 Journal of Analytical Sciences, Methods and Instrumentation, 2012, 2, 60-67 http://dx.doi.org/10.4236/jasmi.2012.22012 Published Online June 2012 (http://www.SciRP.org/journal/jasmi) Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS Haisheng Zhang1, Weiping Zhou2, Hongqing Wang1*, Yuyuan Wang1, Fangfang He1, Zhiqiang Cheng1, Honglin Li1, Jinhui Tang1 1School of Chemistry and Chemical Engineering, University of South China, Hengyang, China; 2College of Mathematics and Physi- cal, University of South China, Hengyang, China. Email: *hqwang2009cn@yahoo.com.cn Received March 2nd, 2012; revised March 20th, 2012; accepted April 10th, 2012 ABSTRACT A new surface ion-imprinted Multi-walled carbon nanotubes (MCNTs), which was 6,6’-((1E,1’E)-(pyridine-2,6-diyl- bis(azanylyl))bis(methanylylidene))bis(2-allyl-phenol) and Pb(II) complex as functional monomer and template ion was presented for extracting and enrichment traces of Pb(II) ion. Parameters affecting the recovery of Pb(II) have been in- vestigated in detail. The novel IMCNTs display high affinity, specificity, and selectivity for Pb(II) with a maximum uptake capacity of 115.5 mg·g–1 at pH 4.0. Meanwhile, only 11 mins was enough for extracting 98.5% Pb(II) for the IMCNTs. No significant loss in adsorption capacity is observed when the IMCNTs are reused for eleven times. Separa- tion and preconcentration with IMCNTs particles results in a limit of detection of 0.47 μg·L–1 (3σ) and RSD (n = 8) of 1.16% by using atomic absorption spectrophotometer (AAS). Keywords: Lead Determination; Ion Imprinted Particles; Carbon Nanotube; Selective Recognition 1. Introduction Soil and water pollution, caused by toxic heavy metals, is a major environmental concern [1]. Among toxic heavy elements, lead is one of the commonest for animals and humans, even at lower concentrations [2,3]. Unlike organic compounds, lead is non-biodegradable and accumulative through its interaction with inorganic and organic materials, including adsorption, formation of complexes and chemical combinations etc. [4]. Consequently, the development of reliable methods for the removal and determination of lead from environment is of particular significance [5,6]. However, for conventional techniques, determining lead is very difficult due to its low concentrations in environmental samples and matrix interference. Thus, an