Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 201-205 doi:10.4236/jep.2010.12024 Published Online June 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP 1 Catalytic Dehydration of Glycerol under Mild Condition: An Environmentally Benign Acrolein Production Ágnes Zsigmond1, Péter Bata1, Mónika Fekete1, Ferenc Notheisz2 1Department of Organic Chemistry, University of Szeged, Szeged, Hungary; 2Organic Catalysis Research Group of the Hungarian Academy of Sciences, University of Szeged, Szeged, Hungary. Email: {azsig, notheisz}@chem.u-szeged.hu, bata_peter@freemail.hu Received February 8th, 2010; revised March 29th, 2010; accepted March 31st, 2010. ABSTRACT The increase of biodiesel production results in the accumulation of glycerol, which requires an increasing demand to- wards the study of chemical application of glycerol. Glycerol has to be transformed to other valuable chemicals, which can be used as starting materials for organic synthesis. With the final goal to find a reasonable solution for this prob- lem we have studied the dehydration of glycerol in liquid phase using a supported HPA catalyst and developed an en- vironmentally benign production of acrolein. Our method does not have any extreme conditions and produces a total conversion with high (93%) selectivity. Keywords: Consuming of Excess Glycerol, Production of Acrolein, Heterogeneous Method 1. Introduction Since people around the world almost exclusively cover its increasing energy demand with fossil basic materials (crude oil, natural gas, coal), sooner or later we are going to run out of these materials. To avoid this situation, the only possibility is to use biomass as a carbon source for the synthesis of fuels [1]. However, the use of biomass industrially in economically relevant chemical synthesis, new processes have to develop so that crude oil can be substituted [2]. Crude oil can be substituted by biodiesel where rape-oil is converted with methanol. However, there is a problem accompanying the bio- diesel production, namely the increase of the production of glycerol as a by-product, which can cause environ- mental and economical problems, as well. The effective utilization of this byproduct will be a key issue to pro- mote the bio-diesel commercialization. Consequently an increasing demand has been developed towards the study of chemical application of glycerol to other valuable chemicals, which can be used as starting materials for organic synthesis [3]. One of the possibilities is to perform an acid-induced dehydration of glycerol to acrolein, as it can be seen in Scheme 1. CH2 CH CH2 OH OH OH HPA CH CH CH2 O +2 H2O Scheme 1. Heteropolyacid catalyzed dehydration of glycerol to acrolein Acrolein is an important chemical intermediate so it is very important to develop a sustainable and cost- effective production route via glycerol from biodiesel, which offers an alternative for the presently dominating petrochemical process based on propylene. The dehydration of glycerol to acrolein became a rela- tively well studied subject recently, when the biodiesel production started to grow. However, most of these works applying drastic conditions, e.g. the dehydration of glycerol in sub- and supercritical water. Bühler et al. found only low glycerol conversion (31%) and similar selectivity (37 mol%) meanwhile they used relatively high pressure (25-45 MPa) and temperature (300-400oC) [4]. Ramayya et al. have studied the effect of the addition of sulfuric acid on the dehydration of glycerol and found that the addition of acid dramatically enhances the ac- rolein yield [5]. They have also used high temperature (300-350oC) and pressure (34.5 MPa) and the obtained acrolein conversion was not too high. The other disad- vantage of this method was the application of the highly corrosive sulfuric acid. Meanwhile it was proven that high reaction tempera- ture was necessary for the dehydration of glycerol, since no acrolein formation was detected at 250oC, 34.5 MPa using sulfuric acid as a catalyst [6]. Some other work can be found dealing with gas phase dehydration of glycerol on mesoporous ZSM-5 [7], or Nb2O5 catalyst [8], but either the low selectivity or the expensive catalyst makes these methods practically un-  Catalytic Dehydration of Glycerol under Mild Condition: An Environmentally Benign Acrolein Production 202 applicable. H. Atia, et al. have also studied the gas phase dehydra- tion of glycerol to acrolein using different supported HPA, as catalysts [9,10]. The influence of selected sup- port materials, catalyst loading and reaction temperature on acrolein formation was studied at standardized reac- tion conditions. Alumina was found to be superior to silica as support material with regard to catalyst activity and selectivity. Some other works were dealing with also the gas phase dehydration of glycerol using zirconia and silica supported heteropoly acid catalysts [11-13]. The authors compared the different catalyst preparation methods and they found that independently of the preparation history of ZrO2, the Keggin-anion at the catalyst surface ap- peared as a key to the selectivity for acrolein production. Acrolein selectivity as high as 70 mol% was obtained over the catalysts having the intermediate densities (0.18-0.65 HPA nm-2). The dehydration of glycerol to produce acrolein was performed over several solid acids [14,15]. Supported heteropoly acids were effective catalysts for the dehydra- tion of glycerol. The catalytic activity depended on the types of heteropoly acid and on the size of the mesopores in the silica support. Silicotungstic acid supported on silica with mesopores of 10 nm showed the highest cata- lytic activity with the acrolein selectivity of > 85 mol% at an ambient pressure and 275°C. According to our knowledge there is no environmen- tally benign process for the utilization of the excess glycerol. With the final goal to develop an environmen- tally benign production of acrolein, we have prepared a supported HPA catalyst and studied the dehydration of glycerol in liquid phase. For our study relatively mild conditions were selected and a new heterogeneous cata- lyst, which could replace the very corrosive sulfuric acid in this process. 2. Experimental 2.1 Catalyst Preparation 7.0 g of Al2O3 was suspended in 30 mL of methanol with stirring. 1.93 g (0.67 mmol) of phosphotungstic acid hy- drate (PTA) was dissolved in 25 mL of methanol and this solution was added dropwise into the alumina suspension with efficient stirring. The stirring was continued for an additional hour at room temperature, under an Ar at- mosphere. The solution was removed from the solid ma- terial and the solid residue was washed with methanol several times to remove the weakly adsorbed PTA from the surface of Al2O3. The prepared catalyst was characterized by FT-IR spectroscopy, taking the spectrum of the phosphotungstic acid hydrate and the anchored PTA/Al2O3, as well. The FT-IR spectra were recorded on a Bio-Rad FTS–65 A spectrophotometer, in the range of 400-4000 cm-1, in KBr pellets. 