Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 8, No.5, pp 339-347, 2009 jmmce.org Printed in the USA. All rights reserved Model for the Calculation of the Concentration of Dissolved Hematite during Hydrogen Peroxide Leaching of Iron Oxide Ore C. I. Nwoye* 1 , C. C. Nwakwuo 2 , C. Nlebedim 3 , U.C. Nwoye 4 , R. A. Umana 5 and G. C. Obasi 6 1 Department of Materials and Metallurgical Engineering, Federal University of Technology, Owerri, Nigeria. 2 Department of Material Science, Oxford University. United Kingdom. 3 Department of Material Science, University of Cadiff, Wales, United Kingdom. 4 Data Processing, Modelling and Simulation Unit, Weatherford Nig. Ltd. Port-Harcourt Nigeria. 5 Department of Mathematics and Computer Science, Federal University of Technology, Owerri, Nigeria. 6 Department of Material Science, Aveiro University, Portugal. *Corresponding Author, contact: chikeyn@yahoo.com ABSTRACT Model for the calculation of the concentration of dissolved haematite during hydrogen peroxide leaching of iron oxide ore has been derived. The model was found to depend on both the % concentration of dissolved iron and weight input of iron oxide ore from experiment. The validity of the model is rooted on the expression %Fe 2 O 3 ≈ %Fe√(µ) 1/3 where both sides of the relationship are correspondingly almost equal. The deviation of the model-predicted concentration of dissolved haematite from the corresponding experimental values is less than 30% which is quite within the acceptable range of deviation limit of experimental results. The model indicates that the dissolved % ratio of extreme oxidation stage of iron to that of its extreme reduction stage is approximately equal to one-sixth (1/6th) power of the weight input of iron oxide ore during the leaching process. Key Words: Model, Calculation, Haematite dissolution, Hydrogen Peroxide, Iron Oxide Ore, Leaching  340 C. I. Nwoye, C. C. Nwakwuo, C. Nlebedim, U.C. Nwoye, R. A. Umana and G. C. Obasi Vol.8, No.5 1. INTRODUCTION Several works have been reported [1-4] involving the use of different acids for the leaching of iron oxide ore. Also attempts have been made to leach pyrite using strong oxidizing agent like hydrogen peroxide because of the inert nature of the pyrite. Ambikadevi and Lalithambika [5] found oxalic acid (0.05-0.15M) to be the best extractant for removing iron from iron compounds. The dissolution