Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 7, No.1, pp 59-70, 2007 jmmce.org Printed in the USA. All rights reserved 59 Characterization of Chromium in Contaminated Soil Studied by SEM, EDS, XRD and Mössbauer Spectroscopy L. R. Reyes-Gutiérrez 1,2 , E. T. Romero-Guzmán 3 , A. Cabral-Prieto 3 and R. Rodríguez-Castillo 1 1 Universidad Nacional Autónoma de México, Instituto de Geofísica, Posgrado en Ciencias de la Tierra, UACPyP-CCH. México. lreyes@uaeh.reduaeh.mx; rrdz@tonatiuh.igeofcu.unam.mx 2 Universidad Autónoma del Estado de Hidalgo. Centro de Investigaciones en Ciencias de la Tierra. Carretera Pachuca-Tulancingo Km. 4.5, Pachuca Hidalgo. C.P. 42184. Tel: 01 771 71 72000 ext. 6622, Fax: 01 771 71 721 33. lreyes@uaeh.reduaeh.mx 3 Instituto Nacional de Investigaciones Nucleares. Gcia. Ciencias Básicas, Depto. de Química Carretera México-Toluca km36.5, Salazar Estado de México AP 18-1027. C.P. 52045. Tel: +52 (55) 53 29 72 00, ext. 2266, Fax: +52 (55) 53 29 73 01. etrg@nuclear.inin.mx ABSTRACT Chromium is important from the environmental point of view since its behavior and toxicity properties depend on its oxidation states. The Cr(VI) concentration in wells of Buenavista, Guanajuato, Mexico, is higher than the permissible level of it for drinking water, 0.05mg/L. The objective of this research was to determine the elution of chromium with deionized water from contaminated soil samples and to determine the oxidation state of Fe, which is an element that can limit the mobility of chromium. These results will be considered in a pump and treat remediation scheme for this site. Chromium contaminated soil samples were obtained from an industrial area of Leon, Guanajuato, México. O, Na, Mg, K, Al, Si, Ca, Cr and Fe were found in the chemical analysis by EDS of the contaminated samples. In the soluble species only O, Na, S, Ca and Cr were found. The oxidation state of iron was determined by Mössbauer spectroscopy (MS) in the soil contaminated with chromium, in the soil washed with deionizer water and also in the soluble samples. CaCrO 4 was found in the soluble fraction, as a single crystalline phase by XRD. MS indicated that at least two iron species were present, one insoluble and the other sparingly soluble. Keywords: Cr(VI), soil contamination, Mössbauer, EDS, SEM, DRX  60 L. R. Reyes-Gutiérrez,, E. T. Romero-Guzmán, A. Cabral-Prieto and R. Rodríguez-Castillo Vol.7, No.1 1. INTRODUCTION Chromium (Cr) is present in nature mainly in the form of highly insoluble Cr(III) minerals. The other stable oxidation state of chromium is VI, which forms very soluble chromate compounds. However, the ocurrence of appreciable amounts