Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 8, No.5, pp 349-357, 2009 jmmce.org Printed in the USA. All rights reserved Growth and Characterization Studies of MnHP Single Crystal in Silica Gel Medium P. Suresh 2 , G. Kanchana 1 , P. Sundaramoorthi* 2 1 Department of Biochemistry, Muthayammal College of Arts and Science, Rasipuram, Namakkal, India-637408. 2 Department of Physics, Thiruvalluvar Govt. Arts College, Rasipuram, Namakkal, India-637401. *Corresponding Author, contact: moorthi.sundara@gmail.com Phone: 04287231802, Fax: 04287231882 ABSTRACT MnHP (Manganese Hydrogen Phosphate) crystals were grown in silica gel medium using different gel densities, various concentrations of ortho- phosphoric acid and supernatant solutions in single diffusion process. The gel pH plays an important role in the formation of different HPO 4 species in the phosphoric system. The pH range in which HPO 42- ions dominates were considered which in turn is necessary for the growth of MnHP crystals. The characterizations of grown crystals were studied by FTIR, SEM, XRD and etching. The results are reported and discussed in detailed manner. Key Words: MnHP, calculi, surface morphology, growth parameters, trace element and SMS gel medium. 1. INTRODUCTION SHP (Strontium hydrogen phosphate) and BHP (Barium hydrogen phosphate) were grown in silica gel medium at room temperature and reported [1-2]. In the present investigation, single crystal of trace element was grown in silica gel medium at different parameters, which contains one major element (Phosphate), and one minor or trace element (Manganese). MnHP is a single crystal which typically represents the biological crystals formed in the human urinary tracts called renal stones. The body of a normal man weighing 70kg contains about 12-20mg manganese. It is distributed throughout the body tissues and fluids. Bone, kidney, liver, pancreas and pituitary contain more manganese than other tissues. The manganese  350 P.Suresh, G.Kanchana, P.Sundaramoorthi Vol.8, No.5 content of human blood is very low ranging from 2 to 3 micrograms per 100ml. Only 3-4 per cent of the manganese present in the regular diet is absorbed and mixed in plasma and the remaining being excreted in faeces. A normal human body requirement of manganese ranges from about 2.5 to 