2.2 Dehydration Experiments The reaction was carried out in a two neck round-bottom flask, equipped with a dropping funnel and a column. The dried catalyst (8.93 g) was suspended in 70 ml high boiling point solvent (diesel oil) in a round-bottom flask and was heated gradually to 300oC with efficient stirring. To keep the high temperature, the flask was covered with glass wool. The column was connected to a distillation apparatus and the distilled product was collected in a flask, cooled by ice. Having reached the suitable tem- perature 10 mL (0.13 mol) of glycerol was dropped slowly, meanwhile the product was distilled out. The two phase product was separated and the organic phase was dried by MgSO4. The water phase was extracted by ether and the product distribution of both phases was determined by HP 5890 GC, using HP1 column and ethanol inert standard. 3. Results and Discussion 3.1 Catalyst Characterization With the final goal of developing an environmentally friendly process for the utilization of glycerol, which was produced in a large excess in the biodiesel production, we have anchored the phosphotungstic acid hydrate on Al2O3 support. The prepared catalyst was characterized by FT-IR spectroscopy, taking the spectrum of the free phosphotungstic acid hydrate and the anchored PTA, as well (Figure 1). The comparison of the two spectra above shows con- vincingly that the “free” acid and the heterogenized sam- ples have several similar bands (1090 and 890 cm-1), indicating the anchoring of the same acid. 3.2 Dehydration Experiments The preliminary experiments were done using H3PO4/ Al2O3 catalyst, which was prepared according to a patent description [16]. For the preparation of acrolein the same protocol was used as described in the patent and our pre- liminary experiments showed an increase in acrolein se- lectivity with increasing temperature. The dehydration reaction has not started below 240oC, and it seems that a good acrolein production needs at least 280oC or even higher temperature. In other words, the most difficult problem in the laboratory to find a good, neutral solvent with a high boiling point. We have tried several solvents e.g. triglykoldimethyl ether, methyldiglykol-tert-butyl ether and finally a distilled fraction of diesel oil was se- lected. Based on data of our preliminary experiments and Copyright © 2010 SciRes. JEP 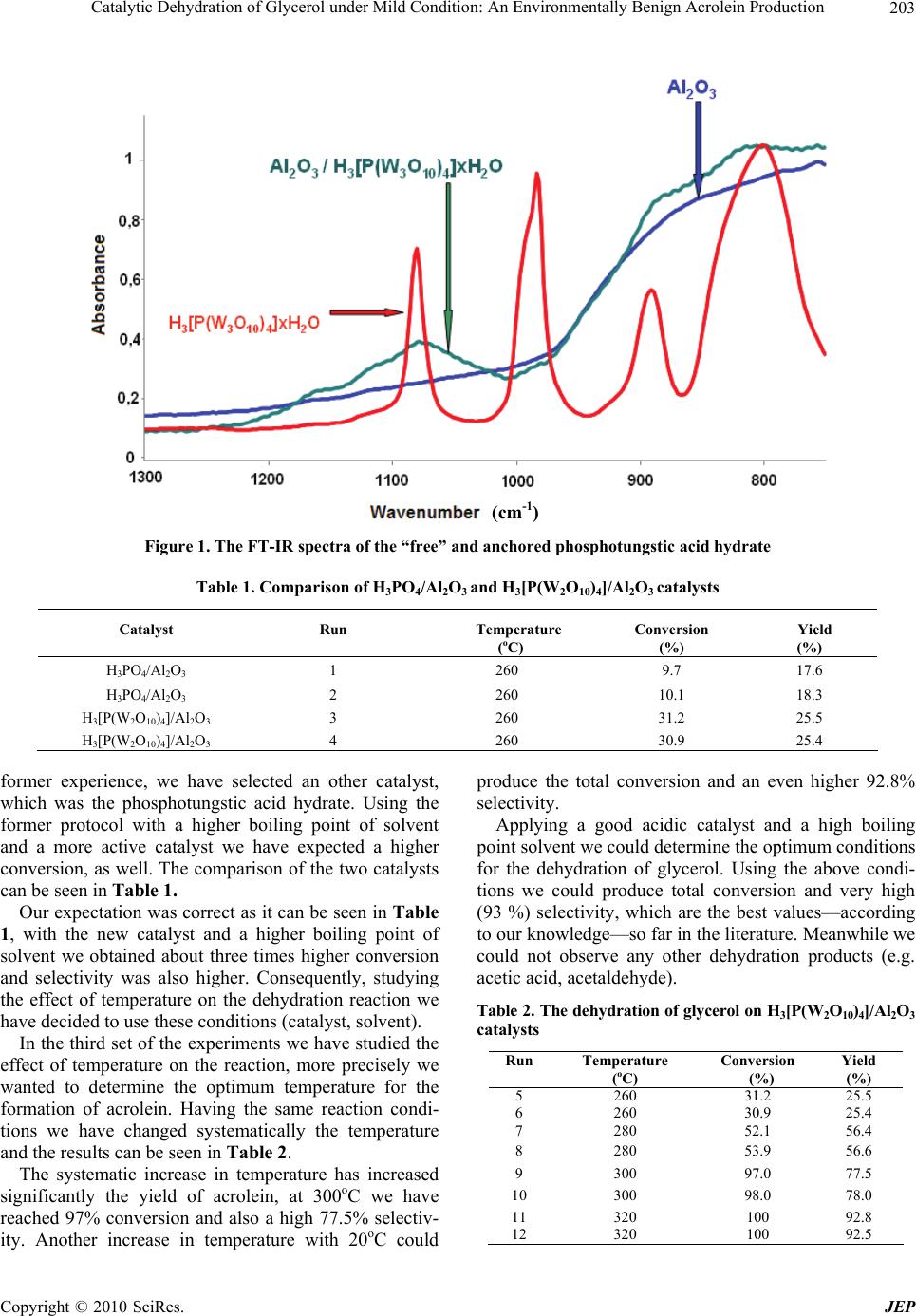 Catalytic Dehydration of Glycerol under Mild Condition: An Environmentally Benign Acrolein Production 203 Figure 1. The FT-IR spectra of the “free” and anchored phosphotungstic acid hydrate Table 1. Comparison of H3PO4/Al2O3 and H3[P(W2O10)4]/Al2O3 catalysts Catalyst Run Temperature ( oC) Conversion (%) Yield (%) H3PO4/Al2O3 1 260 9.7 17.6 H3PO4/Al2O3 2 260 10.1 18.3 H3[P(W2O10)4]/Al2O3 3 260 31.2 25.5 H3[P(W2O10)4]/Al2O3 4 260 30.9 25.4 former experience, we have selected an other catalyst, which was the phosphotungstic acid hydrate. Using the former protocol with a higher boiling point of solvent and a more active catalyst we have expected a higher conversion, as well. The comparison of the two catalysts can be seen in Table 1. Our expectation was correct as it can be seen in Table 1, with the new catalyst and a higher boiling point of solvent we obtained about three times higher conversion and selectivity was also higher. Consequently, studying the effect of temperature on the dehydration reaction we have decided to use these conditions (catalyst, solvent). In the third set of the experiments we have studied the effect of temperature on the reaction, more precisely we wanted to determine the optimum temperature for the formation of acrolein. Having the same reaction condi- tions we have changed systematically the temperature and the results can be seen in Table 2. The systematic increase in temperature has