of Cr(VI) in soil and water suggests that artificial addition of chromate ions due to industrial activities has ocurred. For instance, the leather industry generates solid residues with a high content of Cr(III) oxides. These Cr(III) oxides dissolve at pH values below 6. The Cr(VI) compounds dissolve in acid and basic media and they are readily reduced to Cr(III) species under acid conditions. Thus, chromium as contaminat may be found in water, either as dissolved chromate (VI) species and/or as poorly soluble Cr(III) oxides deposited on the sediments. Cr(VI) is potentially more dangerous to living organisms than Cr(III), because of its high solubility. The highest permissible level of total soluble chromium concentration in drinking water has been set to 0.05 mg/L [1-4]. The chromite, FeCr 2 O 4 , a natural source of Cr [5], can be oxidized to Cr(VI) in presence of Mn (III/IV) minerals, which are frequently found in soils [6-8]. On the other hand, organic compounds, ferrous species and sulfide compounds may be responsible for the reduction of Cr(VI) to Cr(III), i. e., these redox reactions may occur in contaminated waters, sediments and soils [9,10]. In this paper a soil contaminated with Cr from an industry that produces chromium compounds located in Buenavista, Guanajuato State, México was analyzed. In this site, there are two large deposites of Cr(III) and Cr(VI). One deposit is on protected surface without connecting with the other, a container in an excavated place. The Cr(VI) container was not properly designed and built into a clayey unit. Rainwater may enter to the container, forming a leakage that reaches the local aquifer. This aquifer is not mostly used as drinking water source [2,11]. The wastes of this last container were place in other one by the end of 1994. The lack of strictict environmenatl regulations has allowed that the permisible levels of total soluble chromium concentrations in drinking water had been higher than 0.05 mg/L. There are several examples where high concentrations of Cr(VI) has been the cause of cancer in childs and building workers [1,2,4]. Armienta et al., (1996) detected Cr in local vegetation but reduced Cr(III) was not observed in soils [12]. This research is the second part of the previous study [3] looking for the remediation of this contaminated site. These studies are important to prevent risks to the human health since chromium can be accumulated on skin, lungs, muscles fat, and it accumulates in liver, dorsal spine, hair, nails and placenta [1]. Therefore, the aim of this work was to identify the chemical specie of chromium as well as to determine the degree of association