Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 7, No.3, pp 215-231, 2008 jmmce.org Printed in the USA. All rights reserved 215 Growth of Strontium Chromium Magnesium Hydrogen Phosphate (SrCrMHP) Crystal in Silica Gel Medium at Different Growth Environments and Nucleation Reduction Strategy G. Kanchana 1 , P. Suresh 2 , P. Sundaramoorthi 2 *, S. Kalainathan 3 , G.P. Jeyanthi 1 1 Department of Bio-chemistry, Avinashilingam Deemed University,Coimbatore, TamilNadu, India. 2 Department of Physics, A.A.Govt. Arts College, Namakkal – India-637001. (sundara78@rediffmail.com) 3 Department of Physics, SSH, VIT University, Vellore - India ABSTRACT Kidney stone consists of various organic, inorganic and semi organic compounds. Mineral oxalate monohydrate and di-hydrate are the main inorganic constituents of kidney stones. However, mechanisms leading to the formation of mineral oxalate kidney stones are not clearly understood. In this field of study, there are several hypotheses including nucleation, crystal growth and/or aggregation of formation of AOMH (Ammonium oxalate monohydrate) and AODH (Ammonium oxalate di-hydrate) crystals. The effect of some urinary species such as ammonium oxalate, calcium citrate, proteins and trace elements were reported by the author. The kidney stone constituents are grown in silica gel medium (SMS) which provides the necessary growth simulation (in-vivo). In the artificial urinary stone growth process, identification of growth parameters within the different chemical environment was carried out and reported for the urinary crystals such as CHP, SHP, BHP and MHP. In the present study, SrCrMHP (Strontium chromium magnesium hydrogen phosphate) crystals are grown in three different growth faces to attain the total nucleation reduction. Extension of this research, many characterization studies have been carried out and the results are reported. Key words: CHP, MHP, AMHP, SHP, SrCrMHP, trace element, major minerals, minor minerals.  216 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 1. INTRODUCTION SHP (Strontium hydrogen phosphate), CHP (Calcium hydrogen phosphate) and BHP (Barium hydrogen phosphate) were grown in silica gel medium at room temperature. The next approach is to grow mixed crystal in silica gel medium at different environments, which contains one major element (Phosphate), two minor or trace elements (Strontium, Chromium) and one inhibitor (Magnesium). SrCrMHP is a mixed crystal, which typically represent the biological crystals formed in the human urinary tracts called renal stones. One can obtain the periodic precipitation of Liesegang rings of biological crystal named as Brushite, Struvite, HAP, BMHP and SMHP [1-9]. 