was found to increase with acid concentration within the range (0.05-0.15M) studied. Both hydrogen ions and oxalate were increased in this case. Using 0.15M oxalic acid approximately, 70% of the iron could be extracted from a slurry (20%w/v) containing 0.93% iron oxide (of goethite and haematite phases) at 100 0 C within 90mins. The iron oxide concentration in the leach is equivalent to 1.86g/L Fe 2 O 3 . A study of the electrochemical dissolution of haematite (α-Fe 2 O 3 ), maghetite, (γ-Fe 2 O 3 ), goethite, (α-FeOOH) and lepidochrocite, (γ-FeOOH) inhydrochloric and oxalic acid using voltammetry [6] indicated that the hydroxyl-oxides of FeOOH can be reduced also via soluble Fe(III) species at 0.6-0.8 V (vs Ag-AgCl), where as haematite and maghetite dissolve only via direct reduction of the solid at -0.55 to -0.60V (vs AG-AgCl). These potentials were determined as peaks on voltammograms conducted with stationary electrodes made from these iron oxides and hydroxyl-oxides. This fundamental study [6] on the electrchemical behaviour of different types of iron oxides confirms the electrochemical nature of haematite reductive dissolution. This further explains why it is easier to dissolve hydroxyl-oxides such as goethite where dissolution can take place via both reduction (solid and aqueous species) and complexation [7] whereas haematite dissolves mainly via solid reduction [8]. Oxalate can easily be reductant for such a process, as shown in its Eh-pH diagram [9]. Lee et al.[10] reported that dissolution of haematite in oxalic acid is via a reductive mechanism which made Fe(III) non-existent in the solution. The overall reaction was therefore reported [10] as a redox reaction, forming two half cells: Oxidation of oxalate to form carbonic acid or carbon dioxide: HC 2 O 4- = H + 2CO 2 + 2e - (1) Reduction of haematite forming Fe(II) oxalate: 2H + + Fe 2 O 3 + 4HC 2 O 4- + 2e - = 2Fe(C 2 O 4 ) 22 - + 3H 2 O (2) The dissolution reaction is therefore: H + + Fe 2 O 3 + 5 HC 2 O 4- = 2Fe(C 2 O 4 ) 22 - + 3H 2 O + 2CO 2 (3) The overall reaction indicates that species involved in the leaching would be hydrogen ions, oxalate and iron oxide (haematite particles). Model for computational analysis of the concentration of dissolved haematite and heat absorbed by oxalic acid solution during leaching of iron oxide ore has been derived [11]. 