that exists between the chromium and iron, this knowledge will be used in a treatment scheme for this site. 2. EXPERIMENTAL 2.1. Site Description: The container of Cr(VI) wastes under study is located near the Turbio river, a few kilometers away from Buenavista, Leon City, Guanajuato state, Mexico. The Cr(VI) wastes from the industrial processes are located inside the industry area facilities. The stratigraphic nature of the site is composed of fine to coarse grain sand with variable quantities of silt, clays and graves [13]. The hydraulic conductivity (Kx, Ky), the distribution coefficient (Kd) and the α L /α TV ratio of this site have been reported by Reyes (1998) [3]. The container consists of an excavation without a membrane; 70 m long, 30 m width, and 6 m deep and it is located only 3 m over watertable (Figure 1). The initial Cr concentration of the leakage, 90 mg/L was 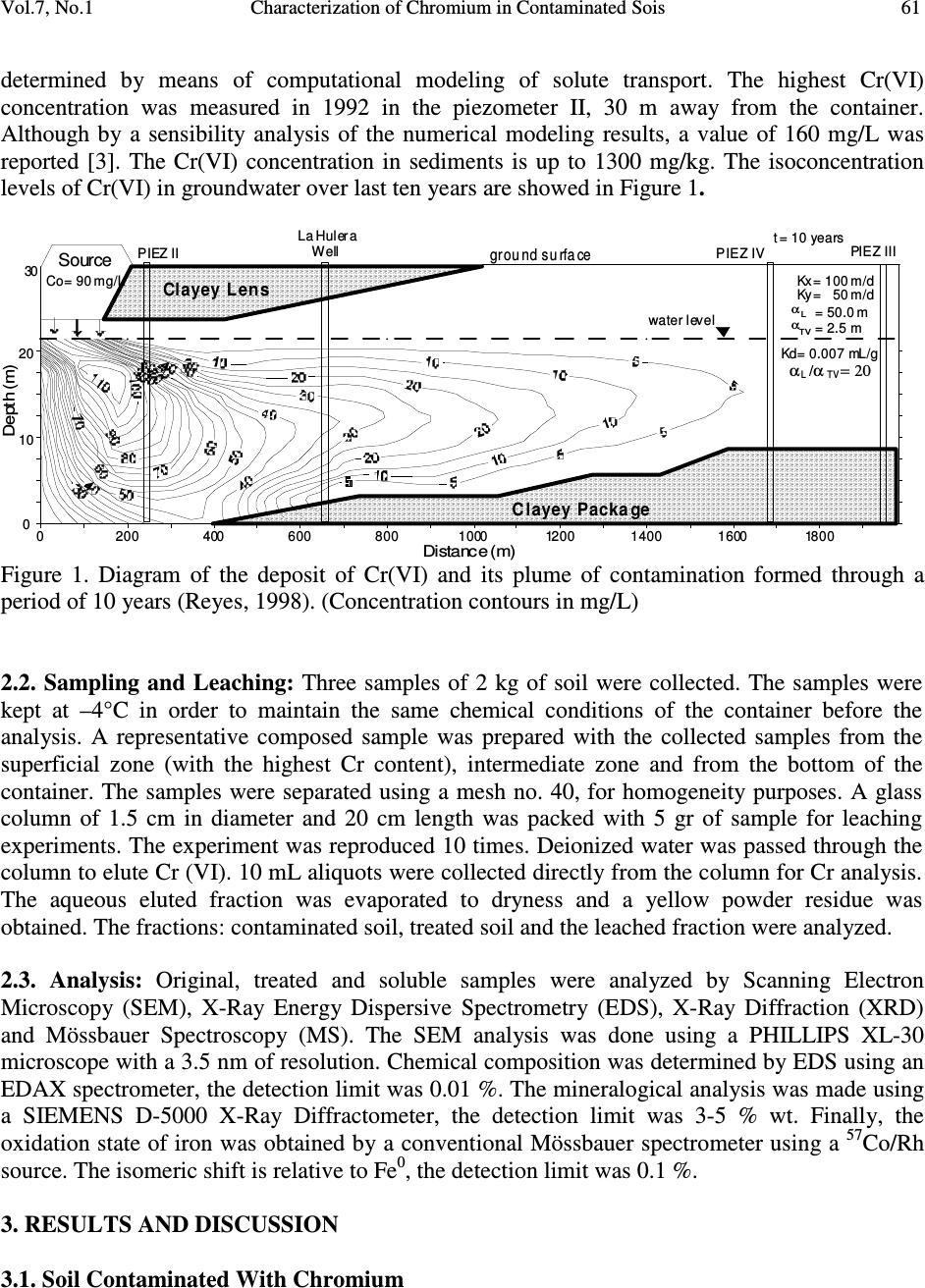 Vol.7, No.1 Characterization of Chromium in Contaminated Sois 61 determined by means of computational modeling of solute transport. The highest Cr(VI) concentration was measured in 1992 in the piezometer II, 30 m away from the container. Although by a sensibility analysis of the numerical modeling results, a value of 160 mg/L was reported [3]. The Cr(VI) concentration in sediments is up to 1300 mg/kg. The isoconcentration levels of Cr(VI) in groundwater over last ten years are showed in Figure 1. 0200 400 600 80010001200140016001800 Distance (m) D e p t h ( m ) 20 ground surface water level Source 0 30 t = 10 years 10 La Hulera Well α α L TV = 50.0 m = 2.5 m Kx= 100 m/d Ky= 50 m/d Kd= 0.007 mL/g Clayey Lens Clayey Package Co= 90 mg/L α /α = 20 TVL PIEZ IVPIEZ III PIEZ II Figure 1. Diagram of the deposit of Cr(VI) and its plume of contamination formed through a period of 10 years (Reyes, 1998). (Concentration contours in mg/L) 2.2. Sampling and Leaching: Three samples of 2 kg of soil were collected. The samples were kept at –4°C in order to maintain the same chemical conditions of the container before the analysis. A representative composed sample was prepared with the collected samples from the superficial zone (with the highest Cr content), intermediate zone and from the bottom of the container. The samples were separated using a mesh no. 40, for homogeneity purposes. A glass column of 1.5 cm in diameter and 20 cm length was packed with 5 gr of sample for leaching experiments. The experiment was reproduced 10 times. Deionized water was passed through the column to elute Cr (VI). 10 mL aliquots were collected directly from the column for Cr analysis. The aqueous eluted fraction was evaporated to dryness and a yellow powder residue was obtained. The fractions: contaminated soil, treated soil and the leached fraction were analyzed. 