2. EXPERIMENTAL PROCEDURES The dissociation of orthophosphoric acid system can be represented by three dissociation equilibrium and the presence of various ions at various pH values is studied. Based on these results, gel medium of pH in the range from 6 to 10 has been used in which the HPO 42- ions dominates or alone exist. This decreases the possibility of SrCrMP crystals occurring during the SrCrMHP growth. The crystallization apparatus employed were glass test tubes of 25 mm diameter and 150 mm length for single diffusion process (SDP) and thick walled glass U tubes of 30 mm diameter and 180 mm length for double diffusion process (DDP). The chemicals used were EXCELAR-Qualigens (E-Q) AR grade CrCl 2 , SrCl 2 , Mg (NO 3 ) 2 .2H 2 O (MW-258.41) and orthophosphoric acid (Sp.gr.1.75). The SMS gel or water glass was prepared as per the literature. One of the reactants orthophosphoric acid was mixed with silica gel at desired gel density and elevated temperatures. After the gel set, the supernatant mixture (Chromium chloride +Strontium chloride + Magnesium nitrate) at a required mole solutions was slowly added along the walls of the growth columns (test tubes, U-tubes) over the set gels and tightly closed to prevent evaporation. Then the growth systems were allowed to react within the gel medium and the following chemical reaction takes place [10-14]. (CrCl 2 +Mg (NO 3 ) 2 .2H 2 O) + SrCl 2 + H 3 PO 4 SrCrMgHPO 4 + Waste. Table-1 and Table-2 represent the growth parameters of SrCrMHP crystal and the bold letters represent the optimum growth parameters in SDP and DDP growth processes. The growth column of different growth environments are shown in Fig 1-6. 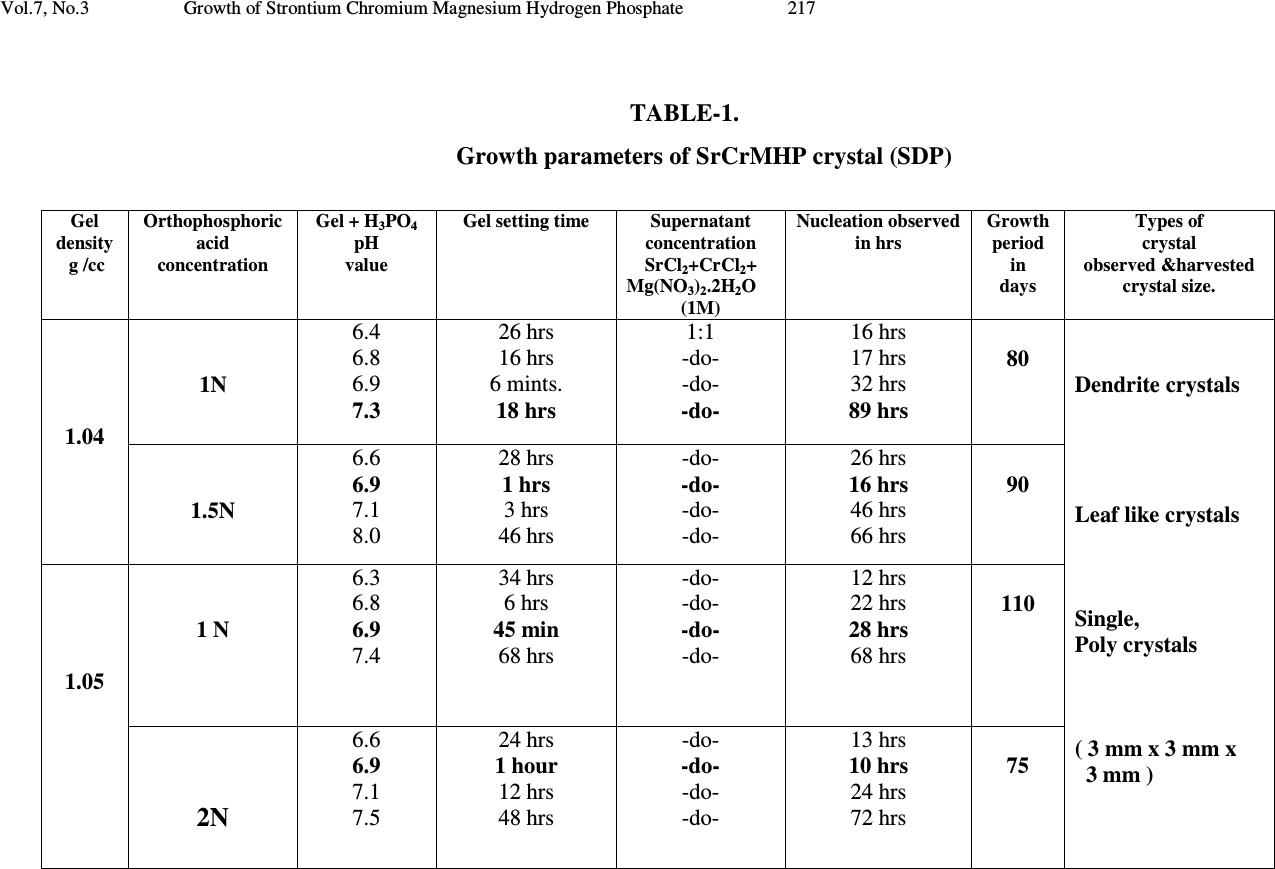 Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 217 TABLE-1. Growth parameters of SrCrMHP crystal (SDP) Gel density g /cc Orthophosphoric acid concentration Gel + H3PO4 pH value Gel setting time Supernatant concentration SrCl2+CrCl2+ Mg(NO3)2.2H2O (1M) Nucleation observed in hrs Growth period in days Types of crystal observed &harvested crystal size. 1.04 1N 6.4 6.8 6.9 7.3 26 hrs 16 hrs 6 mints. 