effective enrichment and separation process is usually necessary prior to determination. In recent years, Surface imprinting technique (SIT) has become a powerful method for high selectivity adsorption of target metal ions [7]. It is flexible, economical, environ- mental-friendly, highly selective, speedy, simple, rich in accessible sites, safe, easily automatic, and quick in mass transfer and binding kinetics [8-10]. The particularly promising applications of SIT are trace enrichment and trace separation [8-12]. SIT is mainly based on the utiliza- tion of inorganic and organic solid sorbents which possess a stable and insoluble porous matrix having suitable active groups (typically organic groups) to interact with metal ions. Silica gel [13,14] and MCNTs [15] are ideal supports for organic groups. There are some reports about the enrichment and separation of Pb(II), using SIT with silica gel as the support [2,3,6]. However, there is few report on the synthesis of IMCNTs for Pb(II) extrac- tion. The classical preparation procedure of ion-imprinted polymer is collecting template molecule, functional monomer and cross-linker together to compounding. But the template molecule with functional monomer often isn’t monogamous combination in ion-imprinted polymer. The coordination effect between metal ion and ligand is forceful, combination quickly, steady, convenient [16]. So, the coordination effect between metal ion and ligand is a top choice in SIT progress. The kinds of o-Hydroxyphenol Schiff base chemical compound have very well coordi- nation capability with metal ions [17,18]. In order to increase relative selectivity of absorbent for Pb(II), a novel complex of 6,6’-((1E,1’E)-(pyridine-2,6-diylbis (azanylyl))bis(methanylylidene))bis(2-allylphenol)w(PD *Corresponding author. Copyright © 2012 SciRes. JASMI  Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS 61 ABMBAPOL) with Pb(II) was synthesized as functional monomer and template molecule of ion-imprinted polymer in this study. Meanwhile, a new surface-grafted Pb(II)- imprinted carbon nanotubes sorbent was presented. Parameters affecting the separation and pre-concentration of Pb(II) from aqueous solution were also discussed. Finally, a new method for determining traces of Pb(II) was developed. 