2.3. Analysis: Original, treated and soluble samples were analyzed by Scanning Electron Microscopy (SEM), X-Ray Energy Dispersive Spectrometry (EDS), X-Ray Diffraction (XRD) and Mössbauer Spectroscopy (MS). The SEM analysis was done using a PHILLIPS XL-30 microscope with a 3.5 nm of resolution. Chemical composition was determined by EDS using an EDAX spectrometer, the detection limit was 0.01 %. The mineralogical analysis was made using a SIEMENS D-5000 X-Ray Diffractometer, the detection limit was 3-5 % wt. Finally, the oxidation state of iron was obtained by a conventional Mössbauer spectrometer using a 57 Co/Rh source. The isomeric shift is relative to Fe 0 , the detection limit was 0.1 %. 3. RESULTS AND DISCUSSION 3.1. Soil Contaminated With Chromium 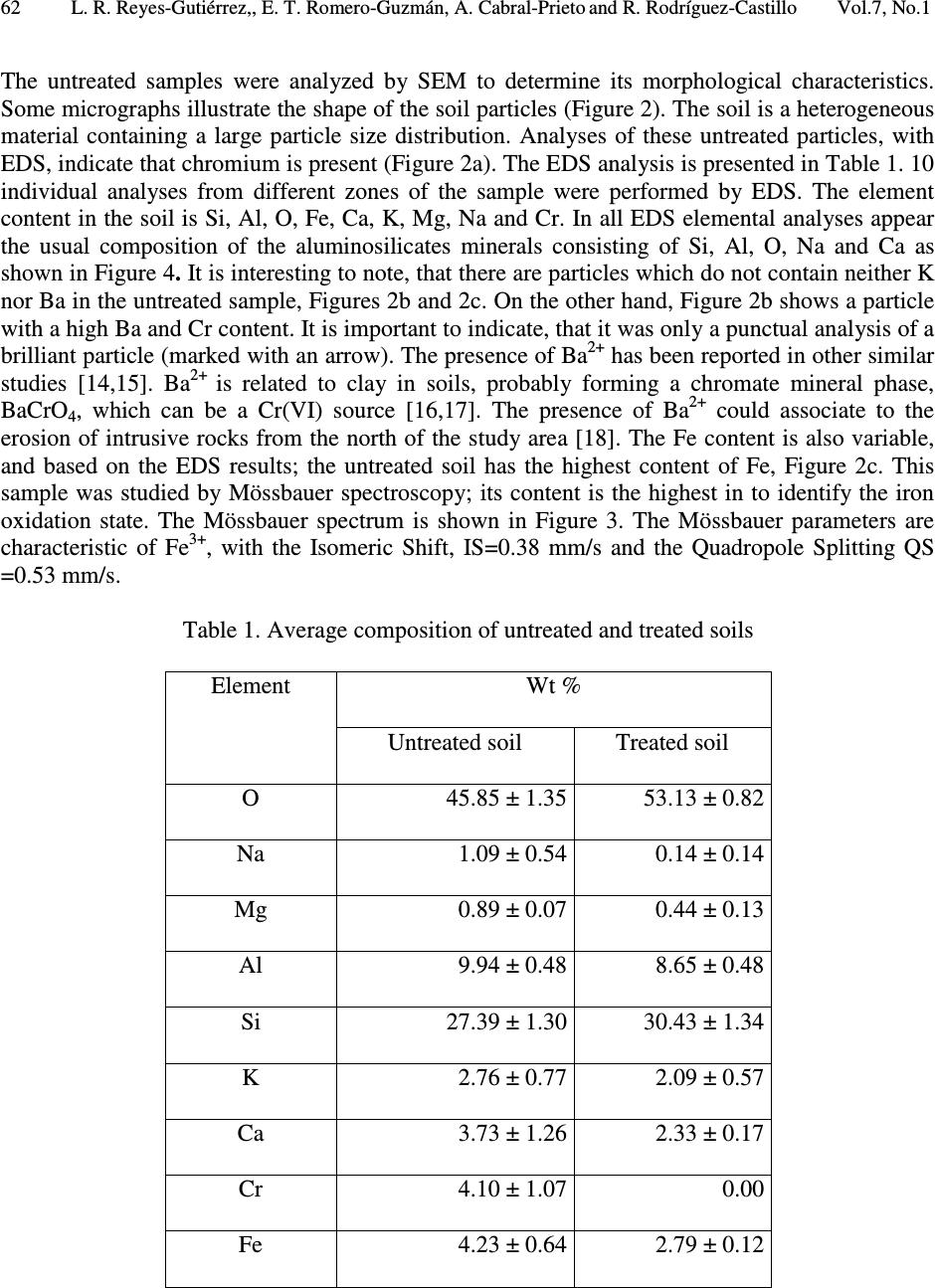 62 L. R. Reyes-Gutiérrez,, E. T. Romero-Guzmán, A. Cabral-Prieto and R. Rodríguez-Castillo Vol.7, No.1 The untreated samples were analyzed by SEM to determine its morphological characteristics. Some micrographs illustrate the shape of the soil particles (Figure 2). The soil is a heterogeneous material containing a large particle size distribution. Analyses of these untreated particles, with EDS, indicate that chromium is present (Figure 2a). The EDS analysis is presented in Table 1. 10 individual analyses from different zones of the sample were performed by EDS. The element content in the soil is Si, Al, O, Fe, Ca, K, Mg, Na and Cr. In all EDS elemental analyses appear the usual composition of the aluminosilicates minerals consisting of Si, Al, O, Na and Ca as shown in Figure 4. It is interesting to note, that there are particles which do not contain neither K nor Ba in the untreated sample, Figures 2b and 2c. On the other hand, Figure 2b shows a particle with a high Ba and Cr content. It is important to indicate, that it was only a punctual analysis of a brilliant particle (marked with an arrow). The presence of Ba 2+ has been reported in other similar studies [14,15]. Ba 2+ is related to clay in soils, probably forming a chromate mineral phase, BaCrO 4 , which can be a Cr(VI) source [16,17]. The presence of Ba 2+ could associate to the erosion of intrusive rocks from the north of the study area [18]. The Fe content is also variable, and based on the EDS results; the untreated soil has the highest content of Fe, Figure 2c. This sample was studied by Mössbauer spectroscopy; its content is the highest in to identify the iron oxidation state. The Mössbauer spectrum is shown in Figure 3. The Mössbauer parameters are