18 hrs 1:1 -do- -do- -do- 16 hrs 17 hrs 32 hrs 89 hrs 80 Dendrite crystals Leaf like crystals Single, Poly crystals ( 3 mm x 3 mm x 3 mm ) 1.5N 6.6 6.9 7.1 8.0 28 hrs 1 hrs 3 hrs 46 hrs -do- -do- -do- -do- 26 hrs 16 hrs 46 hrs 66 hrs 90 1.05 1 N 6.3 6.8 6.9 7.4 34 hrs 6 hrs 45 min 68 hrs -do- -do- -do- -do- 12 hrs 22 hrs 28 hrs 68 hrs 110 2N 6.6 6.9 7.1 7.5 24 hrs 1 hour 12 hrs 48 hrs -do- -do- -do- -do- 13 hrs 10 hrs 24 hrs 72 hrs 75 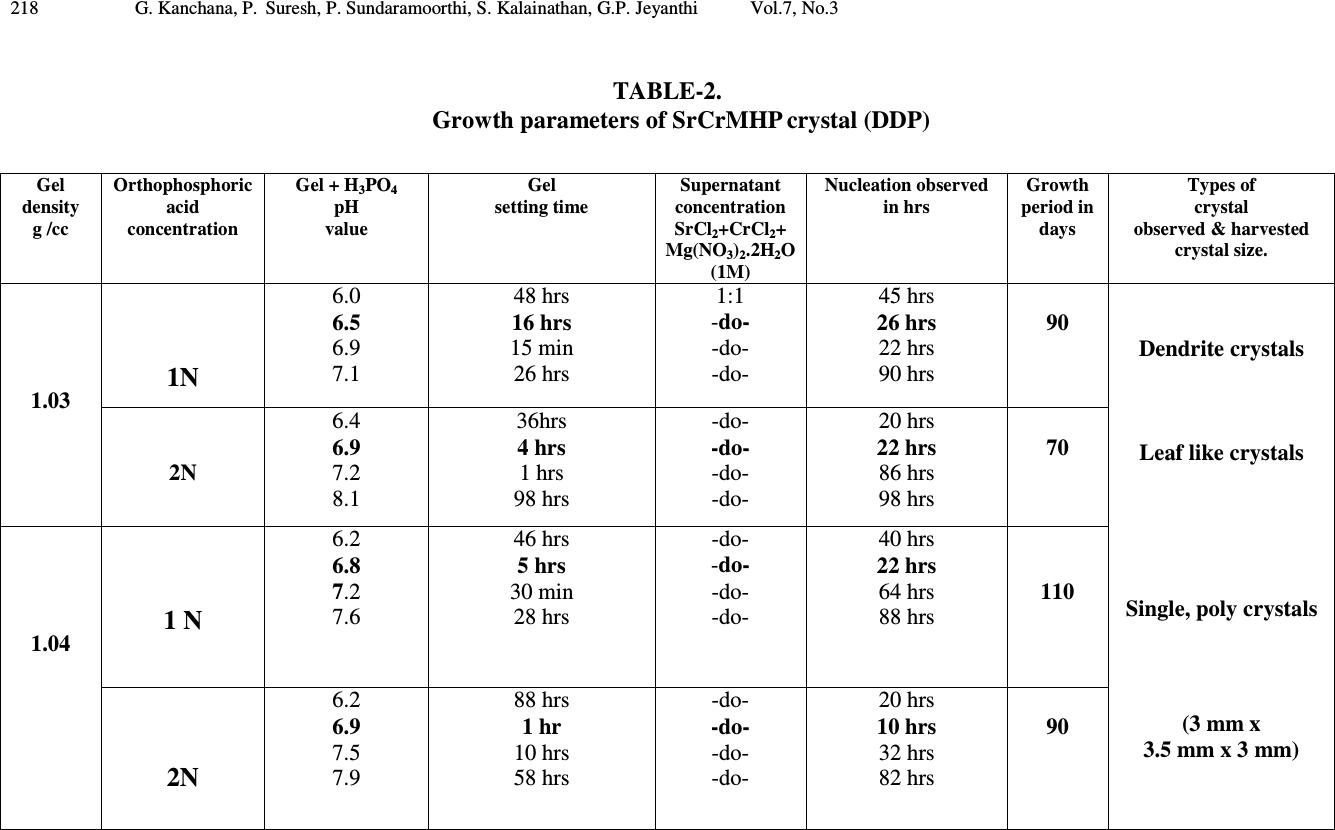 218 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 TABLE-2. Growth parameters of SrCrMHP crystal (DDP) Gel density g /cc Orthophosphoric acid concentration Gel + H3PO4 pH value Gel setting time Supernatant concentration SrCl2+CrCl2+ Mg(NO3)2.2H2O (1M) Nucleation observed in hrs Growth period in days Types of crystal observed & harvested crystal size. 1.03 1N 6.0 6.5 6.9 7.1 48 hrs 16 hrs 15 min 26 hrs 1:1 -do- -do- -do- 45 hrs 26 hrs 22 hrs 90 hrs 90 Dendrite crystals Leaf like crystals Single, poly crystals (3 mm x 3.5 mm x 3 mm) 2N 6.4 6.9 7.2 8.1 36hrs 4 hrs 1 hrs 98 hrs -do- -do- -do- -do- 20 hrs 22 hrs 86 hrs 98 hrs 70 1.04 1 N 6.2 6.8 7.2 7.6 46 hrs 5 hrs 30 min 28 hrs -do- -do- -do- -do- 40 hrs 22 hrs 64 hrs 88 hrs 110 2N 6.2 6.9 7.5 7.9 88 hrs 1 hr 10 hrs 58 hrs - do - -do- -do- -do- 20 hrs 10 hrs 32 hrs 82 hrs 90  Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 219 Fig-1. Growth of SrCrMHP crystal within laboratory environment (SDP). Fig -2. Growth of SrCrMHP crystal within laboratory environment (SDP).  220 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 Fig-3. Growth of SrCrMHP crystal within sunlight exposed medium (SDP). Fig-4. Growth of SrCrMHP crystal under laser exposed medium (SDP).  Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 221 Fig-5. Harvested SrCrMHP crystals in SDP (3 mm x 3 mm x 3 mm). Fig-6. Harvested SrCrMHP crystals in DDP (3 mm x 3.5 mm x 3 mm).  222 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 3. CHARACTERIZATION STUDIES of SrCrMHP CRYSTAL 3.1 FTIR Spectral Analysis of SrCrMHP Crystal FTIR spectrometer having KBr pellets sample holder and KBr detector was used for the analysis. The KBr pellet samples were used and the absorption frequencies range from 400 to 4000 cm -1 . Fig-7 shows the FTIR spectrum of SrCrMHP crystal. The results matched with the reported values. The absorption bonds, absorption frequencies and percentage of transmittance were compared with the reported values. The values are tabulated in Table-3. The functional groups confirm the SrCrMHP crystal constituents [15-19]. 3.2 Thermo Gravimetric (TGA and DTA) Analysis of SrCrMHP Crystal The TGA and DTA of SrCrMHP crystal was carried out by STA 11500-PLTS instrument. SrCrMHP crystal sample of 1.290 mg was taken for TGA process. The TGA was performed from room temperature to 1000ºC by heating it at a constant rate. Fig.