5.0 mg per day. Manganese is present naturally in many foods such as spices, sea foods, cereals, grains and leafy vegetables. There are some evidences that manganese is essential for the growth of animals, especially for the normal skeletal growth in the prenatal period. Manganese appears to play an important role in the functioning of central nervous system. It is also essential for the normal reproductive function. If the mineral level of the body fluid increases, automatic mineral deposition starts leading to the development of renal stones. Authors have done a series of experiments with silica gel crystal growth medium at different pH values ranging from 5.5 to 11. One can obtain the periodic precipitation, Liesegang rings [3-5] of biological crystals named as HAP, Brushite, Struvite, BMHP, SMHP etc. 2. MATERIALS AND METHODS The dissociation of ortho-phosphoric acid system can be represented by three-dissociation equilibrium and the presence of various ions at various pH values are reported [6]. Based on these results, the gel pH in the range from 6 to 11 has been used (Milwaukee QS-MN pH-600, packet digital pH-meter used for measurements) in which the HPO 42- ion dominates or alone exists. This decreases the possibility of the occurrence of MnP crystals during MnHP growth. The crystallization apparatus employed were glass test tubes of 25 mm diameter and 150 mm long for single diffusion method (SDM). The chemicals used were Excelar-Qualigens(E-Q) AR grade MnCl 2 and E-Q AR grade ortho-phosphoric acid (Sp.gr.1.75). The SMS gel or water glass was prepared as per the literature [7]. One of the reactant ortho-phosphoric acid was mixed with silica gel at desired gel density and elevated temperatures. After the gel set, the supernatant mixture (Manganese chloride) at a required mole solution was slowly added along the walls of the growth columns (test tubes) over the set gels and tightly closed to prevent evaporation. Then the growth systems were allowed to react within the gel medium and the following chemical reaction take place. MnCl 2 + [H 3 PO 4 + gel]MnHPO 4 + By- products 2.1. The following observations have been made in the present investigation The reaction starts immediately after the addition of supernatant solutions. But the nucleation was observed only after 26 hours