characteristic of Fe 3+ , with the Isomeric Shift, IS=0.38 mm/s and the Quadropole Splitting QS =0.53 mm/s. Table 1. Average composition of untreated and treated soils Element Wt % Untreated soil Treated soil O 45.85 ± 1.35 53.13 ± 0.82 Na 1.09 ± 0.54 0.14 ± 0.14 Mg 0.89 ± 0.07 0.44 ± 0.13 Al 9.94 ± 0.48 8.65 ± 0.48 Si 27.39 ± 1.30 30.43 ± 1.34 K 2.76 ± 0.77 2.09 ± 0.57 Ca 3.73 ± 1.26 2.33 ± 0.17 Cr 4.10 ± 1.07 0.00 Fe 4.23 ± 0.64 2.79 ± 0.12 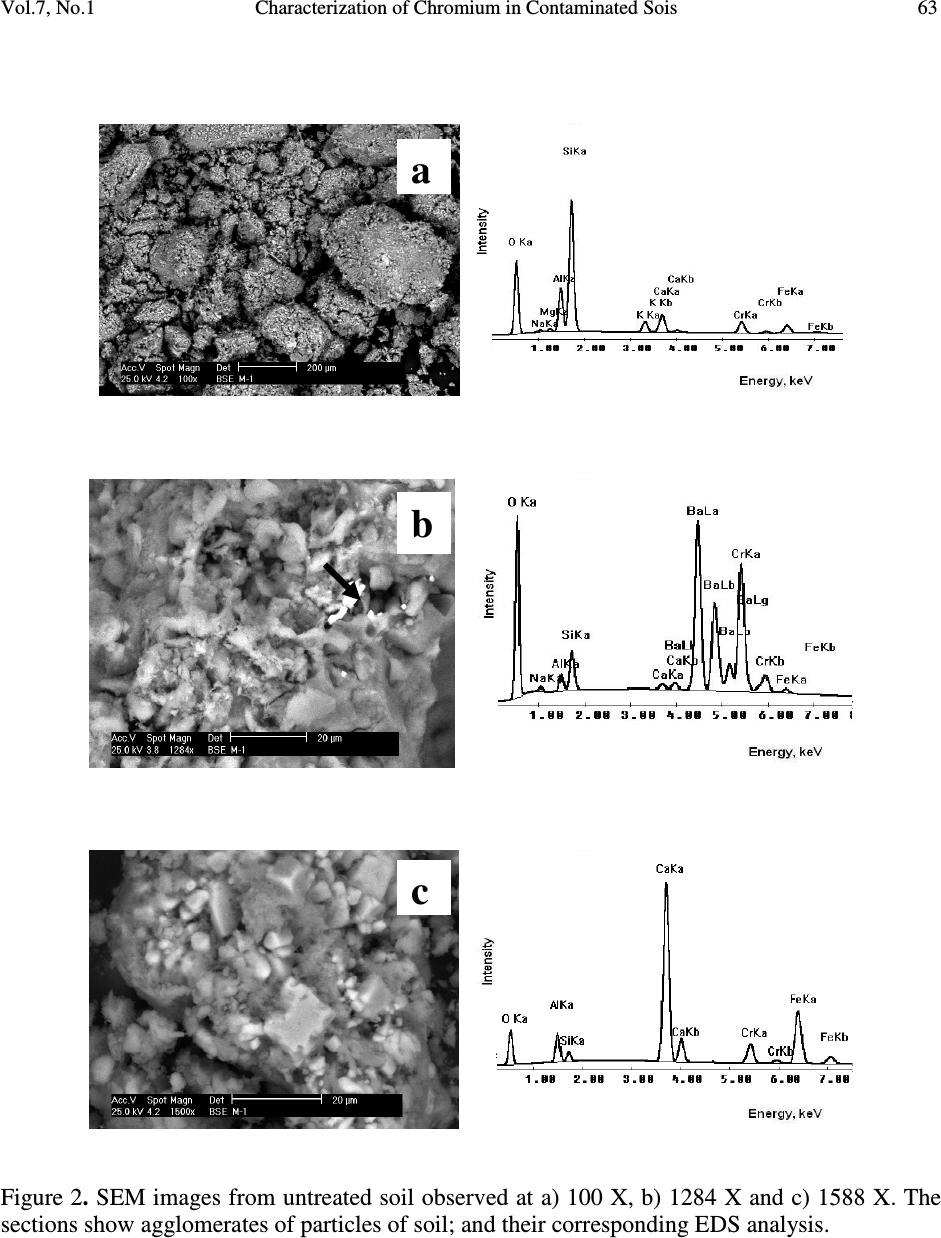 Vol.7, No.1 Characterization of Chromium in Contaminated Sois 63 Figure 2. SEM images from untreated soil observed at a) 100 X, b) 1284 X and c) 1588 X. The sections show agglomerates of particles of soil; and their corresponding EDS analysis. a b c 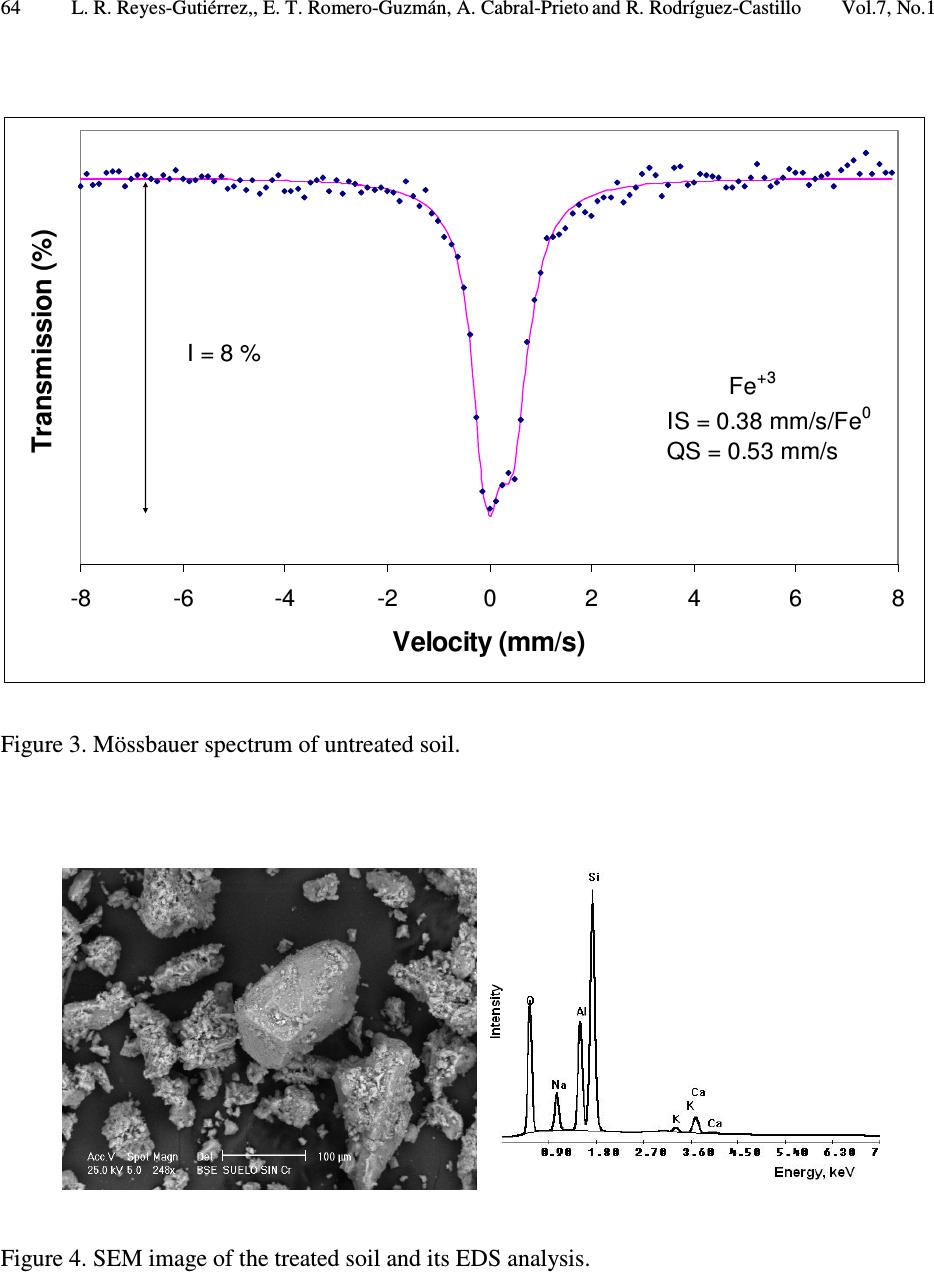 64 L. R. Reyes-Gutiérrez,, E. T. Romero-Guzmán, A. Cabral-Prieto and R. Rodríguez-Castillo Vol.7, No.1 Figure 3. Mössbauer spectrum of untreated soil. Figure 4. SEM image of the treated soil and its EDS analysis. -8 -6-4 -202468 Velocity (mm/s) Transmission (%) Fe + 3 IS = 0.38 mm/s/Fe 0 QS = 0.53 mm/s I = 8 % 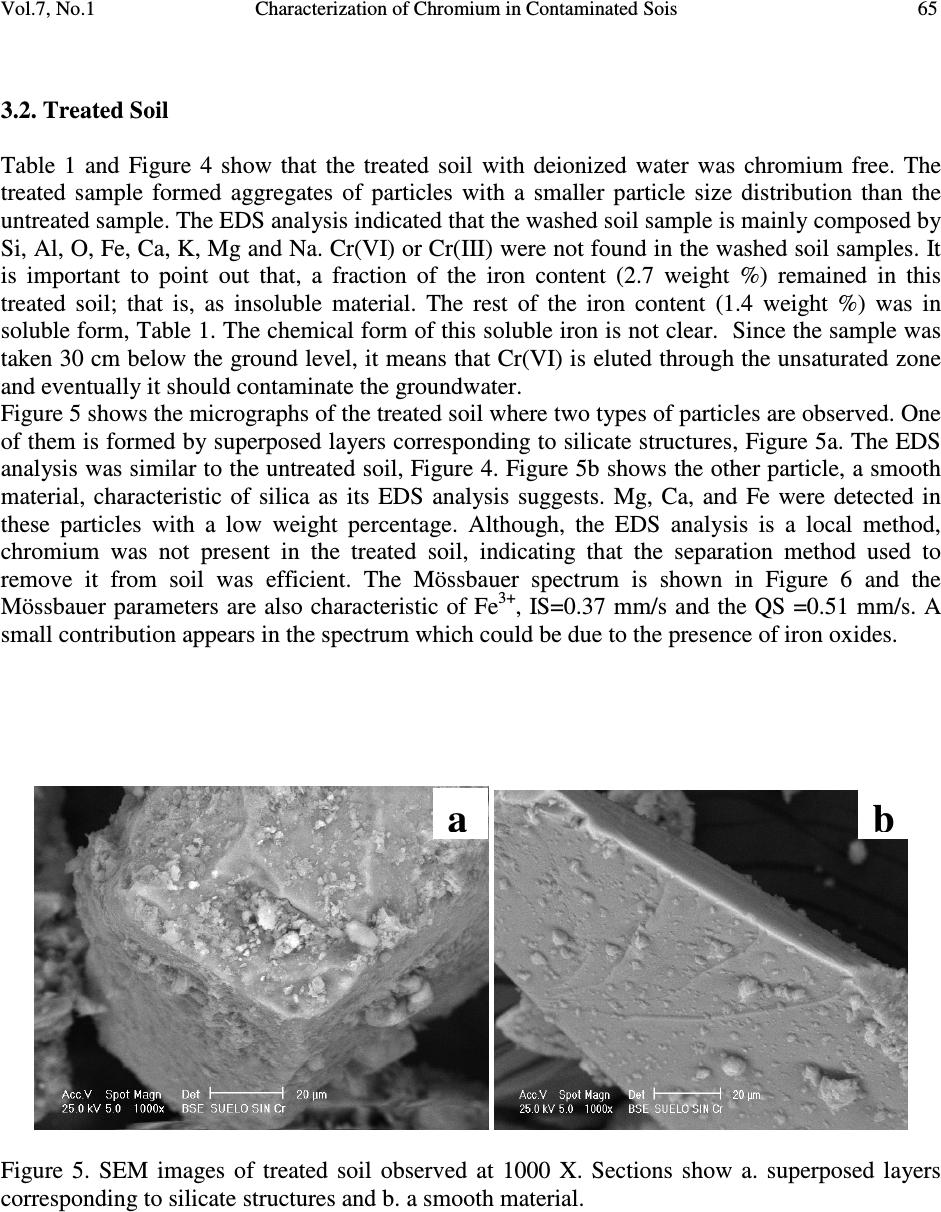 Vol.7, No.1 Characterization of Chromium in Contaminated Sois 65 3.2. Treated Soil Table 1 and Figure 4 show that the treated soil with deionized water was chromium free. The treated sample formed aggregates of particles with a smaller particle size distribution than the untreated sample. The EDS analysis indicated that the washed soil sample is mainly composed by Si, Al, O, Fe, Ca, K, Mg and Na. Cr(VI) or Cr(III) were not found in the washed soil samples. It is important to point out that, a fraction of the iron content (2.7 weight %) remained in this treated soil; that is, as insoluble material. The rest of the iron content (1.4 weight %) was in soluble form, Table 1. The chemical form of this soluble iron is not clear. Since the sample was taken 30 cm below the ground level, it means that Cr(VI) is eluted through the unsaturated zone and eventually it should contaminate the groundwater. Figure 5 shows the micrographs of the treated soil where two types of particles are observed. One of them is formed by superposed layers corresponding to silicate structures, Figure 5a. The EDS analysis was similar to the untreated soil, Figure 4. Figure 5b shows the other particle, a smooth material, characteristic of silica as its EDS analysis suggests. Mg, Ca, and Fe were detected in these particles with a low weight percentage. Although, the EDS analysis is a local method, chromium was not present in the treated soil, indicating that the separation method used to remove it from soil was efficient. The Mössbauer spectrum is shown