Paper Menu >>
Journal Menu >>
 Journal of Environmental Protection, 2010, 1, 179-182 doi:10.4236/jep.2010.12022 Published Online June 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP 1 Preparation of High Effective Flocculant for High Density Waste Drilling Mud Fanghui Wang, Jing Zou, Hong Zhu*, Kefei Han, Jiantao Fan School of Science, Beijing University of Chemical and Technology, Beijing, China. Email: zhuho128@126.com Received February 2nd, 2010; revised May 4th, 2010; accepted May 5th, 2010. ABSTRACT To satisfy the requirement on the separation of solid and liquid in waste drilling mud, prepare a high effective floccu- lant for high density waste drilling mud used starch, 2-Trimethylammonium ethyl methacrylate chloride (DMC) and acrylamide (AM). The result showed that when the ratio of starch, DMC and AM was 2:1:3, the weight of initiator (po- tassium persulfate) was 0.2% of the AM, reaction temperature was 65℃ and reaction time was 5h, the performance of product was the best. The water content in filter cake was 27.6% after the waste drilling mud disposed by the optimiza- tion flocculant. The flocculent effect of optimization flocculant was superior to that of other flocculant in market. Keywords: High Density, Waste Drilling Mud, Dispose, Cation 1. Introduction The waste drilling mud and drilling wastewater were the inevitable industrial waste while Oil and Gas Exploration Drilling. They has become one of the most severe pollu- tion sources, whose effect on environment has been concerned gradually [1-3].There were many methods [4-7] to dispose the waste drilling mud and drilling wastewater, such as, Gravity Separation, Impact, Baffle, or Spray Separation, Parallel-Plate and Thin-Film Sepa- ration and Vacuum Separation. The Solid-Liquid Separa- tion has such advantages as large capacity, simple opera- tion and using a few equipments [8], it was the most im- portant and Widely Application method. At deep well drilling, the component of waste drilling mud was more and more complexity, the density of waste drilling mud was higher, and the waste drilling mud treatment was more harder [9]. The flocculant in market has not deal with the high density waste drilling mud. So a new starch-based cation copolymer flocculant for high density waste drilling mud was prepared. 2. Methods 2.1 Agent Edible corn starch, 2-Trimethylammonium ethyl metha- crylate chloride (CP), acrylamide (A.R), potassium per- sulfate (A.R), sodium hydroxide (A.R). High density waste drilling mud, the density was 1.639 g/mL and the Solid content was 56.72%. 2.2 Synthesis of Starch-Based Cation Coploymer Flocculant Add some deionized water, corn starch and sodium hy- droxide into a three-neck flask with stirrer. Heat the sys- tem to 70˚C and stir until starch dissolved completely (about 30 min), then cool the system to room temperature and removal of oxygen dissolved in the solution by blowing continuously nitrogen. After add some AM and DMC into above system, add the potassium persulfate solution drop by drop. Keep the system react for a certain time at a given temperature to gain product. 2.3 Flocculent Treatment Add some gel breaker to some waste drilling mud, then add some flocculant to above system and stir, centrifugal separation (3500 r/min) for 10min. Weight the quality of filter cake (m1) and soild phase (m2) after baking, gain the water content in filter cake by the formula as follows: the water content in filter cake = (m1-m2)/m1. 