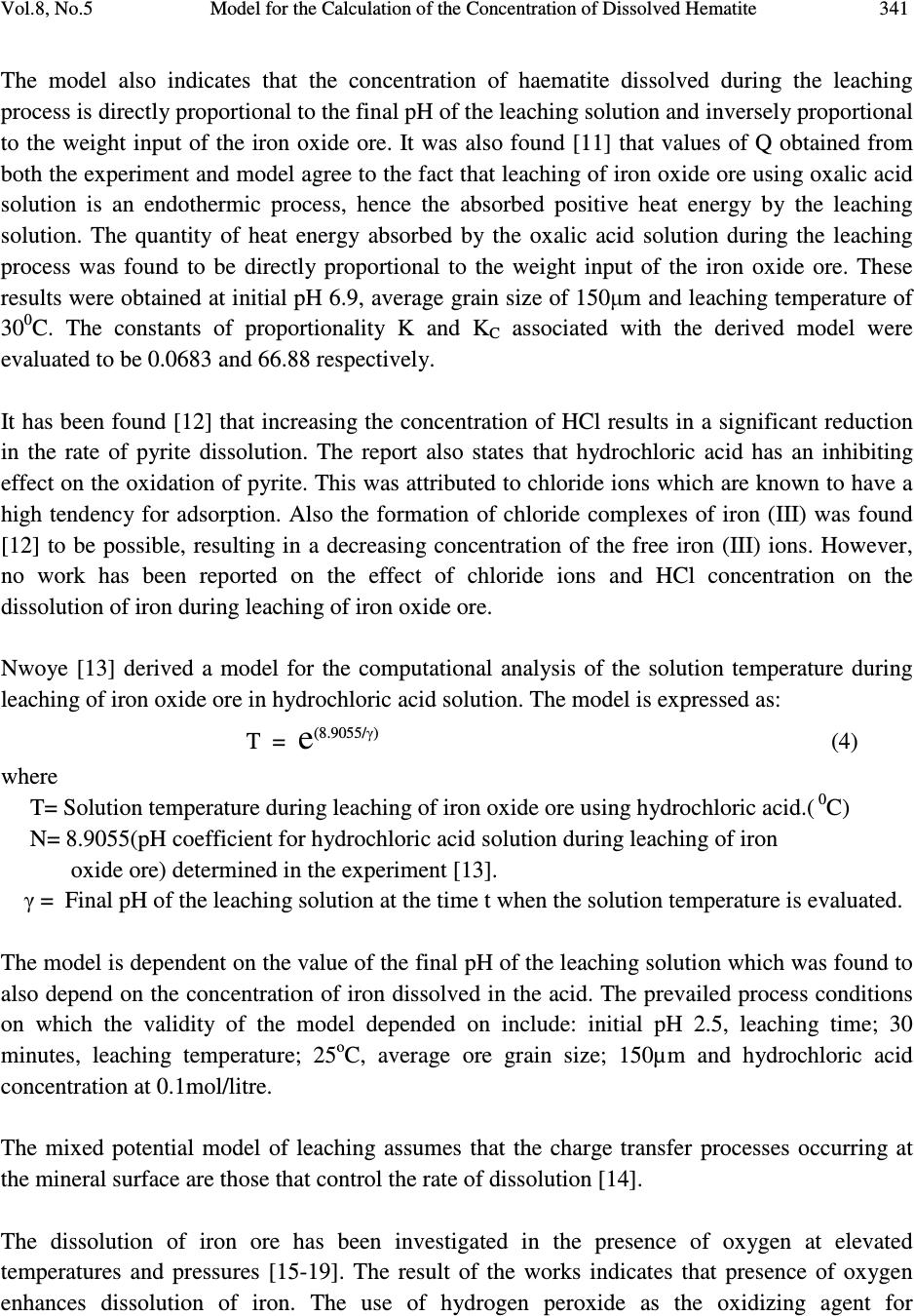 Vol.8, No.5 Model for the Calculation of the Concentration of Dissolved Hematite 341 The model also indicates that the concentration of haematite dissolved during the leaching process is directly proportional to the final pH of the leaching solution and inversely proportional to the weight input of the iron oxide ore. It was also found [11] that values of Q obtained from both the experiment and model agree to the fact that leaching of iron oxide ore using oxalic acid solution is an endothermic process, hence the absorbed positive heat energy by the leaching solution. The quantity of heat energy absorbed by the oxalic acid solution during the leaching process was found to be directly proportional to the weight input of the iron oxide ore. These results were obtained at initial pH 6.9, average grain size of 150µm and leaching temperature of 30 0 C. The constants of proportionality K and K C associated with the derived model were evaluated to be 0.0683 and 66.88 respectively. It has been found [12] that increasing the concentration of HCl results in a significant reduction in the rate of pyrite dissolution. The report also states that hydrochloric acid has an inhibiting effect on the oxidation of pyrite. This was attributed to chloride ions which are known to have a high tendency for adsorption. Also the formation of chloride complexes of iron (III) was found [12] to be possible, resulting in a decreasing concentration of the free iron (III) ions. However, no work has been reported on the effect of chloride ions and HCl concentration on the dissolution of iron during leaching of iron oxide ore. Nwoye [13] derived a model for the computational analysis of the solution temperature during leaching of iron oxide ore in hydrochloric acid solution. The model is expressed as: T = e (8.9055/γ) (4) where T= Solution temperature during leaching of iron oxide ore using hydrochloric acid.( 0 C) N= 8.9055(pH coefficient for hydrochloric acid solution during leaching of iron oxide ore) determined in the experiment [13]. γ = Final pH of the leaching solution at the time t when the solution temperature is evaluated. The model is dependent on the value of the final pH of the leaching solution which was found to also depend on the concentration of iron dissolved in the acid. The prevailed process conditions on which the validity of the model depended on include: initial pH 2.5, leaching time; 