-8 shows the TGA and DTA graph of SrCrMHP crystal. The % of weight of SrCrMHP sample present at a particular temperature is tabulated in Table-4 [20-24]. Strontium, chromium and magnesium are stable with respect to temperature up to 900 ºC. About 21.2% of SrCrMHP crystal sample was decomposed and 78.8% of the sample remains stable. 3.3 Etching Study of SrCrMHP Crystal A well-grown SrCrMHP crystal was immersed in HCl solution at a desired concentration. The dissolution of SrCrMHP crystal depends upon the etchant concentration, temperature, and crystal morphology and etching time. The etch pits are shown in Fig-9. The etch pits observed in the photo are knife pits, cone pits, leaf pits and step pits [25-29]. 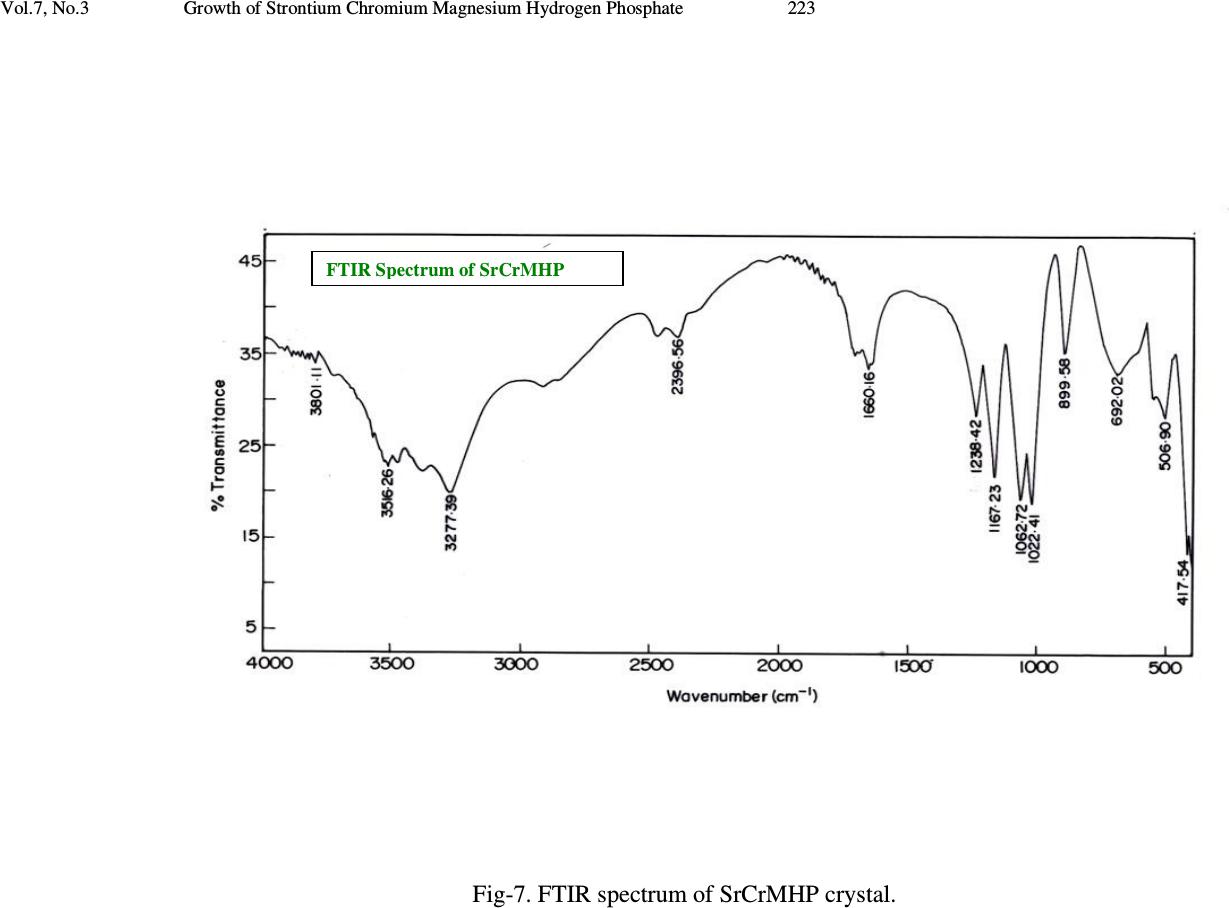 Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 223 Fig-7. FTIR spectrum of SrCrMHP crystal. FTIR Spectrum of S r C r MHP 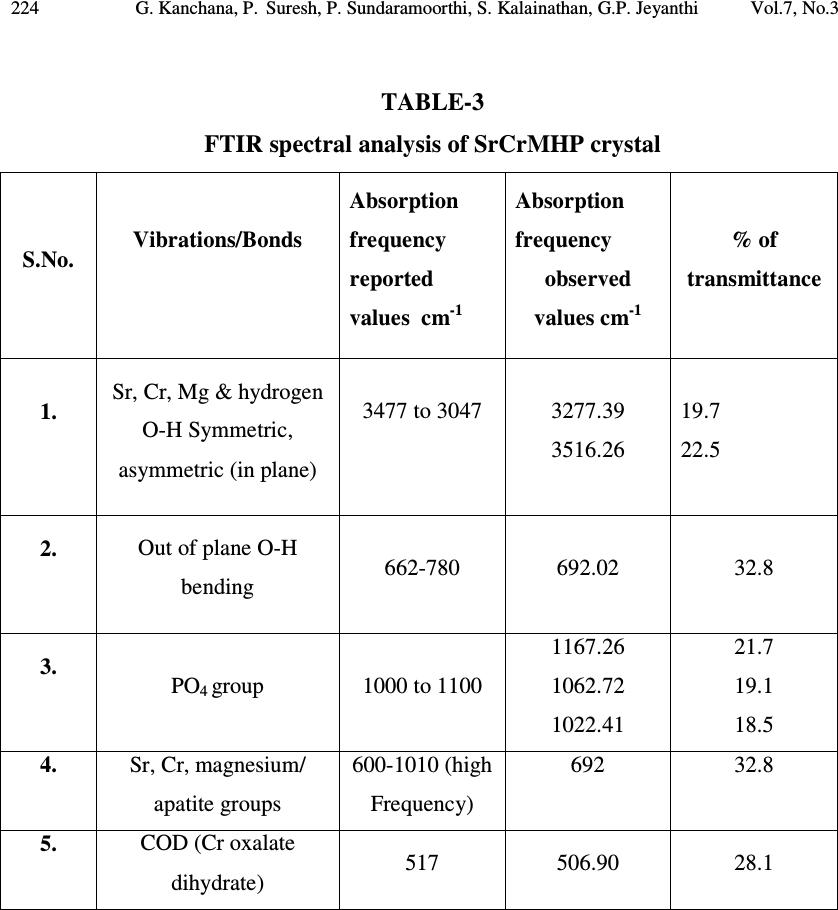 224 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 TABLE-3 FTIR spectral analysis of SrCrMHP crystal S.No. Vibrations/Bonds Absorption frequency reported values cm -1 Absorption frequency observed values cm -1 % of transmittance 1. Sr, Cr, Mg & hydrogen O-H Symmetric, asymmetric (in plane) 3477 to 3047 3277.39 3516.26 19.7 22.5 2. Out of plane O-H bending 662-780 692.02 32.8 3. PO 4 group 1000 to 1100 1167.26 1062.72 1022.41 21.7 19.1 18.5 4. Sr, Cr, magnesium/ apatite groups 600-1010 (high Frequency) 692 32.8 5. COD (Cr oxalate dihydrate) 517 506.90 28.1 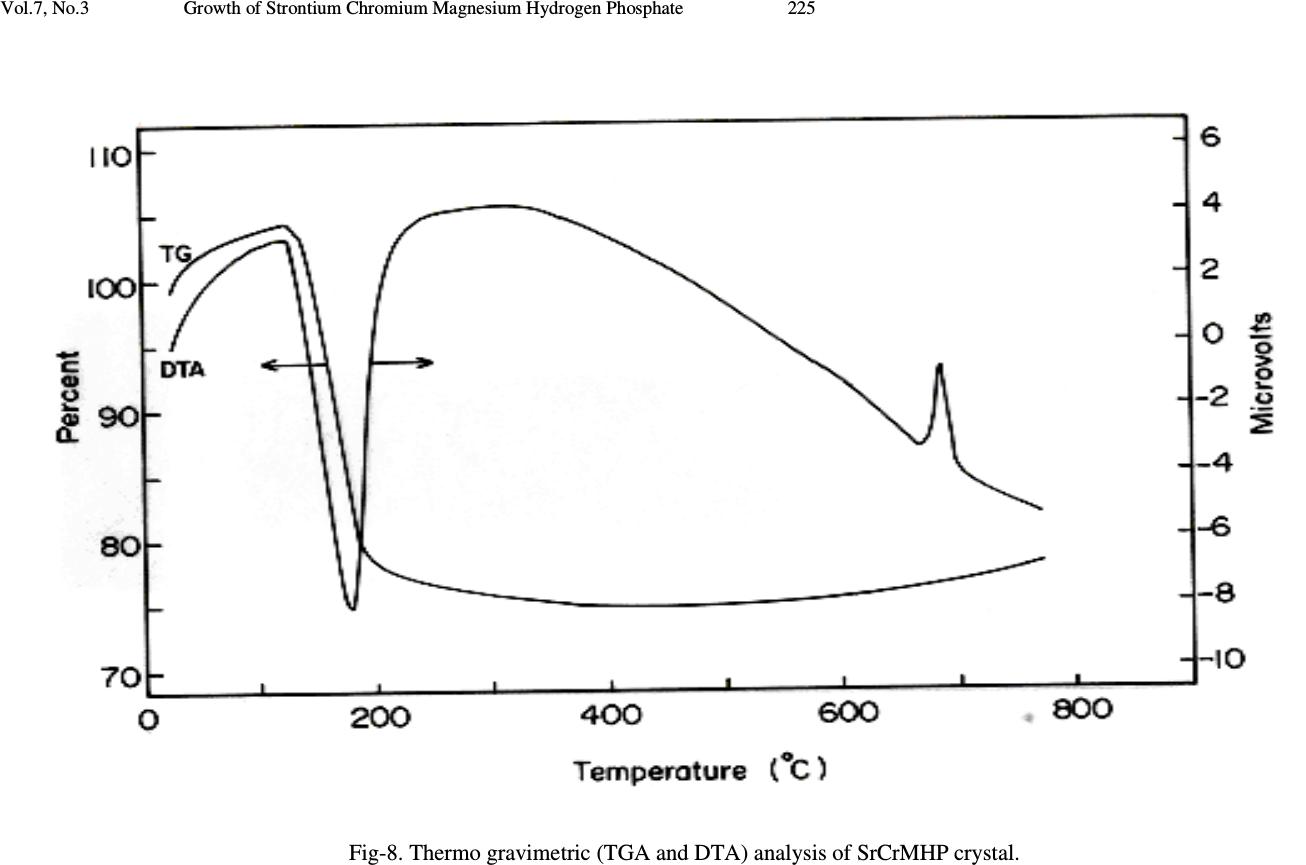 Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 225 Fig-8. Thermo gravimetric (TGA and DTA) analysis of SrCrMHP crystal.  226 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 TABLE- 4. Thermal analysis of SrCrMHP crystal Points TGA DTA in 0 C Temperature ( 0 C) % of SrCrMHP crystal present Quantity of sample remaining (mg) 1 2 3 4 5 35 122.19 190.69 - - 100 104.4 78.8 78.8 78.8 1.290 1.35 1.02 1.02 1.02 122.94 170.33 237.58 668.33 686.87 Fig-9. Chemical etching of SrCrMHP crystal at room temperature (1.5 mm - 40X). 3.4 Scanning Electron Microscopic Study of SrCrMHP Crystal A well-grown SrCrMHP single crystal was selected for the investigation of surface morphology by using SEM. The SEM photograph was taken in the version S-300- I instrument. The sample named VCA-600 was kept in lobe middle; the data size was 640 x 480 µm. The minor and major magnification of SEM was about 250 times. SEM accelerating voltage was 25000 volts and the sample was kept in a high vacuum at 18200 µm working distance and monochromatic colour mode was employed. 50 µm  Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 227 focusing of SrCrMHP crystal SEM is shown in Fig-10. In the surface analysis of SEM- SrCrMHP crystal, smooth, fine grain boundaries and few valley regions were observed [30-38]. 