and the growth process took a period of nearly one year for completion. Some well-developed single, poly crystals were observed in the SDM growth columns. The gel density above 1.06gm/cc and with pH above 8 yielded no crystals, but mean time reaction takes place. Some of the test tubes of gel density 1.03gm/cc with pH around 6.5  Vol.8, No.5 Growth and Characterization Studies of MnHP Single Crystal in Silica Gel Medium 351 and below 6 were allowed for the reactions to take place. After a period of five months, a less number of nucleation was observed near the bottom and middle of the test tubes. Some of them grew as a needle and well transparent single and platelet crystals. From these investigations, the optimum growth parameters of MnHP crystals were identified and reported in Table-1. Table-1 MnHP crystal growth parameters in SDM Gel density gm /cc Ortho- phosphoric acid concentration in N Gel+ H 3 PO 4 pH value Gel setting time in hrs Supernatant concentration MnCl 2 (M) Nucleation observed in hrs Growth period in days Types of crystals observed in all conditions and harvested crystal size 1.04 1 6.4 6.8 6.9 7.3 26 16 1 18 2 -do- -do- -do- 26 37 42 99 280 Dendrite crystals Leaf like crystals Single, Poly crystals, (2mmx 2mm x2mm) 1.5 6.6 6.9 7.1 8.0 28 1 3 46 -do- -do- -do- -do- 16 26 56 67 290 1.05 1 6.3 6.8 6.9 7.4 34 6 1 68 -do- -do- -do- -do- 22 72 48 88 210 2 6.6 6.9 7.1 7.5 24 1 12 48 -do- -do- -do- -do- 43 30 44 82 275 An extension of the reaction period even up to one and a half year did not improve the size of these crystals. The growth columns (SDM) of MnHP crystals and harvested MnHP crystal are shown in Fig-1 and Fig-2 respectively. The maximum dimension of the crystals obtained is 2 mm x 2 mm x 2 mm. In this investigation fast gel set shows non-transparent gel medium and gives less nucleation and yield. 3. CHARACTERIZATION STUDIES OF MnHP CRYSTALS 3.1. FTIR spectral analysis of MnHP crystal FTIR spectrometer having KBr pellets sample holder and KBr detector was used for the analysis. The KBr pellet samples were used and the absorption frequencies range from 600 to 4000 cm -1 [8-12]. The absorption bonds, absorption frequencies and percentage of transmittance were 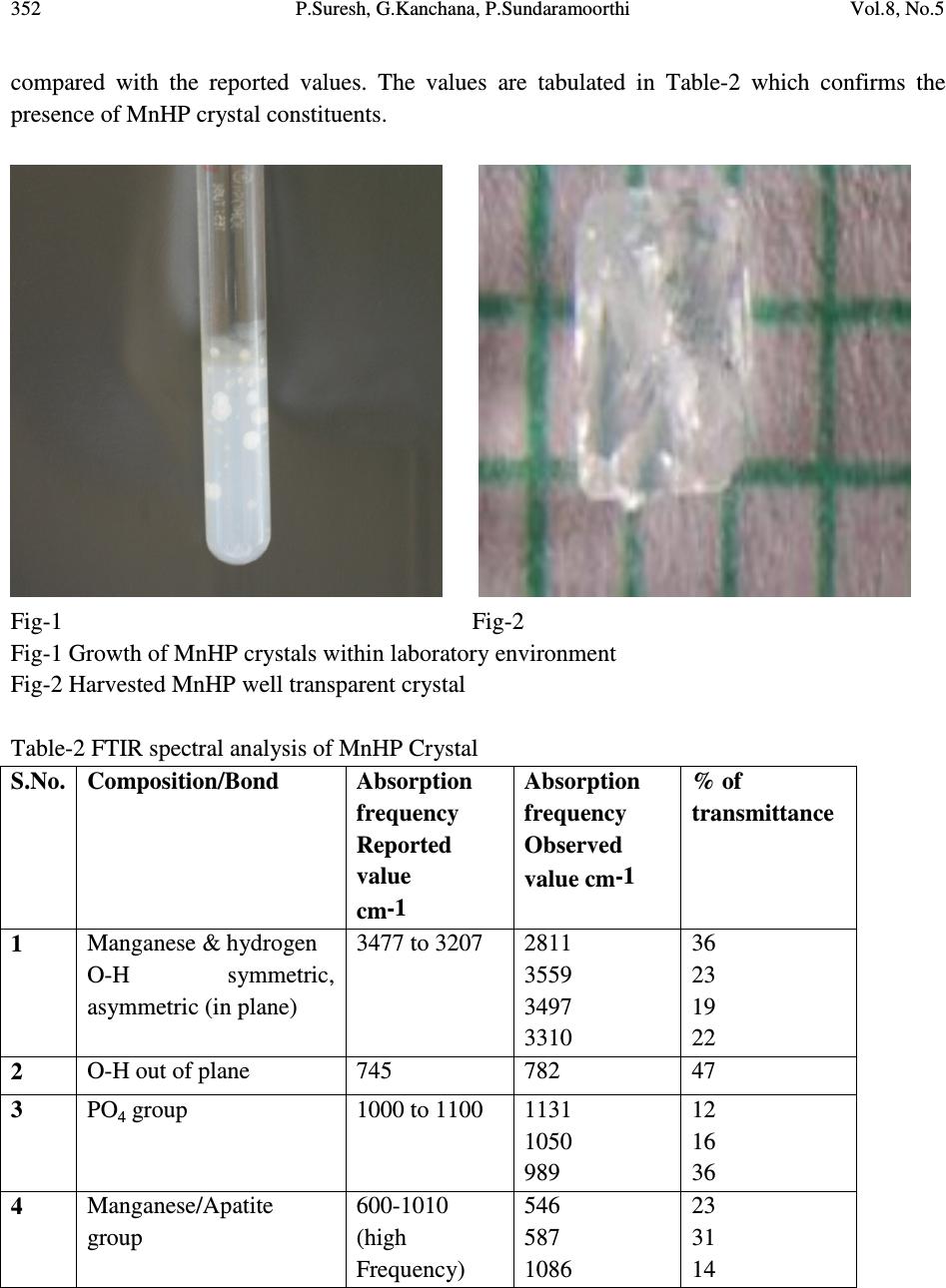 352 P.Suresh, G.Kanchana, P.Sundaramoorthi Vol.8, No.5 