3. Result and Discussion 3.1 Preparing Conditions of Starch-Based Cation Copolymer Flocculant 3.1.1 Appropriate Weight Ratio of AM and Starch The amount of starch was 3 g, DMC dosage was 1 mL, pH = 7, the reaction temperature was 70˚C, reaction time was 3 h, the amount of acrylamide was respectively 3 g, 6 g, 9 g, 12 g, the amount of potassium persulfate was 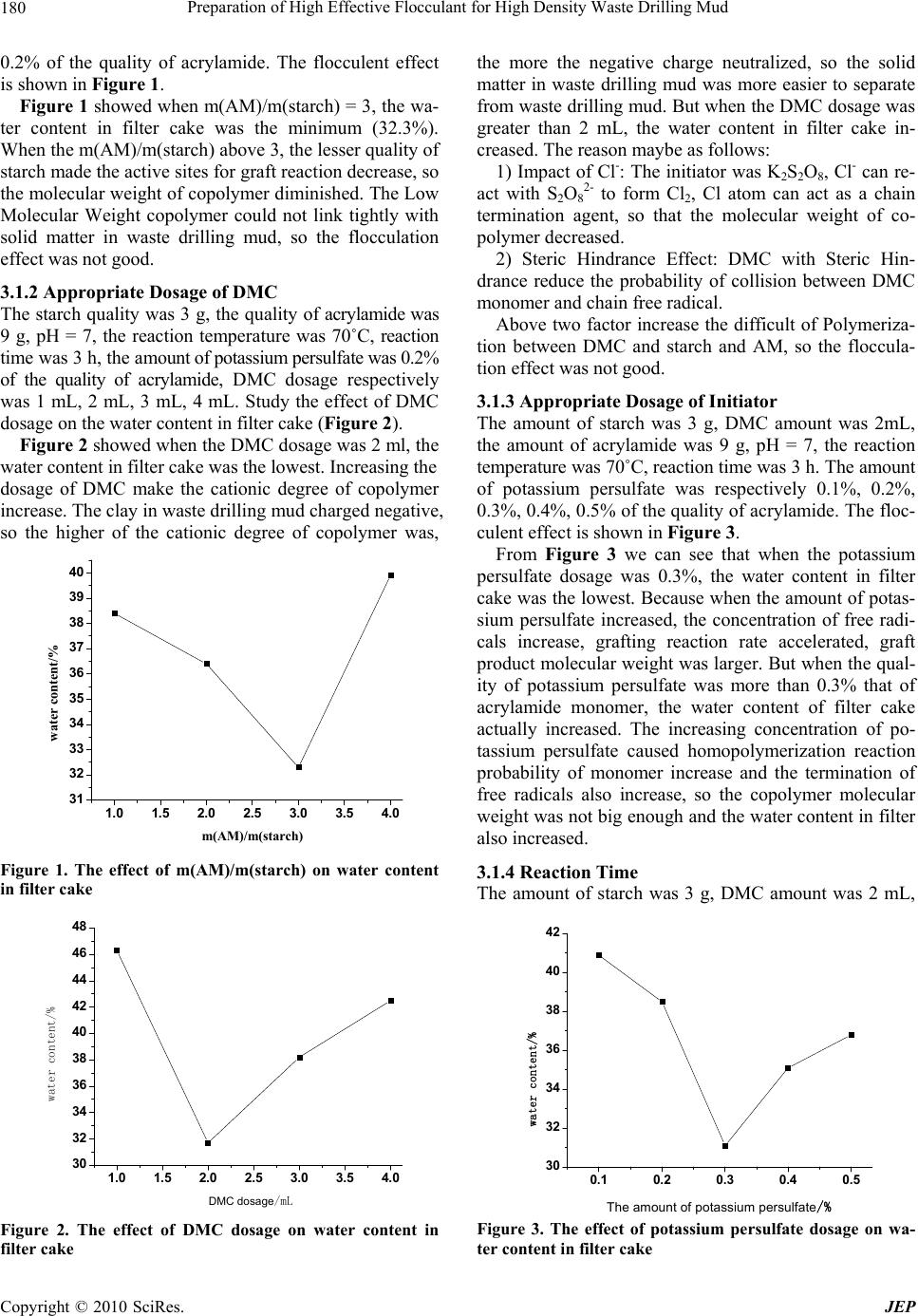 Preparation of High Effective Flocculant for High Density Waste Drilling Mud 180 0.2% of the quality of acrylamide. The flocculent effect is shown in Figure 1. Figure 1 showed when m(AM)/m(starch) = 3, the wa- ter content in filter cake was the minimum (32.3%). When the m(AM)/m(starch) above 3, the lesser quality of starch made the active sites for graft reaction decrease, so the molecular weight of copolymer diminished. The Low Molecular Weight copolymer could not link tightly with solid matter in waste drilling mud, so the flocculation effect was not good. 3.1.2 Appropriate Dosage of DMC The starch quality was 3 g, the quality of acrylamide was 9 g, pH = 7, the reaction temperature was 70˚C, reaction time was 3 h, the amount of potassium persulfate was 0.2% of the quality of acrylamide, DMC dosage respectively was 1 mL, 2 mL, 3 mL, 4 mL. Study the effect of DMC dosage on the water content in filter cake (Figure 2). Figure 2 showed when the DMC dosage was 2 ml, the water content in filter cake was the lowest. Increasing the dosage of DMC make the cationic degree of copolymer increase. The clay in waste drilling mud charged negative, so the higher of the cationic degree of copolymer was, 1.0 1.5 2.0 2.5 3.0 3.5 4.0 31 32 33 34 35 36 37 38 39 40 water content/% m(AM)/m(starch) Figure 1. The effect of m(AM)/m(starch) on water content in filter cake 1.0 1.5 2.0 2.5 3.0 3.5 4.0 30 32 34 36 38 40 42 44 46 48 water content/% DMC dosage/mL Figure 2. The effect of DMC dosage on water content in filter cake the more the negative charge neutralized, so the solid matter in waste drilling mud was more easier to separate from waste drilling mud. But when the DMC dosage was greater than 2 mL, the water content in filter cake in- creased. The reason maybe as follows: 1) Impact of Cl-: The initiator was K2S2O8, Cl- can re- act with S2O8 2- to form Cl2, Cl atom can act as a chain termination agent, so that the molecular weight of co- polymer decreased. 2) Steric Hindrance Effect: DMC with Steric Hin- drance reduce the probability of collision between DMC monomer and chain free radical. Above two factor increase the difficult of Polymeriza- tion between DMC and starch and AM, so the floccula- tion effect was not good. 3.1.3 Appropriate Dosage of Initiator The amount of starch was 3 g, DMC amount was 2mL, the amount of acrylamide was 9 g, pH = 7, the reaction temperature was 70˚C, reaction time was 3 h. The amount of potassium persulfate was respectively 0.1%, 0.2%, 0.3%, 0.4%, 0.5% of the quality of acrylamide. The floc- culent effect is shown in Figure 3. From Figure 3 we can see that when the potassium persulfate dosage was 0.3%, the water content in filter cake was the lowest. Because when the amount of potas- sium persulfate increased, the concentration of free radi- cals increase, grafting reaction rate accelerated, graft product molecular weight was larger. But when the qual- ity of potassium persulfate was more than 0.3% that of acrylamide monomer, the water content of filter cake actually increased. The increasing concentration of po- tassium persulfate caused homopolymerization reaction probability of monomer increase and the termination of free radicals also increase, so the copolymer molecular weight was not big enough and the water content in filter also increased. 