30 minutes, leaching temperature; 25 o C, average ore grain size; 150µm and hydrochloric acid concentration at 0.1mol/litre. The mixed potential model of leaching assumes that the charge transfer processes occurring at the mineral surface are those that control the rate of dissolution [14]. The dissolution of iron ore has been investigated in the presence of oxygen at elevated temperatures and pressures [15-19]. The result of the works indicates that presence of oxygen enhances dissolution of iron. The use of hydrogen peroxide as the oxidizing agent for  342 C. I. Nwoye, C. C. Nwakwuo, C. Nlebedim, U.C. Nwoye, R. A. Umana and G. C. Obasi Vol.8, No.5 hydrometallurgical processes has been increasingly studied. McKibben [15] studied the kinetics of aqueous oxidation of pyrite by hydrogen peroxide at pH 1-4 and 293-313K. Model for computational analysis of heat absorbed by hydrogen peroxide solution (relative to the weight of iron oxide ore added) has been derived [20]. The values of the heat absorbed Q as predicted by the model were found to agree with those obtained from the experiment that the leaching process is endothermic in nature hence the positive values of Q and the absorbed heat. The deviations of the predicted Q values from the experimental values were found to be within the acceptable range. The model was found to be dependent on the weight of iron oxide ore added to solution in the course of leaching. The model is stated as: Q = e 1.04(√W) (5) where Q= Quantity of heat energy absorbed by hydrogen peroxide solution during the leaching process (J) N= 1.04 (Weight-input coefficient) determined in the experiment[20]. W = Weight of iron oxide ore used (g) The present work is aimed at deriving a model for the calculation of the concentration of dissolved haematite during leaching of Itakpe (Nigeria) iron oxide ore in hydrogen peroxide solution. 2. MODEL Hydrogen ions from the hydrogen peroxide attack the iron oxide ore within the liquid phase in the presence of oxygen. This simultaneously facilitates and enhances the reductive dissolution of haematite. The ore (solid phase) was assumed to be stationary, and contains the un-leached iron. 2.1. Model Formulation Results of research work [21] previously reported (as in Table 1) were used for this work for the formulation of the model. Computational analysis carried out on the experimental data from the previous work [21] as shown in Table 1, resulted to Table 2 which indicate that; %Fe 2 O 3 ≈ %Fe√(µ) 1/3 (6) Rearranging equation (1) 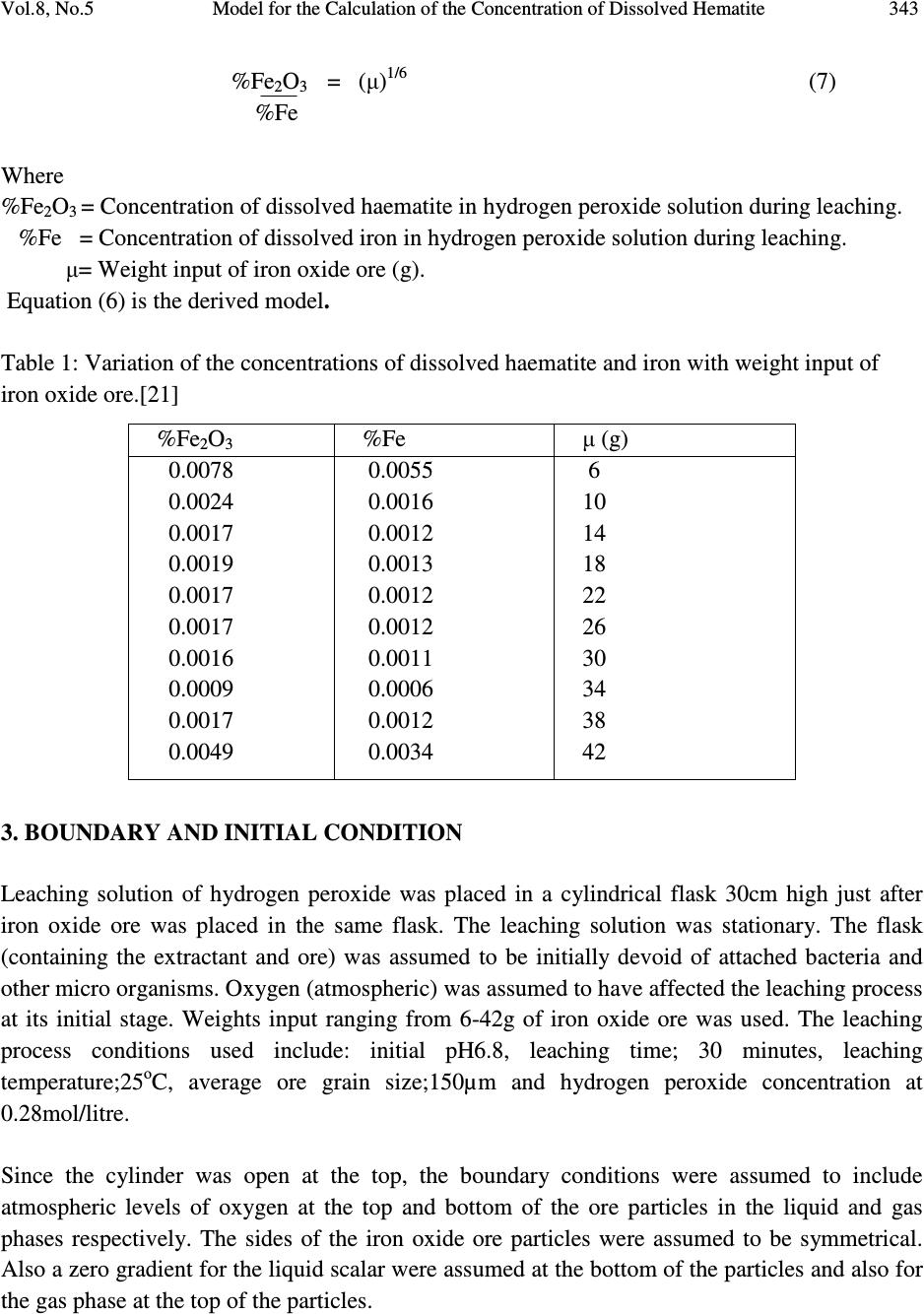 Vol.8, No.5 Model for the Calculation of the Concentration of Dissolved Hematite 343 %Fe 2 O 3 = (µ) 1/6 (7) %Fe Where %Fe 2 O 3 = Concentration of dissolved haematite in hydrogen peroxide solution during leaching. %Fe = Concentration of dissolved iron in hydrogen peroxide solution during leaching. µ= Weight input of iron oxide ore (g). Equation (6) is the derived model. Table 1: Variation of the concentrations of dissolved haematite and iron with weight input of iron oxide ore.[21] 3. BOUNDARY AND INITIAL CONDITION Leaching solution of hydrogen peroxide was placed in a cylindrical flask 30cm high just after iron oxide ore was placed in the same flask. The leaching solution was stationary. The flask (containing the extractant and ore) was assumed to be initially devoid of attached bacteria and other micro organisms. Oxygen (atmospheric) was assumed to have affected the leaching process at its initial stage. Weights input ranging from 6-42g of iron oxide ore was used. The leaching process conditions used include: initial pH6.8, leaching time; 30 minutes, leaching temperature;25 o C, average ore grain size;150µm and hydrogen peroxide concentration at 0.28mol/litre. Since the cylinder was open at the top, the boundary conditions were assumed to include atmospheric levels of oxygen at the top and bottom of the ore particles in the liquid and gas phases respectively. The sides of the iron oxide ore particles were assumed to be symmetrical. Also a zero gradient for the liquid scalar were assumed at the bottom of the particles and also for the gas phase at the top of the particles. %Fe 2 O 3 %Fe µ (g) 0.0078 0.0024 0.0017 0.0019 0.0017 0.0017 0.0016 0.0009 0.0017 0.0049 0.0055 0.0016 0.0012 0.0013 0.0012 0.0012 0.0011 0.0006 0.0012 0.0034 6 10 14 18 22 26 30 34 38 42 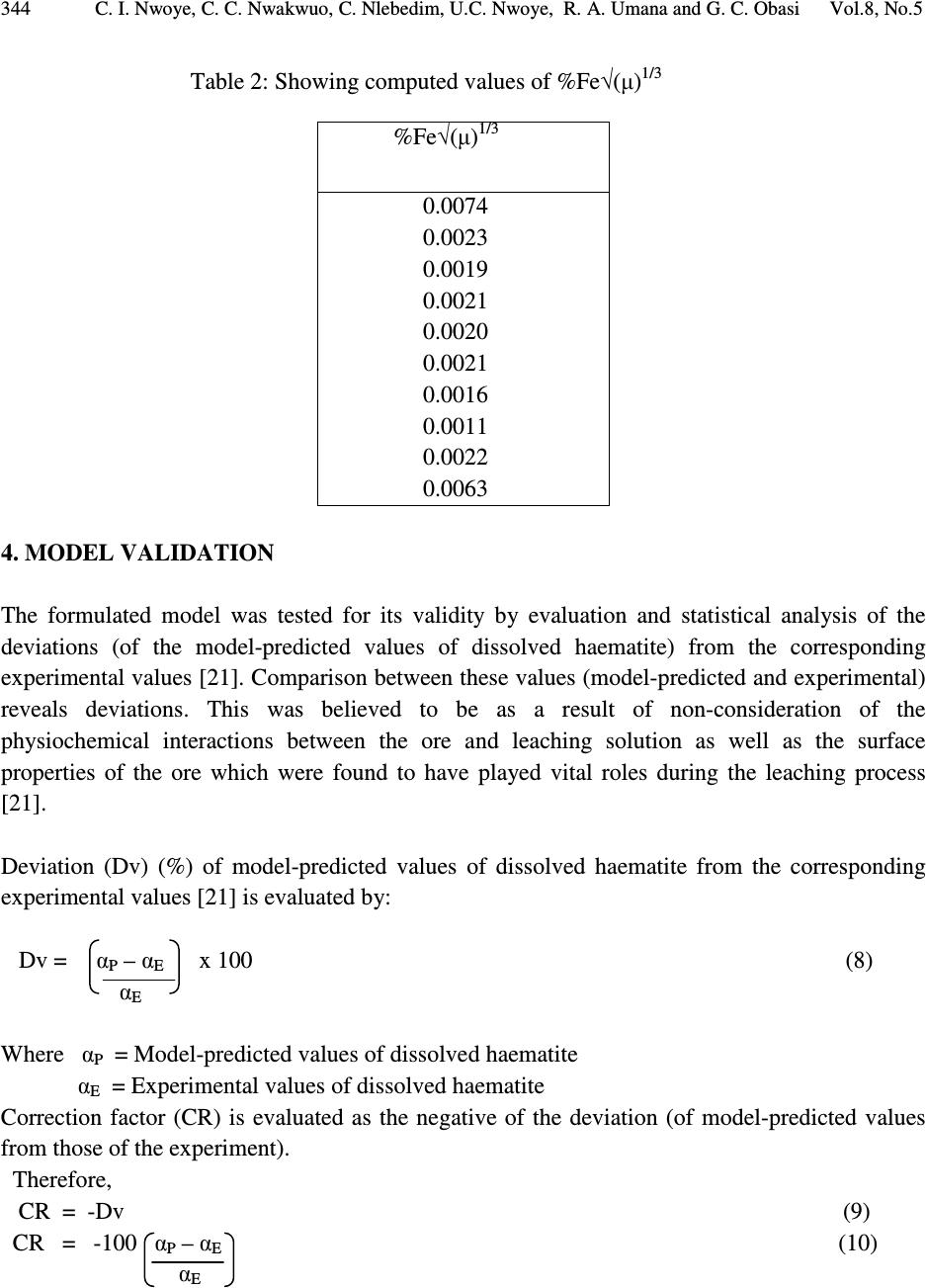 344 C. I. Nwoye, C. C. Nwakwuo, C. Nlebedim, U.C. Nwoye, R. A. Umana and G. C. Obasi Vol.8, No.5 Table 2: Showing computed values of %Fe√(µ) 1/3 4. MODEL VALIDATION The formulated model was tested for its validity by evaluation and statistical analysis of the deviations (of the model-predicted values of dissolved haematite) from the corresponding experimental values [21]. Comparison between these values (model-predicted