increased significantly the yield of acrolein, at 300oC we have reached 97% conversion and also a high 77.5% selectiv- ity. Another increase in temperature with 20oC could produce the total conversion and an even higher 92.8% selectivity. Applying a good acidic catalyst and a high boiling point solvent we could determine the optimum conditions for the dehydration of glycerol. Using the above condi- tions we could produce total conversion and very high (93 %) selectivity, which are the best values—according to our knowledge—so far in the literature. Meanwhile we could not observe any other dehydration products (e.g. acetic acid, acetaldehyde). Table 2. The dehydration of glycerol on H3[P(W2O10)4]/Al2O3 catalysts Run Temperature (oC) Conversion (%) Yield (%) 5 260 31.2 25.5 6 260 30.9 25.4 7 280 52.1 56.4 8 280 53.9 56.6 9 300 97.0 77.5 10 300 98.0 78.0 11 320 100 92.8 12 320 100 92.5 (cm-1) Copyright © 2010 SciRes. JEP  Catalytic Dehydration of Glycerol under Mild Condition: An Environmentally Benign Acrolein Production 204 The other advantage of this method that it is not ap- plying drastic conditions- high pressure and temperature- as the methods described in the literature. So, using the above conditions we could develop an environmentally benign method for the utilization of the extra amount of glycerol produced by the biodiesel formation. To check the efficiency of our catalyst we have made a blind experiment, too. During the blind experiment we have followed the same protocol without catalyst and we have not observed any acrolein formation. In other words applying these conditions the thermal dehydration is not occurring. 3.3 Recycling of the H3[P(W2O10)4]/Al2O3 Catalyst It is well known that the most important advantage of heterogeneous catalysts is their recyclability. To check the reusability of our catalyst, we have applied our cata- lyst in three subsequent runs and the results can be seen on Figure 2. As it can be seen on Figure 2, (light column represents conversion, dark column selectivity) we could apply successfully our catalyst in three subsequent runs. Nei- ther the conversion nor the selectivity has changed dur- ing the three subsequent experiments, which means that our catalyst has the advantage of the heterogeneous cata- lyst. The material balance is a very important fact from the point of view the practical application. For the experi- ments we have used 10 ml (0.13 mol) of glycerol for each run and the amount of isolated acrolein has in- creased as a function of temperature. Table 3 clearly shows that the isolated amount of ac- rolein has increased with increasing temperature. At the optimum temperature the material balance is reasonable. The loss of material is about 10%, which means the method is still practically useful. 4. Conclusions With the final goal of the utilization of the extra amount of glycerol, produced by the biodiesel production, we Figure 2. The selectivity and conversion in three subsequent runs at 300oC Table 3. The material balance as a function of temperature Temparature (oC) Amount of acrolein (g) (mol) Yield (%) 260 0.84 0.02 15.38 280 4.04 0.07 53.84 300 5.80 0.10 76.92 320 6.69 0.12 92.30 have developed a method to prepare valuable starting material. This starting material is the acrolein, having two reactive double bonds, which make it possible to apply in various organic synthesis. The method devel- oped by us is an environmentally benign method, does not have any extreme conditions and producing a total conversion with high (93%) selectivity. According to our knowledge this is the only environmentally benign method which produces such a high conversion and se- lectivity. REFERENCES [1] P. Gallezot, “Process Options for Converting Renewable Feedstocks to Bioproducts,” Green Chemistry, Vol. 9, No. 4, 2007, pp. 295-302. [2] A. Corma, S. Iborra and A. Velty, “Chemical Routes for the Transformation of Biomass into Chemicals,” Chemi- cal Reviews, Vol. 107, No. 6, 2007, pp. 2411-2502. [3] C.