2. Materials and Methods 2.1. Reagents and Chemicals Analytical and spectral grade chemicals and doubly dis- tilled water (DDW) were used throughout the experi- ments. 0.1 M HNO3 or 0.1 M NH3·H2O were utilized for adjusting pH of solutions. Standard solutions (1.0 mg· mL –1) of Pb(II), Cd(II), Cu(II) and Ni(II), containing 1.0% HNO3, were prepared by dissolving corresponding amounts of nitrate salts in DDW. 3-Allylsalicylaldehyde, 2,6-Diaminopyridine, Acrolein, and 2,2’-azobisisobutyronitrile (AIBN) (98%) were offered by Aladdin. 3-Aminopropy ltrimethoxysilane (APS) and ethylene glycol dimethacrylate (EGDMA) were pur- chased from Golden Dragon Industrial (HK) Co., Ltd. MCNTs was supplied by Shenzhen Nanotech Port Co. Ltd. Lead standard liquid (GBW08619) was supplied by National Institute of Metrology. Other chemicals were purchased from J&K scientific LTD. 2.2. Apparatus Scanning Electron Microscope (SEM, JEOL JSM 6700 F) was used to study the morphology and shape of the MCNTs. A Perkin-Elmer Lambda 45 AAS spectrometer was used for determining concentrations of metal ions. IR Spectra (4000 - 400 cm–1) in KBr pellets were re- corded using IR Prestige-21 from Shimadzu. 1H-NMR spectra were taken on a Varian XL-300 Spectrometer with TMS as the internal reference and CD3COCD3 as the solvents. A pHs-3 C digital pH meter was used for measuring pH. 2.3. Preparation of Samples Natural water samples were taken locally. River water came from Xiangjiang River, Hengyang, China. The Tap water was collected from our laboratory. The waste water was obtained from lead-zinc mine of Songbai, Hengyang, China. Before filled with the water samples, polyethylene bottles were cleaned with detergent, water, diluted nitric acid and DDW in sequence. All water samples were filtered through a 0.45 μm membrane filter and adjusted to pH 4 with the diluted HNO3 and NH3·H2O. 2.4. Preparation of IMCNTs and Non-IMCNTs 2.4.1. Synthesis of 6,6’-((1E,1’E)-(pyridine-2,6-diylbis(azanylyl)) bis(methanylylidene))bis-(2-allylphenol) The functional monomer was prepared as the literature [19]. Firstly, 100 mL methanol (MeOH) and 3-Allyl- salicylaldehyde (2.97 g) were added to a three-neck flask one after another. Then, 2,6-Diaminopyridine (1.00 g) in 60 mL MeOH was added dropwise to the above solution under stirring at room temperature. After being continu- ously stirred for 2 h at room temperature, many yellow solids were obtained by