and experimental) reveals deviations. This was believed to be as a result of non-consideration of the physiochemical interactions between the ore and leaching solution as well as the surface properties of the ore which were found to have played vital roles during the leaching process [21]. Deviation (Dv) (%) of model-predicted values of dissolved haematite from the corresponding experimental values [21] is evaluated by: Dv = α P – α E x 100 (8) α E Where α P = Model-predicted values of dissolved haematite α E = Experimental values of dissolved haematite Correction factor (CR) is evaluated as the negative of the deviation (of model-predicted values from those of the experiment). Therefore, CR = -Dv (9) CR = -100 α P – α E (10) α E %Fe√(µ) 1/3 0.0074 0.0023 0.0019 0.0021 0.0020 0.0021 0.0016 0.0011 0.0022 0.0063 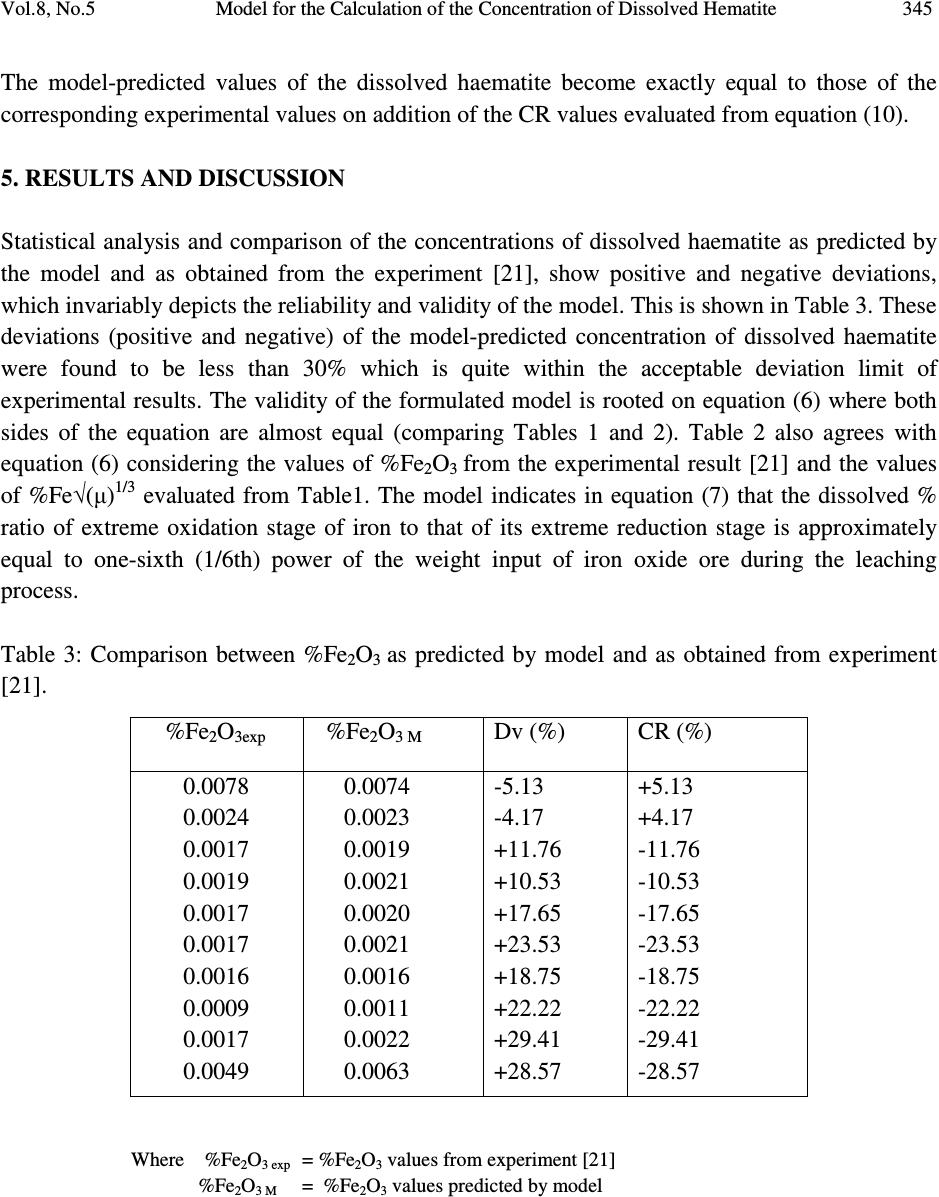 Vol.8, No.5 Model for the Calculation of the Concentration of Dissolved Hematite 345 The model-predicted values of the dissolved haematite become exactly equal to those of the corresponding experimental values on addition of the CR values evaluated from equation (10). 5. RESULTS AND DISCUSSION Statistical analysis and comparison of the concentrations of dissolved haematite as predicted by the model and as obtained from the experiment [21], show positive and negative deviations, which invariably depicts the reliability and validity of the model. This is shown in Table 3. These deviations (positive and negative) of the model-predicted concentration of dissolved haematite were found to be less than 30% which is quite within the acceptable deviation limit of experimental results. The validity of the formulated model is rooted on equation (6) where both sides of the equation are almost equal (comparing Tables 1 and 2). Table 2 also agrees with equation (6) considering the values of %Fe 2 O 3 from the experimental result [21] and the values of %Fe√(µ) 1/3 evaluated from Table1. The model indicates in equation (7) that the dissolved % ratio of extreme oxidation stage of iron to that of its extreme reduction stage is approximately equal to one-sixth (1/6th) power of the weight input of iron oxide ore during the leaching process. Table 3: Comparison between %Fe 2 O 3 as predicted by model and as obtained from experiment [21]. Where %Fe 2 O 3 exp = %Fe 2 O 3 values from experiment [21] %Fe 2 O 3 M = %Fe 2 O 3 values predicted