-H. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. Lu, “Chemoselective Catalytic Conversion of Glycerol as a Biorenewable Source to Valuable Commodity Chemicals,” Chemical Society Reviews, Vol. 37, No. 3, 2008, pp. 527- 549. [4] W. Bühler, E. Dinjus, H. J. Ederer, A. Kruse and C. Mas, “Ionic Reactions and Pyrolysis of Glycerol as Competing Reaction Pathways in Near- and Supercritical Water,” Journal of Supercritical Fluids, Vol. 22, No. 1, 2002, pp. 37-53. [5] S. Ramayya, A. Brittain, C. DeAlmeida, W. Mok and M. J. Antal, “Acid-Catalysed Dehydration of Alcohols in Supercritical Water,” Fuel, Vol. 66, No. 10, 1987, pp. 1364-1371. [6] M. Watanabe, T. Iida, Y. Aizawa, T. M. Aida and H. Inomata, “Acrolein Synthesis from Glycerol in Hot-Com- pressed Water,” Bioresource Technology, Vol. 98, No. 6, 2007, pp. 1285-1290. [7] C. Márquez-Alvarez, E. Sastre and J. Pérez-Pariente, “Solid Catalysts for the Synthesis of Fatty Esters of Glycerol, Polyglycerols and Sorbitol from Renewable Resources Sorbitol from Renewable Resources,” Topics in Catalysis, Vol. 27, No. 1-4, 2004, pp. 105-117. [8] S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, “Sus- tainable Production of Acrolein: Gas-Phase Dehydration of Glycerol over Nb2O5 Catalyst,” Journal of Catalysis, Vol. 250, No. 2, 2007, pp. 342-349. [9] H. Atia, U. Armbruster and A. Martin, “Dehydration of Glycerol in Gas Phase Using Heteropolyacid Catalysts as Active Compounds,” Proceedings of the DGMK-Confe- Copyright © 2010 SciRes. JEP  Catalytic Dehydration of Glycerol under Mild Condition: An Environmentally Benign Acrolein Production Copyright © 2010 SciRes. JEP 205 rence “Future Feedstocks for Fuels and Chemicals”, Ber- lin, 29 September-1 October 2008, pp. 177-184. [10] H. Atia, U. Armbruster and A. Martin, “Dehydration of Glycerol in Gas Phase Using Heteropolyacid Catalysts as Active Compounds,” Journal of Catalysis, Vol. 258, No. 1, 2008, pp. 71-82. [11] S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, “Sus- tainable Production of Acrolein: Preparation and Charac- terization of Zirconia-Supported 12-Tungstophosphoric Acid Catalyst for Gas-Phase Dehydration of Glycerol,” Applied Catalysis A: General, Vol. 353, No. 2, 2009, pp. 213-222. [12] S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, “Sus- tainable Production of Acrolein: Investigation of Solid Acid-Base Catalysts for Gas-Phase Dehydration of Glyc- erol,” Green Chemistry, Vol. 9, No. 10, 2007, pp. 1130- 1136. [13] S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, “Sus- tainable Production of Acrolein: Gas-Phase Dehydration of Glycerol over 12-Tungstophosphoric Acid Supported on ZrO2 and SiO2,” Green Chemistry, Vol. 10, No. 10, 2008, pp. 1087-1093. [14] L. Ning, Y. Ding, W. Chen, L. Gong, R. Lin, L. Yuan and Q. Xin, “Glycerol Dehydration to Acrolein over Acti- vated Carbon-Supported Silicotungstic Acids,” Chinese Journal of Catalysis, Vol. 29, No. 3, 2008, pp. 212-214. [15] E. Tsukuda, S. Sato, R. Takahashi and T. Sodesawa, “Pro- duction of Acrolein from Glycerol over Silica-Supported Heteropoly Acids,” Catalysis Communications, Vol. 8, No. 9, 2007, pp. 1349-1353. [16] H. E. Hoyt and T. H. Manninen, “Production of Acrolein from Glycerol,” U.S. Patent 2558520, U.S. Industrial Chemicals, Arvada, 1948. |

