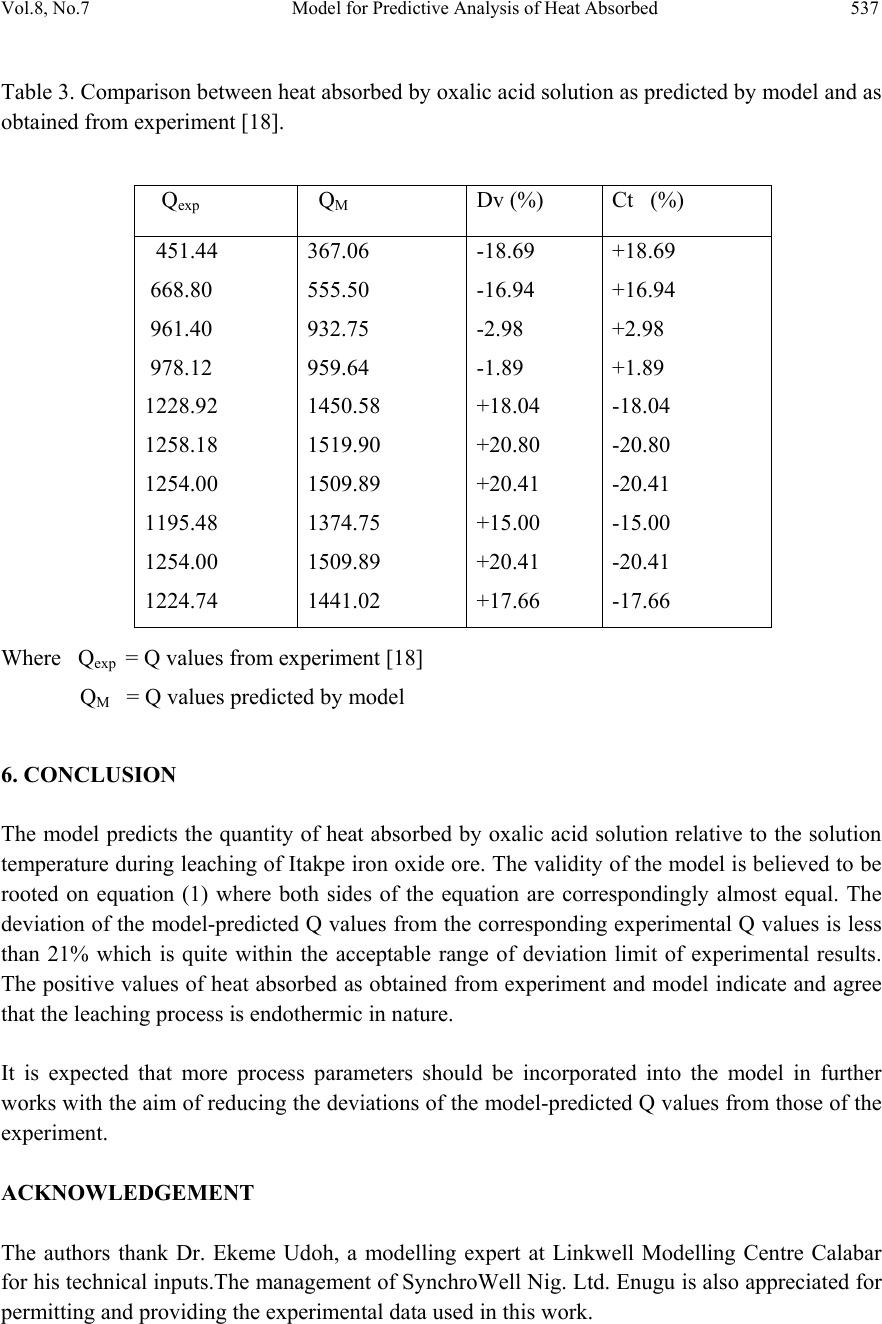 Vol.8, No.7 Model for Predictive Analysis of Heat Absorbed 537 Table 3. Comparison between heat absorbed by oxalic acid solution as predicted by model and as obtained from experiment [18]. Where Qexp = Q values from experiment [18] QM = Q values predicted by model 6. CONCLUSION The model predicts the quantity of heat absorbed by oxalic acid solution relative to the solution temperature during leaching of Itakpe iron oxide ore. The validity of the model is believed to be rooted on equation (1) where both sides of the equation are correspondingly almost equal. The deviation of the model-predicted Q values from the corresponding experimental Q values is less than 21% which is quite within the acceptable range of deviation limit of experimental results. The positive values of heat absorbed as obtained from experiment and model indicate and agree that the leaching process is endothermic in nature. It is expected that more process parameters should be incorporated into the model in further works with the aim of reducing the deviations of the model-predicted Q values from those of the experiment. ACKNOWLEDGEMENT The authors thank Dr. Ekeme Udoh, a modelling expert at Linkwell Modelling Centre Calabar for his technical inputs.The management of SynchroWell Nig. Ltd. Enugu is also appreciated for permitting and providing the experimental data used in this work. Qexp QM Dv (%) Ct (%) 451.44 668.80 961.40 978.12 1228.92 1258.18 1254.00 1195.48 1254.00 1224.74 367.06 555.50 932.75 959.64 1450.58 1519.90 1509.89 1374.75 1509.89 1441.02 -18.69 -16.94 -2.98 -1.89 +18.04 +20.80 +20.41 +15.00 +20.41 +17.66 +18.69 +16.94 +2.98 +1.89 -18.04 -20.80 -20.41 -15.00 -20.41 -17.66  538 C. I. Nwoye, et al. Vol.8, No.7 REFERENCES [1] Sidhu, P.S., Gilkes, R. J., Cronell, R. M., Posner, A. M., Quirk, J.P. (1981) Dissolution of Iron Oxides and Oxydroxides in Hydrochloric and Perchloric Acids. Miner. 29, 269-276. [2]Lim-Numez, R., Gilkes, R. J., (1987) Acid Dissolution of Metal-Containing Geothites and Haematites. Int. J. Miner. Process. 