3.5 X-ray Diffraction of SrCrMHP Crystal The single crystal XRD result reveals the crystalline nature of the grown crystal. The lattice parameters of the SrCrMHP crystal were calculated. The lattice index data are as follows. CD0> LO From to: 1 25 1H 4. -1. 3. * 11.18 S -23.94 11.56 -2.12 **S 2 0.61 771.9 2H 4. 1. 3. * 10.17 S -1.27 10.65 -2.22 **S 2 0.52 691.4 3H 3. 3. 3. * 10.30 S 20.47 8.00 6.41 **S 2 0.55 8629.2 4H 2. 4. 2. * 9.77 S 40.22 8.51 4.22 **S 2 0.63 32860.5 5H 3. -2. 3. * 9.28 S -41.65 6.78 7.84 **S 2 0.65 18929.0 6H 4. 1. 4. * 11.37 S -6.17 9.08 8.04 **S -2 0.60 2185.0 7H 4. -1. 4. * 11.39 S -26.55 8.96 8.33 **S -2 0.64 2317.0 8H 4. -2. 4. * 11.89 S -35.60 9.03 8.10 **S 2 0.68 20586.8 9H 1. 1. 5. * 9.99 S -15.66 -7.75 52.95 **S 2 0.66 479.8 10H -1. 3. 4. * 9.99 S 29.11 -11.67 62.97 **S 2 0.89 15019.5 11H 0. 2. 5. * 10.37 S 0.82 -11.46 63.06 **S 2 0.54 17371.0 12H 1. 0. 6. * 11.69 S -32.48 -7.62 57.31 **S 2 0.67 5865.0 13H 1. 2. 6. * 12.36 S -5.31 -5.73 53.01 **S 2 0.69 1626.3 14H -1. 3. 3. * 8.53 S 41.39 -10.73 56.32 **S -2 0.68 1955.6 15H 1. 0. 4. * 7.90 S -30.41 -8.75 50.74 **S -2 0.64 9272.4 16H 0. 2. 4. * 8.62 S 8.06 -11.83 59.66 **S -2 0.59 1588.7 17H 0. 0. 4. * 7.63 S -38.17 -16.89 71.56 **S -2 0.65 2069.7 18H 0. 2. 6. * 12.18 S -4.63 -10.48 65.17 **S -2 0.76 3917.2 19H -2. 4. 5. * 13.17 S 36.66 -9.78 66.59 **S 2 0.67 9668.4 20H 0. 2. 7. * 14.04 S -8.92 -9.11 66.62 **S 2 0.68 14379.1 21H -1. 3. 4. * 9.93 S 28.98 -11.52 62.95 **S 2 0.65 14779.0 22H 0. 2. 5. * 10.38 S 0.66 -11.32 63.04 **S 2 0.69 17581.7 23H 0. 2. 6. * 12.18 S -4.82 -10.34 65.17 **S -2 0.78 4242.5 24H 1. 0. 6. * 11.68 S -32.30 -7.67 57.26 **S 2 0.76 5968.1 25H 1. 2. 6. * 12.35 S -5.37 -5.66 53.00 **S 2 0.93 16833.1 CD0> REIND Nr S H K L Dev-Ang dTh dPh dCh 0.0093629 1 H 4.009 -1.002 2.995 0.1061 -0.011 0.019 0.105 0.0010538 2 H 3.999 1.000 3.003 0.0266 -0.002 0.001 -0.027 0.0002487 3 H 2.997 2.999 3.002 0.0338 0.003 -0.003 -0.034 0.0003242 4 H 1.999 4.002 2.003 0.0273 -0.005 -0.005 -0.027 0.0003231 5 H 3.000 -2.001 3.004 0.0365 -0.006 -0.006 -0.036 0.0004269 6 H 3.998 0.999 3.995 0.0228 0.009 0.000 0.023 0.0004750 7 H 3.998 -0.998 4.000 0.0158 0.004 -0.012 -0.010 0.0002439 8 H 3.998 -1.998 4.000 0.0226 0.004 -0.014 -0.018 0.0002931  228 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 9 H 1.002 0.999 5.002 0.0261 -0.005 0.026 0.016 0.0003177 10 H -1.001 2.998 4.000 0.0267 0.001 0.000 -0.027 0.0002356 11 H 0.000 2.001 5.000 0.0125 0.000 -0.016 0.006 0.0001124 