3.1.4 Reaction Time The amount of starch was 3 g, DMC amount was 2 mL, 0.1 0.2 0.3 0.4 0.5 30 32 34 36 38 40 42 water content/% The amount of potassium persulfate/% Figure 3. The effect of potassium persulfate dosage on wa- ter content in filter cake Copyright © 2010 SciRes. JEP  Preparation of High Effective Flocculant for High Density Waste Drilling Mud181 the amount of acrylamide was 9 g, pH = 7, the amount of potassium persulfate was 0.3% of the quality of acryla- mide, reaction temperature was 70˚C, the reaction time was respectively 3 h, 4 h, 5 h, 6 h, 7 h. The flocculent effect is shown in Figure 4. As can be seen from Figure 4, the best reaction time was 4 h, this was because early in the reaction system, the active center of grafting reaction was more, the reac- tion rate was quick. But when the reaction time was more than 4 h, the number of grafting points decreased, which made grafting reaction rate slow down, so the grafting yield had fallen and the water content in filter cake cor- respondingly increased. 3.1.5 Reaction Temperature The amount of starch was 3 g, DMC amount was 2 mL, the amount of acrylamide was 9 g, pH = 7, the amount of potassium persulfate was 0.3% of the quality of acryla- mide, reaction time was 4 h, the reaction temperature was respectively 50˚C, 55˚C, 60˚C, 65˚C, 70˚C, 75˚C. The flocculent effect is shown in Figure 5. The reaction temperature at 65˚C, the water content in filter cake was the lowest. Because temperature rising within a certain range can increases the degree of starch 34567 30 32 34 36 38 40 42 44 46 water content/% reaction time/h Figure 4. The effect of reaction time on water content in filter cake 50 55 60 65 70 75 28 32 36 40 44 48 52 water content/% reaction temperature/℃ Figure 5. The effect of reaction temperature on water con- tent in filter cake swelling in water, thus ensure the reaction in the homo- geneous and be propitious to disperse initiator and mono- mer. When the temperature rose exceeds 65˚C, the chain termination reaction rate increased, which lead to lower grafting yield, so the water content in filter cake was higher. 3.2 Analysis of FTIR Figure 6 and Figure 7 are respectively FTIR spectra of starch and copolymer flocculant. As can be seen from the Figure 6, the peak of IR spectra of -OH symmetric stretching vibration in starch glucose unit appeared at 3440 cm-1 and 3442 cm-1. From Figure 7, we can know the peak of IR spectra of C=O stretching vibration and -NH2, -CH2 stretching vibration in copolymer flocculant appeared respectively at 1670 cm-1, 3198 cm-1, 1027 cm-1. There be should have two absorption peak at 3198 cm-1, but it overlapped with the strong broad hydroxyl peak of starch, so there was only a single peak. The absorption peak appeared at 1618 cm-1 was the -NH2 bending vibra- tion characteristic peaks. So, there must have some struc- tural units of AM and DMC in the starch- based cation copolymer flocculant. 