filter. Recrystallization of the yellow compound with CH2Cl2 and n-hexane (2:1), gave 2.38 g yellow crystals. Yield: 2.38 g (60%), mp: 162.1˚C - 162.8˚C, 1H-NMR: (CD3COCD3, d ppm), δ: 3.48 (d, 4H, CH2), 5.04 - 5.14 (m, 4H, CH2), 6.04 - 6.09 (m, 2H, CH), 6.96 - 8.08 (m, 9H, Ar), 9.70 (s, 2H, CH). FT-IR (KBr, cm–1) γ: 3633.89 (O-H); 3045.60 (C-H of Ar-C-H); 2938.10, 2870.67(C-H); 1683.74 (C=N); 1662.32 (C=C); 1608.04 (C=N, pyri- dine); 1487.56, 1450.21 (Ar-C=C). 2.4.2. Synthesis of MCNTs Functionalized by Amino-Group MCNTs were first hydroxylated according to literature [20]. Then, they were functionalized with amino to pre- pare MCNTs-NH2 as described in literature [21]. The typical process was as following. Under stirring, 4 ml of 3-aminopropyltriethoxysilane was added to the mixture of MCNTs-OH (4.01 g) and dry-toluene (100 ml). After the reactions proceeded for 24 h at room temperature, the MCNTs-NH2 was obtained by centrifugation, followed by redispersion in methanol and centrifugation for three times. 2.4.3. Synthesis of Vinyl-Group-Functionalized MCNTs Under stirring, 4 mL acrolein (dissolved in 10 mL MeOH) was added dropwise to a solution of MCNTs-NH2 (1.90 g) and MeOH (90 mL). After reacting for 3 h at room temperature, the reaction solution was centrifugated, re- sulting in the solid nanoparticles Vinyl-Group-Function- alized MCNTs (MCNTs-CH=CH2, 1.99 g) which was then washed with MeOH/DDW/DMF (1:1:1 in volume). 2.4.4. Synthesis of IMCNTs and Non-IMCNTs [22] The synthesis process of IMCNTs, whose surface was grafted by Pb(II)-imprinted group, is presented as Scheme 1. Firstly, 0.76 g (CH3COO)2Pb·3H2O was dis- solved in 25 mL MeOH. Then, this solution was slowly added to a glass reactor containing the functional mono- mer (PDABMBAPOL, 1.59 g) in DMF (25 mL) under Copyright © 2012 SciRes. JASMI 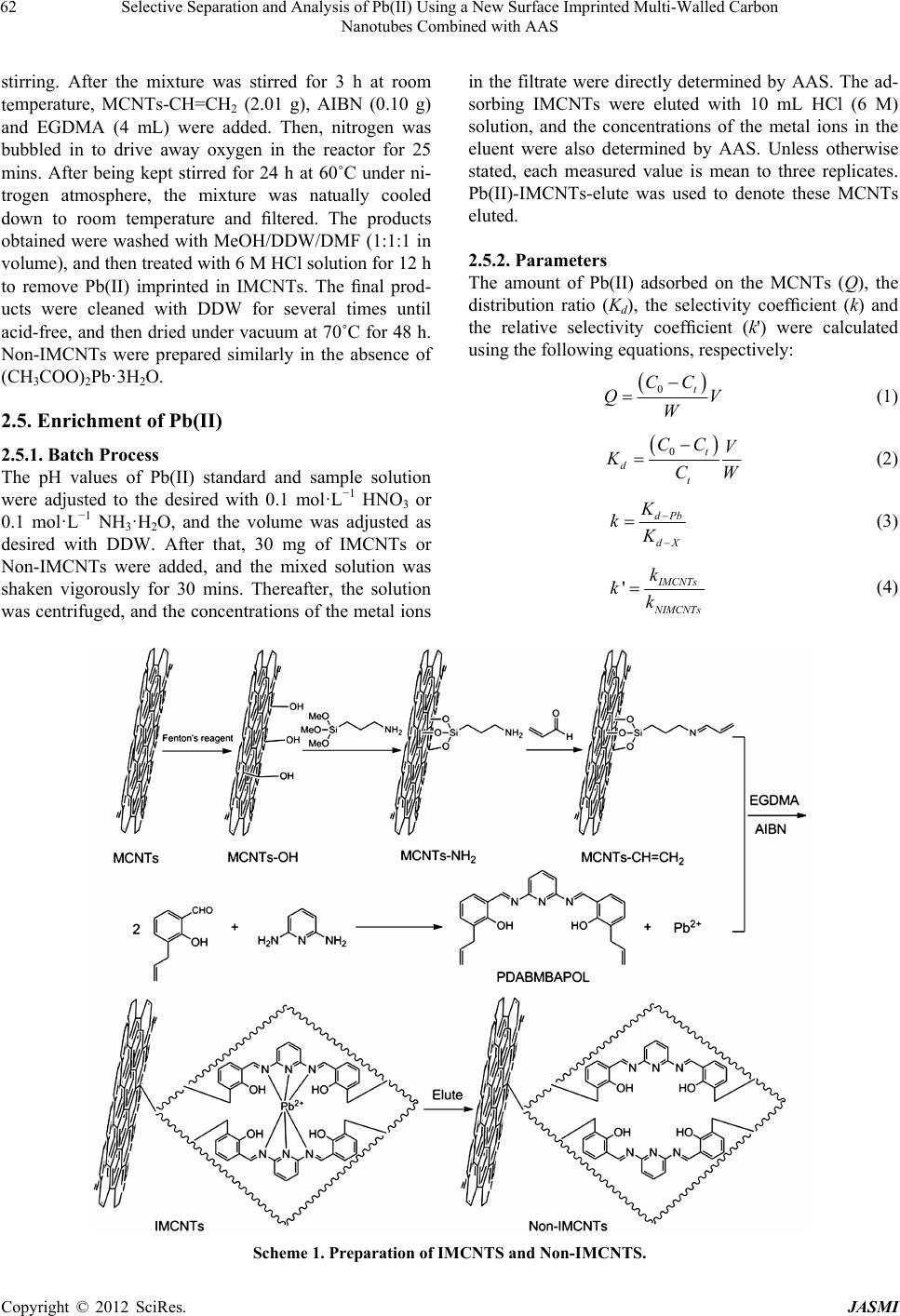 Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS Copyright © 2012 SciRes. JASMI 62 in the filtrate were directly determined by AAS. The ad- sorbing IMCNTs were eluted with 10 mL HCl (6 M) solution, and the concentrations of the metal ions in the eluent were also determined by AAS. Unless otherwise stated, each measured value is mean to three replicates. Pb(II)-IMCNTs-elute was used to denote these MCNTs eluted. stirring. After the mixture was stirred for 3 h at room temperature, MCNTs-CH=CH2 (2.01 g), AIBN (0.10 g) and EGDMA (4 mL) were added. Then, nitrogen was bubbled in to drive away oxygen in the reactor for 25 mins. After being kept stirred for 24 h at 60˚C under ni- trogen atmosphere, the mixture was natually cooled down to room temperature and filtered. The products obtained were washed with MeOH/DDW/DMF (1:1:1 in volume), and then treated with 6 M HCl solution for 12 h to remove Pb(II) imprinted in IMCNTs. The final prod- ucts were cleaned with DDW for several times until acid-free, and then dried under vacuum at 70˚C for 48 h. Non-IMCNTs were prepared similarly in the absence of (CH3COO)2Pb·3H2O. 2.5.2. Parameters The amount of Pb(II) adsorbed on the MCNTs (Q), the distribution ratio (Kd), the selectivity coefficient (k) and the relative selectivity coefficient (k') were calculated using the following equations, respectively: 0t CC Q W V (1) 2.5. Enrichment of Pb(II) 0t d t CC V KCW (2) 2.5.1. Batch Process The pH values of Pb(II) standard and sample solution were adjusted to the desired with 0.1 mol·L−1 HNO3 or 0.1 mol·L−1 NH3·H2O, and the volume was adjusted as desired with DDW. After that, 30 mg of IMCNTs or Non-IMCNTs were added, and the mixed solution was shaken vigorously for 30 mins. Thereafter, the solution was centrifuged, and the concentrations of the metal ions dPb dX K kK (3) ' MCNTs IMCNTs k kk (4) Scheme 1. Preparation of IMCNTS and Non-IMCNTS. 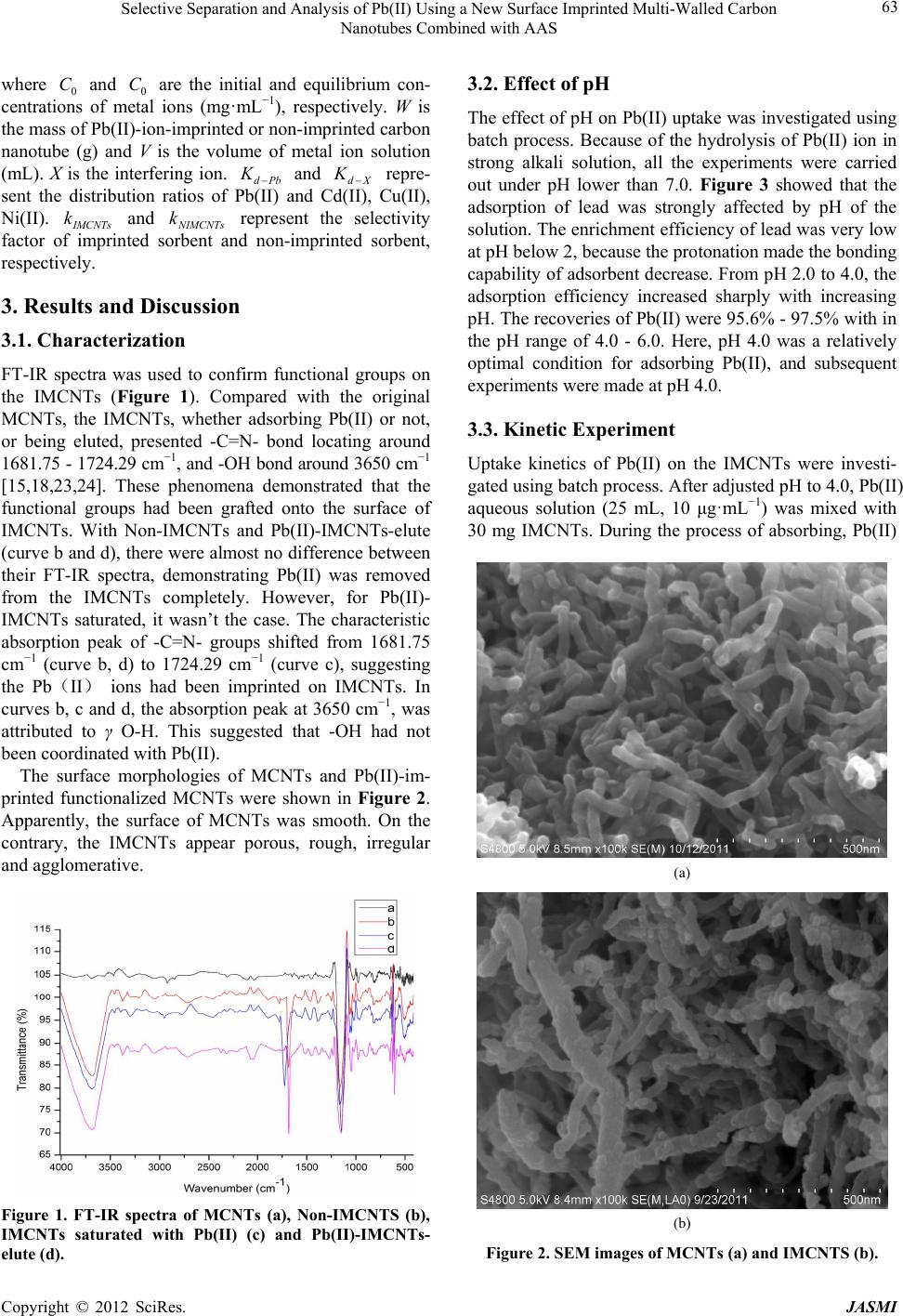 Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS 63 where 0 and 0 are the initial and equilibrium con- centrations of metal ions (mg·mL−1), respectively. W is the mass of Pb(II)-ion-imprinted or non-imprinted carbon nanotube (g) and V is the volume of metal ion solution (mL). X is the interfering ion. dPb and dX C C KK repre- sent the distribution ratios of Pb(II) and Cd(II), Cu(II), Ni(II). MCNTs and k IMCNTs represent the selectivity factor of imprinted sorbent and non-imprinted sorbent, respectively. k 3. Results and Discussion 3.1. Characterization FT-IR spectra was used to confirm functional groups on the IMCNTs (Figure 1). Compared with the original MCNTs, the IMCNTs, whether adsorbing Pb(II) or not, or being eluted, presented -C=N- bond locating around 1681.75 - 1724.29 cm−1, and -OH bond around 3650 cm−1 [15,18,23,24]. These phenomena demonstrated that the functional groups had been grafted onto