12 H 0.997 -0.001 6.005 0.0438 -0.009 0.019 -0.042 0.0006067 13 H 1.001 1.999 6.002 0.0150 -0.003 0.017 0.008 0.0002098 14 H -1.004 2.995 3.003 0.0941 0.002 0.008 -0.094 0.0006959 15 H 0.994 -0.001 4.002 0.0955 0.000 0.017 -0.096 0.0006462 16 H -0.001 1.998 3.998 0.0213 0.005 -0.006 -0.024 0.0002826 17 H -0.006 -0.001 4.000 0.0976 0.000 0.032 -0.097 0.0006389 18 H 0.004 2.000 5.995 0.0424 0.009 -0.001 0.044 0.0006076 19 H -1.998 4.002 5.001 0.0256 -0.002 0.016 0.023 0.0003099 20 H 0.003 2.002 6.997 0.0295 0.004 -0.015 0.029 0.0003975 21 H -1.001 2.999 4.000 0.0111 0.002 -0.004 -0.012 0.0001234 22 H 0.000 2.003 5.002 0.0238 -0.005 -0.028 0.014 0.0003267 23 H 0.003 2.003 5.998 0.0396 0.001 -0.020 0.037 0.0004159 24 H 1.000 -0.001 6.001 0.0082 -0.002 0.007 -0.007 0.0001302 25 H 1.002 2.000 5.998 0.0209 0.004 0.003 0.0229 0.0002846 Reciprocal axis matrix Direct axis matrix 0.019356 -0.095158 0.012389 1.934520 7.88982 -6.028681 0.077469 0.024921 0.054601 -9.932248 2.392297 -0.107989 -0.059732 -0.000905 0.075899 1.421146 6.237479 8.552554 Niggli-values Sigma direct axis matrix 100.5313 103.3842 118.0713 0.004457 0.002937 0.003931 -0.1169 -0.1434 0.0792 0.002609 0.001702 0.002302 0.003732 0.002434 0.003289 Cell parameters Sigma cell parameters a =10.0230Å, b =10.2067Å, c =10.5844Å, α αα α =90.1808˚, β ββ β =90.0243˚, γ γγ γ =90.0752˚. 0.0230 0.0256 0.0274 -0.001071 -0.001339 0.000773 0.000401 0.000449 0.000476 Volume= 1094.6399 . 0.5550 Index-Status: HHHHHHHHHHHHHHHHHHHHHHHHH CD0> The lattice parameters are a=10.0230Å, b=10.2067Å, c=10.5844Å, α αα α=90.1808˚, β ββ β=90.0243˚ and γ γγ γ=90.0752˚. The volume of the unit cell of the SrCMHP crystal is 1094.6399 (Å) 3. From this data, it is confirmed that the SrCMHP crystal system is triclinic. 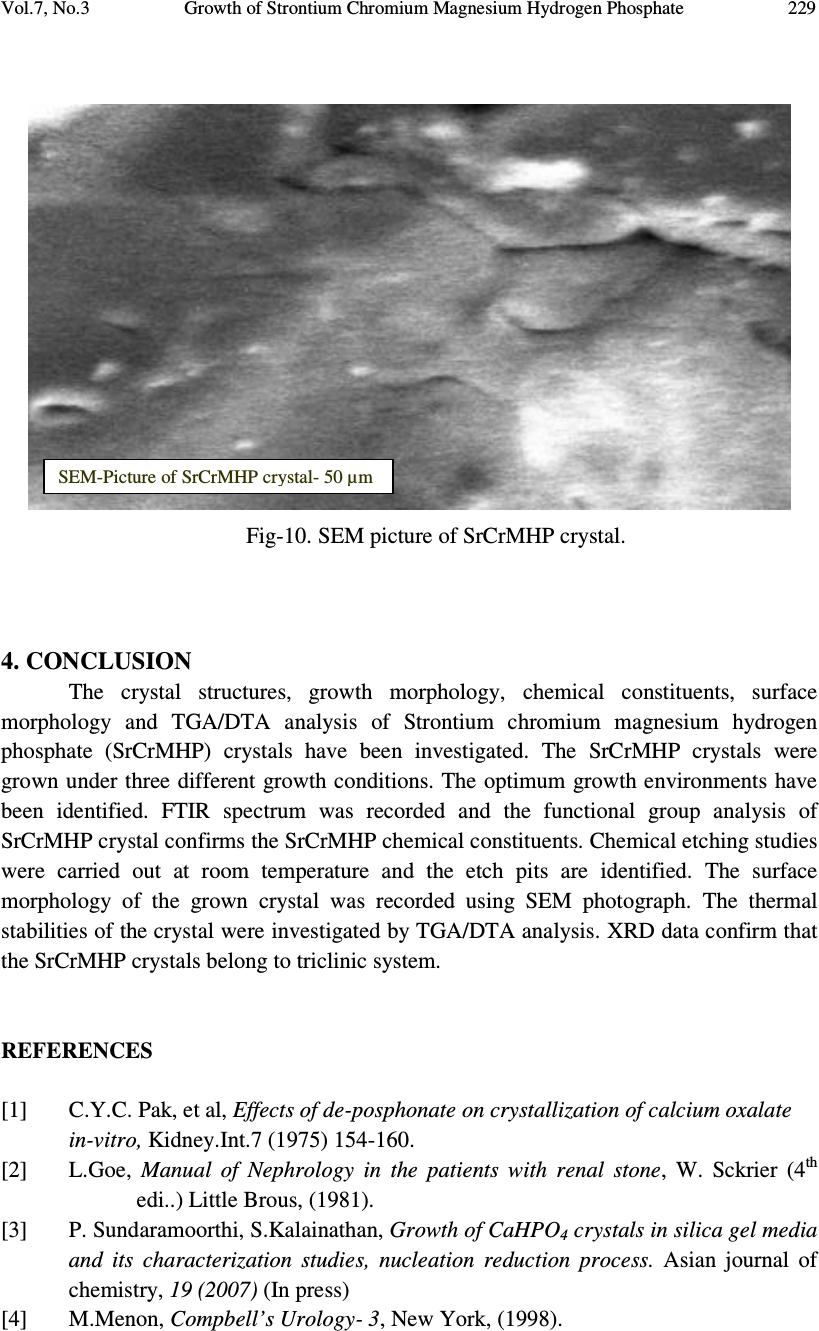 Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 229 Fig-10. SEM picture of SrCrMHP crystal. 4. CONCLUSION The crystal structures, growth morphology, chemical constituents, surface morphology and TGA/DTA analysis of Strontium chromium magnesium hydrogen phosphate (SrCrMHP) crystals have been investigated. The SrCrMHP crystals were grown under three different growth conditions. The optimum growth environments have been identified. FTIR spectrum was recorded and the functional group analysis of SrCrMHP crystal confirms the SrCrMHP chemical constituents. Chemical etching studies were carried out at room temperature and the etch pits are identified. The surface morphology of the grown crystal was recorded using SEM photograph. The thermal stabilities of the crystal were investigated by TGA/DTA