in Figure 6 and the Mössbauer parameters are also characteristic of Fe 3+ , IS=0.37 mm/s and the QS =0.51 mm/s. A small contribution appears in the spectrum which could be due to the presence of iron oxides. Figure 5. SEM images of treated soil observed at 1000 X. Sections show a. superposed layers corresponding to silicate structures and b. a smooth material. a b  66 L. R. Reyes-Gutiérrez,, E. T. Romero-Guzmán, A. Cabral-Prieto and R. Rodríguez-Castillo Vol.7, No.1 -8-6-4-202 46 8 Velocity (mm/s) Transmission (%) Figure 6. Mössbauer spectrum of treated soil. 3.3. Soluble Fraction The micrographs of the soluble residues, the yellow powder, show hexagonal crystals of different size (Figure 7). Their composition includes O, Na, S, Ca and Cr. The X-ray diffraction analysis showed chromatite, CaCrO 4 (Joint Committee on Powder Diffraction Standards, JCPDS card 8- 0458) [19] like a main crystalline phase, also reported by Bajda, 2005 [20], Figure 8. The formation in aqueous solution of the chromatite, CaCrO 4, according Puigdomenech (2004) [21] is Ca 2+ +CrO 42- ↔ CaCrO 4, log = 2.266. Fe was not detected in this fraction by SEM and XRD techniques, but it was detected by Mössbauer spectroscopy. The Mössbauer parameters are also characteristic of Fe 3+ , with IS=0.38 mm/s and the QS =0.54 mm/s, Figure 9. In this case, the FeOHCrO 4 , a salt with Fe 3+ , was determined. The presence of this last compound was reported by Rock et al. (2001) [22]. In agreement with the pH (6.5-8.5) of the eluates during the leaching of chromium from contaminated soil, it was verified that the iron was not as oxide but as hydroxide chemical specie. The FeOHCrO 4 has a log K=-36.5, according to Puigdomenech, (2004) [21], which indicates that in solution this chemical specie should not be formed, nevertheless it precipitated when the eluted solution was evaporated.  Vol.7, No.1 Characterization of Chromium in Contaminated Sois 67 Figure 7. SEM image of chromium removed from soil and its EDS analysis.  68 L. R. Reyes-Gutiérrez,, E. T. Romero-Guzmán, A. Cabral-Prieto and R. Rodríguez-Castillo Vol.7, No.1 10 2030 40 50 60 70 0 50 100 150 200 250 300 350 400 16.09 19.69 24.58 31.09 33.4 37.84 49.18 56.83 61.75 64.54 Intensity (a.u.) 2 θ degrees Figure 8. X-Ray Diffraction pattern of chromatite, CaCrO 4. -8 -6 -4 -202468 Transmission (%) Velocity (mm/s) Fe 3+ IS = 0.38 mm/s/Fe 0 QS = 0.54 mm/s I = 6 % Figure 9. Mössbauer spectrum of the soluble yellow powder fraction.  