80, 144-152. [3]Borghi, E. B., Regazzoni, A. E., Maroto, A. J. G., Blesa, M. A. (1989) Reductive Dissolution of Magnetite by Solution Containing EDTA and Fe(II). J. Colloid Interface Sci. 130(2), 299-310. [4] Chiarizia, R., Horwitz, E. P., (1991) New Formulations of Iron Oxides Dissolution. Hydrometallurgy, 27, 339-360. [5]Ambikadevi, V. R., Lalithambika, M., (2000) Effects of Organic Acids on Ferric Iron Removal from Iron-Stained Kaolinite. Appl. Clay Sci. 16, 133-145. [6] Taxiarchour, M., Panias, D., Doumi, I., Paspaliaris, I., Kontopoulos, A. (1997a) Removal of iron from Silica Sand by Leaching with Oxalic Acid, Hydrometallurgy, 46, 215-227. [7] Vaglio, F., Passariello, B., Barbaro, M., Plescia, P., Marbini, A. M. (1998) Drum Leaching Test in the Iron Removal from Quartz Using Oxalic Acid and Sulphuric Acids. Int. J. Miner. Process. 54,183. [8] Segal, M. G., Sellers, R. M., (1984) In: Sykes, A.G. (Ed.), Advances in Inorganic and Bioinorganic Mechanisms, 1(3). Academic Press, London, 1984, p.97. [9] Frenier, W. W., Growcock, F.B., (1984) Mechanism of Iron Oxide Dissolution, a Review of Recent Literature. Corrosion NACE, 40, pp. 663-668. [10] Jepson, W.B., (1988) Structural Iron in Kaolinite and in Associated Ancillary Materials. In: Stucki, J. W., Goodman, B. A., Schwertmann, U. (Ed.), Iron in Soils and Clay Minerals. NATO ASI Ser, 217. D. Reidel Publ. Co., Dordrecht, Holland, pp. 467-536. [11] Panias, D.,Taxiarchou, M., Paspaliaris, I., Kontopoulos, A. (1996) Mechanism of Dissolution of Iron Oxides in Aqueous Oxalic Acid. Hydrometallurgy 42, 257-265. [12] Blesa, M. A., Morando, P. J., Regazzoni, A. E., (1994) Chemical Dissolution of Metal Oxides. CRC Press, Inc., pp. 269-308. [13] Cornell, R. M.., Schindler, P. W.,(1987) Photochemical Dissolution of Geothite in Acid/Oxalate Solution. Miner. 35(5), 347-352. [14] Lee, S.O., Tran, T., Park Y.Y., Kim S.J., and Kim, M. J. (2006) Study on the Kinetics of Iron Leaching by Oxalic acid. Int. J. Miner process, 80, 144-152. [15] Mandal, S. K., Banerjee, P. C., (2004) Iron Leaching with Oxalic Acid: Effects of Different Physico-Chemical Parameters. Int. J. Miner. Process. 74, 263-270. [16]Nwoye, C. I. (2008) Model for Quantitative Analysis of Dissolved Iron in Oxalic Acid Solution during Leaching of Iron Oxide Ore, Inter. Res. J. Eng. Sc. Tech., 5(1): 37-41. [17] Nwoye, C. I. (2008) Model for Computational Analysis of Dissolved Haematite and Heat Absorbed by Oxalic Acid Solution during Leaching of Iron Oxide Ore, J. Eng.& App. Sc., 4, 22-25. [18]Nwoye, C. I. (2005) SynchroWell Research Work Report, DFM Unit, No 2441162, 60-69.  Vol.8, No.7 Model for Predictive Analysis of Heat Absorbed 539 |