analysis. XRD data confirm that the SrCrMHP crystals belong to triclinic system. REFERENCES [1] C.Y.C. Pak, et al, Effects of de-posphonate on crystallization of calcium oxalate in-vitro, Kidney.Int.7 (1975) 154-160. [2] L.Goe, Manual of Nephrology in the patients with renal stone, W. Sckrier (4 th edi..) Little Brous, (1981). [3] P. Sundaramoorthi, S.Kalainathan, Growth of CaHPO 4 crystals in silica gel media and its characterization studies, nucleation reduction process. Asian journal of chemistry, 19 (2007) (In press) [4] M.Menon, Compbell’s Urology- 3, New York, (1998). SEM - Picture of SrC r MHP crystal - 50 µm  230 G. Kanchana, P. Suresh, P. Sundaramoorthi, S. Kalainathan, G.P. Jeyanthi Vol.7, No.3 [5] S.Joshi and J.Joshi, FTIR spectroscopic, thermal and growth morphological studies of calcium hydrogen phosphate dehydrate crystals, Cryst.Res.Technol.38, (2003) 817-821. [6]. P.Sundaramoorthi, S.Kalainathan, Growth and characterizations studies of SMHP single crystal in silica gel medium and laser induced nucleation reduction process. Biochemical Engineering Journal, 34 (2007) 244-249. [7] P.Sundaramoorthi, S. Kalainathan, Characteristics studies of SHP crystals are grown in silica gel medium. Asian journal of chemistry, 19 (5) (2007) 3739-3746 [8] P.Sundaramoorthi, S.Kalainathan, Crystal growth of some renal stones constituents: I In vitro crystallization of trace element and its characterization studies. The Journal of minerals and materials characterization of Engineering (In press) [9] P.Sundaramoorthi, S.Kalainathan, BMHP single crystal growth in silica gel medium, characterization studies and laser irradiated nucleation reduction process strategy. Asian journal of chemistry, 19 (2007) (In press) [10] J. Dennis, Crystal growths in gels, J.Elec.Che.Soc.14 (1967) 263. [11] J.W. Mullin, Crystallization, Butter worth scientific pub. London 4, (1961). [12] E. Hatscheck, Light affects the crystallization nuclei. Kollid Z.37 (1925) 297. [13] A.J Arminstrong, The scraped sell crystallizer, British chemical engineering, 14 (1969) 647-649. [14] H.K. Henisch, J.I. Hanoka, and J. Dennis, Surface barrier effects of crystals growth in gel medium, J.Electrochem.Soc.112 (1965) 627. [15] G. Socrater, Infrared Cha. Group Friq, Johnwilly, Chichester, (1980). [16] S. Matsuzaki, K. Matsushita, K. Tanykawa, A. Masuda and F. Matsunaga, Sequential analysis of recurrent calcium calculi by infrared spectroscopy, Int.J.Uro.2 (1995) 235-237. [17] Yean-Chin Tsay, Application of infrared spectroscopy to analysis of urinary calculi, J.Urol. 86 (1961) 838-854. [18] C.M. Corns, Infrared