by model %Fe 2 O 3exp %Fe 2 O 3 M Dv (%) CR (%) 0.0078 0.0024 0.0017 0.0019 0.0017 0.0017 0.0016 0.0009 0.0017 0.0049 0.0074 0.0023 0.0019 0.0021 0.0020 0.0021 0.0016 0.0011 0.0022 0.0063 -5.13 -4.17 +11.76 +10.53 +17.65 +23.53 +18.75 +22.22 +29.41 +28.57 +5.13 +4.17 -11.76 -10.53 -17.65 -23.53 -18.75 -22.22 -29.41 -28.57  346 C. I. Nwoye, C. C. Nwakwuo, C. Nlebedim, U.C. Nwoye, R. A. Umana and G. C. Obasi Vol.8, No.5 6. CONCLUSION The model calculates the concentration of dissolved haematite in relation to the concentration of dissolved iron and weight input of iron oxide ore during hydrogen peroxide leaching of Itakpe iron oxide ore. The validity of the model is rooted on equation (6) where both sides of the equation are correspondingly almost equal. The deviation of the model-predicted concentration of dissolved haematite from the corresponding experimental values is less than 30% which is quite within the acceptable range of deviation limit of experimental results. The model indicates that the dissolved % ratio of extreme oxidation stage of iron to that of its extreme reduction stage is approximately equal to one-sixth (1/6th) power of the weight input of iron oxide ore during the leaching process. ACKNOWLEDGEMENTS The authors wish to thank Dr. Ekeme Udoh, a modelling expert at Linkwell Modelling Centre Calabar for his technical inputs. The management of SynchroWell Nig. Ltd. Enugu is also appreciated for permitting and providing the experimental data used in this work. REFERENCES [1] Panias, D., Taxiarchou, M., Paspaliaris, I., and Kontopoulos, A., 1996, “Mechanism of dissolution of iron oxides in aqueous oxalic acid.” Hydrometallurgy, Vol. 42, pp. 257-265. [2] Taxiarchour, M., Panias, D., Doumi, I., Paspaliaris, I., and Kontopoulos, A., 1997, “Removal of iron from silica sand by leaching with oxalic acid.” Hydrometallurgy, Vol. 46, pp. 215- 227. [3] Taxiarchou, M., Parnias, D., Douni. I., Paspaliaris, I., and Kontopoulous, A., 1997, “Dissolution of haematite in acidic oxalate solutions.” Hydrometallurgy, Vol. 44, pp. 287- 299. [4] Lee, S. O., Tran, T., Park, Y.Y., Kim, S. J., and Kim, M.J., 2006a, “Study on the kinetics of iron Leaching by oxalic acid.” Int. J. Miner. Process, Vol. 80, pp. 144-152. [5] Ambikadevi, V. R., and Lalithambika, M., 2000, “Effects of organic acids on ferric iron removal from iron-stained kaolinite.” Appl. Clay Sci., Vol. 16, pp. 