Vol.7, No.1 Characterization of Chromium in Contaminated Sois 69 4. CONCLUSIONS According to the results, the soil contaminated with chromium can be described as aggregates, layers and inorganic particles distributed in the bulk. The soil components are based on O, Na, Mg, K, Al, Si, Ca, Cr and Fe. All soluble species were leached from the soil. The soluble yellow powder was composed by hexagonal crystals indicating that the oxidation state of Cr was VI, which represents the highest risk to the environment for its toxicity. The results revealed the efficient of leaching chromium from soil using deionized water. Fe 3+ was detected by MS in the soluble yellow powder sample but iron was no detected by SEM and XRD. These results indicate that there are two species in the eluted fraction. The compound determined was the chromatite CaCrO 4 which was formed by dissolution of calcium carbonates from soil in the presence of heavily soils polluted with Cr(VI) and FeOHCrO 4 in a lower concentration. Therefore, in this case, the degree of association that exists between the chromium and iron is weak, because the Cr(VI) is leaching easily from the soil particles. ACKNOWLEDGEMENTS The authors would like to thank Chemical Central industry for their technical helpful. REFERENCES [1] González, C. A., Neri, R., Quiñónez, A. and Mendoza, J., 1980, “Posibles daños a la salud de una comunidad abierta, por sales de Cr en el ambiente: Fisiología y patología del Cr.” Sa. Púb. Méx. XXII, p. 85. [2] Gutiérrez, R. M., Castillo, B. S. and Aguilera, E., 1989, “Chromate contamination north of México: proposal for a solution.” UNEP, Industry and Environmental, p . 51. [3] Reyes-Gutiérrez, L. R., 1998, “Factores que controlan la dispersión de compuestos de Cr en un acuífero de conductividad hidráulica variable.” Tesis Maestría UNAM. Posgrado en Geofísica, 125 p. [4] Villalobos, P. M., Gutiérrez, R. M., and Castillo, B. S., 1987, “A study of the factors that influence the interference of Fe(III) in the colourimetric analysis of Cr(VI) in polluted waters.” Rev. Int. de Contam. Ambient. Vol. 3, pp. 7-23. [5] Albert, L., 1988, “Curso Básico de toxicología ambiental: Cromo.” 2ª edición. Editorial LIMUSA S.A. de C. V., pp. 171-183. [6] Bartlett, R. and Bruce, J., 1979, “Behavior of Chromium in soils: III. Oxidation.” J. Environ. Quality, Vol. 8, pp. 31-35. [7] Grove, J. H. and Ellis, B.G., 1980, “Extractable Iron and Manganese as Related to Soil pH and Applied Chromium.” Soil Science Society of America Journal.Vol. 44, pp. 243-246. [8] Mattuck, R. and Nikolaidis, P., 1996, “Chromium mobility in freshwater wetlands.” Journal of Contaminant Hydrology, Vol. 23, pp. 213-232. [9] Bennett, T. A., 1997, “An in situ reactive barrier for the treatment of Cr(VI) and tricloroethylene in groundwater”, Department of Earth Sciences, University of Waterloo. [10] Blowes, D. and Ptacek, C., 2002. 