the surface of IMCNTs. With Non-IMCNTs and Pb(II)-IMCNTs-elute (curve b and d), there were almost no difference between their FT-IR spectra, demonstrating Pb(II) was removed from the IMCNTs completely. However, for Pb(II)- IMCNTs saturated, it wasn’t the case. The characteristic absorption peak of -C=N- groups shifted from 1681.75 cm−1 (curve b, d) to 1724.29 cm−1 (curve c), suggesting the Pb(II) ions had been imprinted on IMCNTs. In curves b, c and d, the absorption peak at 3650 cm−1, was attributed to γ O-H. This suggested that -OH had not been coordinated with Pb(II). The surface morphologies of MCNTs and Pb(II)-im- printed functionalized MCNTs were shown in Figure 2. Apparently, the surface of MCNTs was smooth. On the contrary, the IMCNTs appear porous, rough, irregular and agglomerative. Figure 1. FT-IR spectra of MCNTs (a), Non-IMCNTS (b), IMCNTs saturated with Pb(II) (c) and Pb(II)-IMCNTs- elute (d). 3.2. Effect of pH The effect of pH on Pb(II) uptake was investigated using batch process. Because of the hydrolysis of Pb(II) ion in strong alkali solution, all the experiments were carried out under pH lower than 7.0. Figure 3 showed that the adsorption of lead was strongly affected by pH of the solution. The enrichment efficiency of lead was very low at pH below 2, because the protonation made the bonding capability of adsorbent decrease. From pH 2.0 to 4.0, the adsorption efficiency increased sharply with increasing pH. The recoveries of Pb(II) were 95.6% - 97.5% with in the pH range of 4.0 - 6.0. Here, pH 4.0 was a relatively optimal condition for adsorbing Pb(II), and subsequent experiments were made at pH 4.0. 3.3. Kinetic Experiment Uptake kinetics of Pb(II) on the IMCNTs were investi- gated using batch process. After adjusted pH to 4.0, Pb(II) aqueous solution (25 mL, 10 μg·mL−1) was mixed with 30 mg IMCNTs. During the process of absorbing, Pb(II) (a) (b) Figure 2. SEM images of MCNTs (a) and IMCNTS (b). Copyright © 2012 SciRes. JASMI 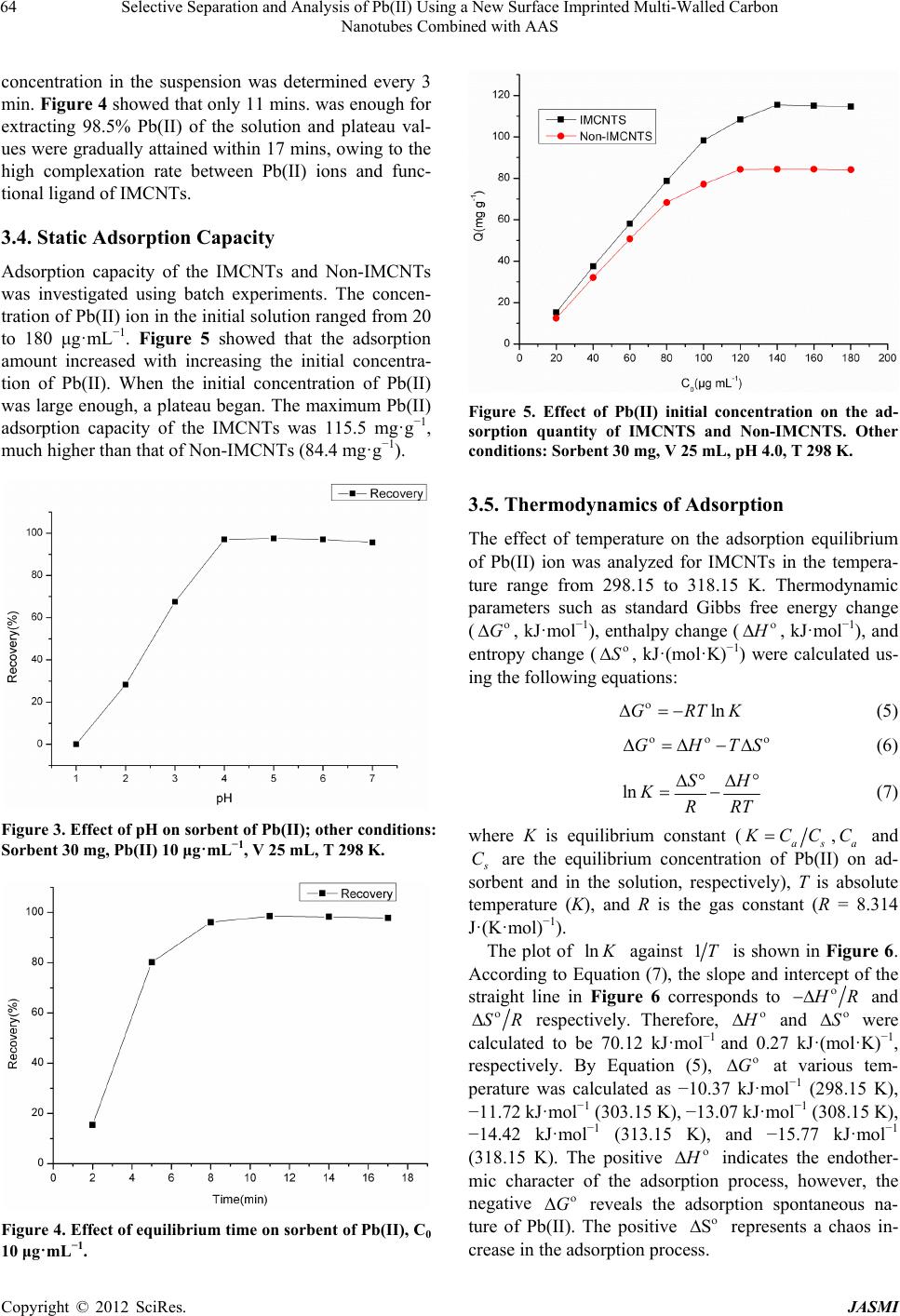 Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS 64 concentration in the suspension was determined every 3 min. Figure 4 showed that only 11 mins. was enough for extracting 98.5% Pb(II) of the solution and plateau val- ues were gradually attained within 17 mins, owing to the high complexation rate between Pb(II) ions and func- tional ligand of IMCNTs. 