133-145. [6] Cepria, G., Uson, A., Perez-Arantgui, J., and Castillo, J. R., 2003, “Identification of Fe(III) oxide and hydroxyl-oxides by voltammetry of immobilized microparticles.” Anal. Chim. Acta., Vol 477, pp. 157-168. [7] Stumm.,W., and Furrer, G., 1987, “The dissolution of oxides and aluminum silicates: examples of surface-coordination-controlled-kinetics.” Aquatic Surface Chemistry. J Wiley and Sons, New York, pp. 97-219. [8] Banwart, S., Davies, S., and Stumm, W., 1989, “The role of oxalate in accelerating the reductive dissolution of haematite (α-Fe 2 O 3 ) by ascorbate.” Colloids Surf. Vol. 39, pp. 303- 309.  Vol.8, No.5 Model for the Calculation of the Concentration of Dissolved Hematite 347 [9] Pourbaix, M., 1958, Atlas of Electrochemical Equilibria in Aqueous Solution, Pergamon Press. [10] Lee, S. O., Tran, T., Park, Y.Y., Kim, S.J., and Kim, M.J., 2006b, “Study on the kinetics of iron Leaching by oxalic acid.” Int. J. Miner Process, Vol. 80, pp.144-152. [11] Nwoye, C. I., 2008, “Model for computational analysis of dissolved haematite and heat absorbed by oxalic acid solution during leaching of iron oxide ore, J. Eng.& App. Sci., Vol. 4, pp. 22-25. [12] Dimitrijevic, M., Antonijevic, M. M., and Dimitrijevic, V., 1999, “Investigation of the kinetics of pyrite oxidation by hydrogen peroxide in hydrochloric acid solutions.” Min. Eng., Vol. 12, pp. 165-174. [13] Nwoye, C. I., 2006, SynchroWell Research Work Report, DFM Unit, No. 2021196, pp. 28- 43. [14] Kanevskii, E. A., and Filippov, A. P., 1963, “Influence of the ionic composition of solutions of Fe(iii) of the solution of uranium dioxide.” Soviet Radiochemistry (Eng. Trans. Of Radiokhimiya). [15] McKibben, M.A., 1984, Ph.D Thesis, Pennsylvania State University. [16] Bailey, L. K., and Peter, E., 1976, Can. Metall. Q., Vol. 15, pp. 333-344. [17] Gerlach, J., Hahne, and H., Pawlek, F., 1966, Erzmetall, Vol. 19, pp. 66-74. [18] McKay, D. R., and Halpern, J., 1958, Trans Metall. Soc. AIME, Vol. 6, pp. 301-309. [19] Vracar, R. Z., 1987, Prikl.Khim. Vol. 60, pp. 1458-465. [20] Nwoye, C. I., 2009, “Model for computational analysis of quantity of heat absorbed by hydrogen peroxide solution relative to weight-input of iron oxide ore during leaching.” J. Eng.& App. Sc., Inter. Res. J. Eng. Sc. Tech., Vol. 5, pp. 40-52. [21] Nwoye, C. I., 2006, SynchroWell Research Work Report, DFM Unit, No. 2021196, pp. 28- 43. |