3.3 Flocculent Effect From Figure 8 we can see that adding the same amount of flocculant, the flocculent effect of organic flocculants was better than that of inorganic coagulant, but the re- sults are not satisfactory, the water content in filter cake 4000 3000 2000 1000 v/cm-1 2928 1651 1021 3442 Figure 6. FTIR spectra of corn starch 4000 3000 2000 1000 v/cm-1 1670 1618 3198 1027 2928 3410 Figure 7. FTIR spectra of starch-based cation copolymer flocculant Copyright © 2010 SciRes. JEP  Preparation of High Effective Flocculant for High Density Waste Drilling Mud Copyright © 2010 SciRes. JEP 182 123456 0 10 20 30 40 50 60 water content/% flocculant REFERENCES [1] M. J. Yang, W. L. Liang, Q. R. Jin, X. H. Wu and G. N. Yu, “Study on Comprehensive Treatment Technique about Waste Drilling Mud,” Mineral Petrology, in Chinese, Vol. 23, No. 1, 2003, pp. 109-112. [2] M. O. Benka and A. Olumagin, “Effects of Waste Drill- ing Fluid on Bacterial Isolates from a Mangrove Swamp Oilfield Location in the Niger Delta of Nigeria,” Biore- source Technology, Vol. 55, No. 3, 1996, pp. 175-179. [3] S. Rehan and H. Tahir, “A Fuzzy-Based Methodology for an Aggregative Environmental Risk Assessment: A Case Study of Drilling Waste,” Environmental Modelling & Software, Vol. 20, No. 1, 2005, pp. 33-46. 1—Non-ionic polyacrylamide; 2—Cationic polyacrylamide; [4] C. G. Street and S. E. Guigard, “Treatment of Oil-Based Drilling Waste Using Supercritical Carbon Dioxide,” Jour- nal of Canadian Petroleum Technology, Vol. 48, No. 6, 2009, pp. 26-29. 3—Anionic polyacrylamide; 4—Polymerization ferric sulphate; 5—Polymerization aluminum sulfate; 6—Starch-based cation copolymer Figure 8. Floccuent effect of some flocculant [5] H. Shang, S. Kingman, C. Snape and J. Robinson, “A New Technology of Microwave Treatment of Oil Con- taminated Drilling Waste in a Single Mode Cavity,” Acta Petrolei Sinica (Petroleum Processing Section), in Chi- nese, Vol. 25, No. 3, 2009, pp. 358-362. was more than 50%. From Figure 8, the flocculent effect of the starch-based cation copolymer flocculant was sig- nificantly better than that of the other coagulant in mar- ket, water content of waste drilling mud after treated rate was 27.6%. [6] J. A. Veil, “Drilling Waste Management: Past, Present, and Future,” Proceedings of SPE Annual Technical Con- ference and Exhibition, San Antonio, 29 September-2 October 2002, pp. 545-551. 4. Conclusions Starch, 2-Trimethylammonium ethyl methacrylate chlo- ride (DMC) and acrylamide (AM) used as raw material and potassium persulfate as initiator to prepare a starch-based cation copolymer flocculant. For high- [7] ASME Shale Shaker Committee, “Drilling Fluids Proc- essing Handbook,” Gulf Professional Publishing, Bur- lington, 2005. [8] Y. Xiao, R. S. Wang and H. Deng, “Treatment of Chemi- cally Enhanced Sold-Liquid Separation for Waste Drilling Fluid,” China Environmental Science, Vol. 20, No. 5, 2000, pp. 453-456. density waste drilling mud, the flocculent effect of the starch-based cation copolymer flocculant flocculation was significantly better than that of the other coagulant in market. [9] G. Li, M. Zhu and J. L. Qian, “Lab Experiments into Solid-Liquid Separation of Waste High Density Drilling Liquid,” Petroleum Drilling Techniques, in Chinese, Vol. 26, No. 1, 1998, pp. 21-22. 5. Acknowledgements This paper is supported by National Hi-Tech Research and Development Program of China (863 Program) (2008BAC43B02). |