compared with the reported values. The values are tabulated in Table-2 which confirms the presence of MnHP crystal constituents. Fig-1 Fig-2 Fig-1 Growth of MnHP crystals within laboratory environment Fig-2 Harvested MnHP well transparent crystal Table-2 FTIR spectral analysis of MnHP Crystal S.No. Composition/Bond Absorption frequency Reported value cm-1 Absorption frequency Observed value cm-1 % of transmittance 1 Manganese & hydrogen O-H symmetric, asymmetric (in plane) 3477 to 3207 2811 3559 3497 3310 36 23 19 22 2 O-H out of plane 745 782 47 3 PO 4 group 1000 to 1100 1131 1050 989 12 16 36 4 Manganese/Apatite group 600-1010 (high Frequency) 546 587 1086 23 31 14  Vol.8, No.5 Growth and Characterization Studies of MnHP Single Crystal in Silica Gel Medium 353 3.2. Etching study of MnHP crystal A well-grown MnHP crystal was immersed in HCl solution at a desired concentration. The dissolution of MnHP crystal depends upon the etchant concentration, temperature, crystal morphology, etching time etc. [13-16]. The etch pits are shown in Fig-3. The etch pits observed in the photo are cone pits, leaf pits and step pits. Fig-3 Etch photo of MnHP crystal at room temperature, HCL as an etchant, etching time - 7 minutes, etchant normality - 2N 3.3. Scanning Electron Microscopic studies of MnHP crystal A well-grown MnHP single crystal was selected for the investigation of surface morphology by using SEM. The SEM photograph was made in the version S-300-I instrument. The sample named VCA-600 kept in lobe middle; the data size was 640x480 µm. The minor and major magnifications of SEM were about 250 times. SEM acceleration voltage was 25000 volts and the sample was kept in a high vacuum. 18200µm working distance and monochromatic color mode was employed. 200µm focusing of MnHP crystal SEM is shown in Fig-4. In the surface analysis of SEM picture of MnHP crystal, smooth, fine grain boundaries and few valley regions are observed [17-20]. 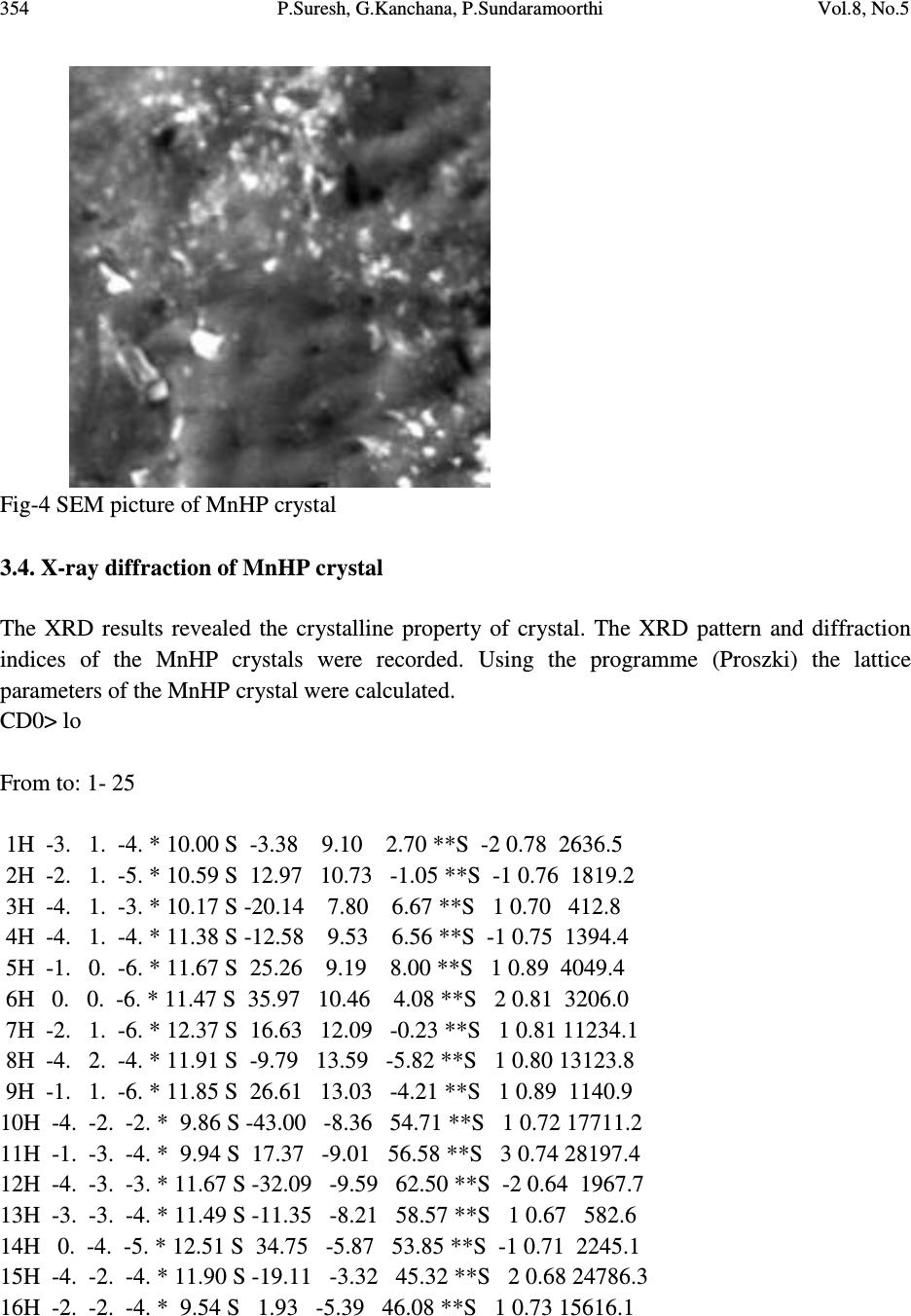 354 P.Suresh, G.Kanchana, P.Sundaramoorthi Vol.8, No.5 Fig-4 SEM picture of MnHP crystal 3.4. X-ray diffraction of MnHP crystal The XRD results revealed the crystalline property of crystal. The XRD pattern and diffraction indices of the MnHP crystals were recorded. Using the programme (Proszki) the lattice parameters of the MnHP crystal were calculated. CD0> lo From to: 1- 25 1H -3. 