analysis of renal calculi-a comparison with conventional technique. J.Ann. Clin. Bio-Cherm 20 (1983) 20-35. [19] A. Hesse and D. Bach, Stone analysis by infrared spectroscopy, In., Urinary stones, Clinical and laboratory Aspects, Edi., Alan Rose, University Park Press, Baltimore, (1982). [20] P.N. Kotru, K.K. Raina and N.K Guptha, Characterizations as thermal behavior of Lanthanum tartar ate crystals grown from gels. Bull. Mater Sci.8 (1986) 547. [21] Anand Kumar Sharma, Shyam Kumar and Narender Kumar Kaushik, Thermall Studies on magnesium, barium and lead, zirconyloxalates, Thermochimica Acta, 47(1981) 149-156. [22] G. Bruzzone, The binary systems calcium-copper, strontium-copper and barium- copper, J. Less Common Metals, 25 (1971) 361-366. [23] G. Bruzzone, The binary systems Sr-In and Ba-In .J. Less Common Metals, 11, (1966) 249-258.  Vol.7, No.3 Growth of Strontium Chromium Magnesium Hydrogen Phosphate 231 [24] Sedat Ilhan, Cem Kahruman and Ibrahim Yusufoglu , Characterization of the thermal decomposition products of ammonium phosphomolybdate hydrate, J. Analytical and Applied Pyrolysis, 78 (2007) 363-370. [25] J.J. Gilman, J. Johnsion and G.W. Sears , Dislocation etch pit formation in Lithium fluoride, J.App. Physics, 295 (1958) 749. [26] J.J. Gilman, et al, Observations Lithium fluoride dislocation glide climb in Lithium fluoride crystals, J.App. Physics, 27 (1956) 1018. [27] J.C. Fisher, In: Dissolutions and Mechanical Properties of Crystals, John Wiley and sons, New York, (1957). [28] J.B. New Kirk, In: Director Observation of Imperfection in crystals, Inter science Publishers, New York (1962). [29] K. Sangwal, Chemical etching principles and the present status, Key Engineering Materials, 58 (1991) 170-187. [30] K. Tasukamot, In situ observation of mono-molecular growth step on crystal growth in aqueous solution-I, J. Cry. Growth, 61 (1983) 199. [31] H.C. Gates, Thirty years of progress in Surface Science, In: Crystal growth and characterization, Edi, North Holland, (1975). [32] H. Bethage, et al, edi, Electron Microscopy in Solid State Physics, Elsever, Amsterdom, (1987). [33] N. Albon et al, In: Growth and Perfection of Crystals, Wiley., New York, (1958). [34] H.K. Henisch, J.M. Garcia-Ruiz, Crystal growth in gels and Lisegang ring formation, J.Crystal Growth, 75 (1986) 195. [35] H.K. Henisch, J.M. Garcia-Ruiz, Crystal growth in gels and Lisegang ring formation-II crystallization criteria and successive precipitation, J.Cry.Growth 75 (1986) 203. [36] H.K. Henisch, Crystals in Gel and Liesegang Rings, Cambridge University press, Cambridge (1986). |