3 rd Intern. Conf. on Groundwater Quality Research, in: Proceedings of the Subsurface Restoration Conf., June 21-24, Dallas Texas, p. 214. [11] Rodríguez, R. and Armienta, A., 1995, “Groundwater chromium pollution in the Rio Turbio Valley, Mexico: Use of pollutants as chemical tracers.” Geofís. Internal., Vol. 34, pp. 417-426.  70 L. R. Reyes-Gutiérrez,, E. T. Romero-Guzmán, A. Cabral-Prieto and R. Rodríguez-Castillo Vol.7, No.1 [12] Armienta, A., Rodríguez, R., Ceniceros, N., Juárez, F. and Cruz, O., 1996, “Distribution, Origin and Fate of Chromium in soils in Guanajuato, México.” Environmental Pollution, Vol. 91, pp. 391-397. [13] Castelán, A. and Villegas, J., 1996, “Control Estratigráfico en la dispersión de Compuestos de Cromo en el Valle de León, Gto.” ESIA-IPN, México. Bachelor Degree Thesis. [14] Thomas, G. W., 1982, “Exchangeable Cations. Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties”, Second Edition. A.L. Page (editor). Agronomy, No. 9, Part 2, American Society of Agronomy, Soil Science Society of America, Madison, Wl: 159-165. [15] Bartlett, R. J., 1991, “Chromium Cycling in Soils: Links, Gaps, and Methods.” Environmental Health Perspectives, Vol. 92, pp. 17-24. [16] James, B. R., 1994, “Hexavalent chromium solubility and reduction in alkaline soils enriched with chromite ore processing residue.” J. Environ. Quality, Vol. 23, pp. 227-233. [17] James, B. R., 1996, “The challenge of remediation chromium-contaminated soil.” Environmental Science & Technology,Vol. 30, pp. 248A-251A. [18] Ramos-Leal, J. A., Durazo, J. González, M. T., Ramírez, G. A., Johannesson K. H., Cortés, A., 2003, “ Producción insostenible de un campo de pozos advertida por lo coincidente de la desmineralización y la profundización del agua subterránea.” En: 1 Congreso de Las Américas Sobre Geofísica Ambiental. Instituto Panamericano de Geografía e Historia. 20 al 24 de octubre, 2003. México, D. F. [19] Bayliss, P., Sabina, S. A., Anderson, R., Cesbron, F., 1986, A Minerals Powder Diffraction File, Databook, International Centre for Diffraction, Editorial Staff, USA. [20] Bajda, T., 2005, “Chromatite CaCrO 4 in soil polluted with electroplating effluents, Zabierzów, Poland.” Science of the Total Environment, Vol. 336, pp. 269-274. [21] Puigdomenech, I., 2004, MEDUSA: Make Equilibrium Diagrams Using Sophisticated Algoritms. http://www.inorg.kth.se/Research/Ignasi;/Index.html. [22] Rock, M. L., James, B. R., and Helz, G. R., 2001, “Hydrogen Peroxide effects on chromium oxidation state and solubility in four diverse, chromium-enriched soils.” Environmental Science & Technology, Vol 35, pp. 4054-4059. |