1. -4. * 10.00 S -3.38 9.10 2.70 **S -2 0.78 2636.5 2H -2. 1. -5. * 10.59 S 12.97 10.73 -1.05 **S -1 0.76 1819.2 3H -4. 1. -3. * 10.17 S -20.14 7.80 6.67 **S 1 0.70 412.8 4H -4. 1. -4. * 11.38 S -12.58 9.53 6.56 **S -1 0.75 1394.4 5H -1. 0. -6. * 11.67 S 25.26 9.19 8.00 **S 1 0.89 4049.4 6H 0. 0. -6. * 11.47 S 35.97 10.46 4.08 **S 2 0.81 3206.0 7H -2. 1. -6. * 12.37 S 16.63 12.09 -0.23 **S 1 0.81 11234.1 8H -4. 2. -4. * 11.91 S -9.79 13.59 -5.82 **S 1 0.80 13123.8 9H -1. 1. -6. * 11.85 S 26.61 13.03 -4.21 **S 1 0.89 1140.9 10H -4. -2. -2. * 9.86 S -43.00 -8.36 54.71 **S 1 0.72 17711.2 11H -1. -3. -4. * 9.94 S 17.37 -9.01 56.58 **S 3 0.74 28197.4 12H -4. -3. -3. * 11.67 S -32.09 -9.59 62.50 **S -2 0.64 1967.7 13H -3. -3. -4. * 11.49 S -11.35 -8.21 58.57 **S 1 0.67 582.6 14H 0. -4. -5. * 12.51 S 34.75 -5.87 53.85 **S -1 0.71 2245.1 15H -4. -2. -4. * 11.90 S -19.11 -3.32 45.32 **S 2 0.68 24786.3 16H -2. -2. -4. * 9.54 S 1.93 -5.39 46.08 **S 1 0.73 15616.1  Vol.8, No.5 Growth and Characterization Studies of MnHP Single Crystal in Silica Gel Medium 355 17H -2. -2. -3. * 8.10 S -7.34 -10.15 55.01 **S -1 0.71 1244.5 18H -3. -2. -2. * 8.25 S -35.75 -12.20 60.50 **S 2 0.72 1817.7 19H -1. 4. -3. * 10.04 S 20.53 28.51 -55.15 **S 2 0.87 8445.4 20H -2. 4. -2. * 9.76 S -2.02 27.31 -51.90 **S 1 1.04 21079.8 21H -3. 5. 1. * 11.90 S -43.69 29.79 -53.96 **S 1 1.00 8956.4 22H -3. 5. -1. * 11.90 S -20.12 29.20 -52.26 **S 1 1.15 8967.8 23H -2. 5. -3. * 12.25 S 7.61 30.13 -53.93 **S 1 1.01 21325.2 24H -2. 5. -2. * 11.47 S -0.90 31.81 -59.61 **S 1 1.19 694.0 25H -1. 4. -1. * 8.45 S 0.84 33.78 -73.19 **S 1 1.13 22465.5 CD0> reind Nr S H K L Dev-Ang dTh dPh dCh 0.0093594 1 H -2.999 1.007 -4.003 0.0757 -0.006 -0.013 0.073 0.0007065 2 H -1.999 1.001 -5.002 0.0211 -0.002 -0.012 0.017 0.0002248 3 H -4.000 0.993 -3.001 0.0819 0.003 -0.020 -0.078 0.0007233 4 H -4.000 1.000 -4.001 0.0109 -0.002 -0.011 -0.008 0.0001326 5 H -0.999 -0.003 -5.999 0.0329 0.003 -0.018 -0.029 0.0003606 6 H 0.001 -0.005 -5.990 0.0476 0.019 -0.020 -0.042 0.0010464 7 H -2.000 0.998 -6.001 0.0226 -0.001 -0.008 -0.0221 0.0002412 8 H -4.002 2.009 -4.002 0.0764 -0.012 0.012 0.076 0.0009725 9 H -1.000 1.001 -6.002 0.0124 -0.005 -0.005 0.012 0.0002521 10 H -3.998 -2.004 -2.001 0.0486 0.000 -0.029 -0.045 0.0004099 11 H -1.000 -2.992 -4.006 0.1106 0.000 0.037 0.108 0.0009394 12 H -4.001 -2.997 -3.003 0.0351 -0.001 -0.011 0.033 0.0003537 13 H -2.994 -2.998 -3.990 0.0425 0.020 0.001 -0.041 0.0010662 14 H -0.003 -3.997 -5.005 0.0578 -0.002 0.063 0.033 0.0006273 15 H -4.000 -2.000 -3.998 0.0161 0.003 0.014 -0.012 0.0002309 16 H -2.001 -2.002 -3.996 0.0523 0.004 0.020 -0.055 0.0004614 17 H -2.005 -2.007 -3.002 0.0712 -0.015 0.039 -0.068 0.0008623 18 H -3.002 -2.005 -2.001 0.0525 -0.009 -0.002 -0.053 0.0005692 19 H -1.004 3.991 -3.005 0.1136 0.007 -0.024 -0.112 0.0010373 20 H -1.999 4.003 -2.000 0.0272 -0.005 -0.002 0.028 0.0003260 21 H -3.003 4.999 1.003 0.0405 -0.002 0.034 -0.032 0.0004221 22 H -3.000 5.001 -0.999 0.0141 0.000 0.016 0.008 0.0001445 23 H -1.999 5.003 -3.001 0.0257 -0.005 -0.010 0.024 0.0003557 24 H -1.998 5.001 -1.999 0.0216 0.000 0.003 0.021 0.0002118 25 H -1.000 3.999 -1.000 0.0046 0.002 -0.007 -0.002 0.0000976 Reciprocal axis matrix Direct axis matrix -0.076371 0.020678 0.056979 -7.659393 -5.683682 -3.047902 -0.056719 0.022058 -0.074083 2.150182 2.314291 -9.716051 -0.030412 -0.093093 -0.005038 6.504328 -8.458021 -0.559365  356 P.Suresh, G.Kanchana, P.Sundaramoorthi Vol.8, No.5 Niggli-values Sigma direct axis matrix 100.2603 104.3808 114.1574 0.002766 0.001097 0.001836 -0.1541 -0.0417 -0.0091 0.005859 0.002324 0.003892 0.004838 0.001919 0.003213 Cell parameters Sigma cell parameters 10.20230 10.2067 10.5844 0.0022 0.0038 0.0034 90.2008 90.0243 90.1752 0.0286 0.0238 0.0291 -0.0014141 -0.000388 -0.000099 0.000499 0.000417 0.000509 Volume= 1102.1727 0.5956 Index-Status: HHHHHHHHHHHHHHHHHHHHHHHHH CD0> The lattice parameters are a=10.02Å, b=10.20Å, c=10.58Å, α=90.2˚, β= 90.0˚ and γ=90.1˚. The volume of the unit cell of the MnHP crystal is 1102.1727 (Å) 3 . From the above data it was confirmed that MnHP crystal system is triclinic [21-22]. 4. CONCLUSION The MnHP crystals were grown at room temperature and found optimum growth parameters. MnHP crystal growth columns and harvested crystal are photographed. FTIR-spectrum recorded the functional group frequencies of MnHP grown crystal constituents. These results were recorded and compared with the reported values. Chemical etching was done at room temperature, which revealed the grown crystal defects. SEM analysis was also done and it revealed the surface morphology of MnHP crystal. MnHP lattice parameters were calculated by XRD. REFERENCE [1] Sundaramoorthi P., and Kalainathan S., 2007, Biochemical Engineering journal, Vol. 34, pp. 244. [2] Sundaramoorthi P., and Kalainathan S., 2007, Journal of Materials and Minerals and Characterization of Engineering, Vol. 6, pp. 33. [3] Sundaramoorthi, P., and Kalainathan S., 2007, Asian journal of chemistry, Vol. 19, pp. 3739. [4] Henisch H. K., Garcia-Ruiz J.M., 1986, J. Crystal Growth, Vol. 75, pp. 195. [5] Henisch H.K., Garcia-Ruiz J.M., 1986, J. Crystal Growth, Vol. 75, pp. 203. [6] Pecsok R.L., Shields L.D., Cairns T., and McWillian I.G., 1976, Modern methods of chemical analysis, John Wily&Sons Inc.,Newyork, pp. 438-442.  Vol.8, No.5 Growth and Characterization Studies of MnHP Single Crystal in Silica Gel Medium 357 [7] Henisch H.K., Crystals in Gel and Liesegang Rings, 1986, Cambridge University press, Cambridge, pp. 11. [8] Socrater, G., 1980, Infrared Cha. Group Friq., Johnwilly- Chichester. [9] Hatscheck E., 1925, J. Kollid Z., Vol. 37, pp. 297. [10] Chin Y., et al., 1961, J. Urology, Vol. 86, pp. 838-854. [11] Corns C.M., 1983, J. Ann. Clin. Bio-Cherm,, Vol. 20, pp. 20-35. [12] Hesse A., and Bach D., 1982, Stone analysis by infrared spectroscopy, In., Urinary stones, Clinical and laboratory Aspects, Edi., Alan Rose, University Park Press, Baltimore, Vol. 87, pp. 105. [13] Gilman, J.J., Johnsion J., and Sears G.W., 1958, J. App. Physics, Vol. 29, pp. 749. [14] Gilman J.J., et al, 1956, J. App. Physics, Vol. 27, pp. 1018. [15] Fisher J.C., 1957, Dissolutions and Mechanical Properties of Crystals, John Wiley and sons, New York. [16] Kirk J.B., 1962, Director Observation of Imperfection in Crystals, Inter science Publishers, New York. [17] Taukamot K., 1983, J. Crystal. Growth, Vol. 61, pp. 199. [18] Gates H.C., 1975, Thirty years of progress in Surface Science, Crystal growth and Characterization, Edi., North Holland. [19] Bethage H., et al., 1987, Electron Microscopy in Solid State Physics, Elsevier, Amsterdom. [20] Albon N. et al, 1958, Growth and Perfection of Crystals, Wiley., New York. [21] Machennan G., and Reevers C.A., 1955, Acta Crystals, Vol. 8, pp. 579. [22] Curry N.A., and Jones D.W., 1971, J. Chem. Soc. A, pp. 3725. |