3.4. Static Adsorption Capacity Adsorption capacity of the IMCNTs and Non-IMCNTs was investigated using batch experiments. The concen- tration of Pb(II) ion in the initial solution ranged from 20 to 180 μg·mL−1. Figure 5 showed that the adsorption amount increased with increasing the initial concentra- tion of Pb(II). When the initial concentration of Pb(II) was large enough, a plateau began. The maximum Pb(II) adsorption capacity of the IMCNTs was 115.5 mg·g−1, much higher than that of Non-IMCNTs (84.4 mg·g−1). Figure 3. Effect of pH on sorbent of Pb(II); other conditions: Sorbent 30 mg, Pb(II) 10 μg·mL−1, V 25 mL, T 298 K. Figure 4. Effect of equilibrium time on sorbent of Pb(II), C0 10 μg·mL−1. Figure 5. Effect of Pb(II) initial concentration on the ad- sorption quantity of IMCNTS and Non-IMCNTS. Other conditions: Sorbent 30 mg, V 25 mL, pH 4.0, T 298 K. 3.5. Thermodynamics of Adsorption The effect of temperature on the adsorption equilibrium of Pb(II) ion was analyzed for IMCNTs in the tempera- ture range from 298.15 to 318.15 K. Thermodynamic parameters such as standard Gibbs free energy change (G , kJ·mol−1), enthalpy change ( , kJ·mol−1), and entropy change (S , kJ·(mol·K)−1) were calculated us- ing the following equations: lnGRT K (5) GHTS (6) ln SH KRRT (7) where K is equilibrium constant (as CC,a C and C are the equilibrium concentration of Pb(II) on ad- sorbent and in the solution, respectively), T is absolute temperature (K), and R is the gas constant (R = 8.314 J·(K·mol)−1). The plot of ln against 1T is shown in Figure 6. According to Equation (7), the slope and intercept of the straight line in Figure 6 corresponds to R and SR respectively. Therefore, and S were calculated to be 70.12 kJ·mol−1 and 0.27 kJ·(mol·K)−1, respectively. By Equation (5), at various tem- perature was calculated as −10.37 kJ·mol−1 (298.15 K), −11.72 kJ·mol−1 (303.15 K), −13.07 kJ·mol−1 (308.15 K), −14.42 kJ·mol−1 (313.15 K), and −15.77 kJ·mol−1 (318.15 K). The positive G indicates the endother- mic character of the adsorption process, however, the negative G reveals the adsorption spontaneous na- ture of Pb(II). The positive represents a chaos in- crease in the adsorption process. S Copyright © 2012 SciRes. JASMI  Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS 65 Figure 6. Effect of temperature on sorbent of Pb(II) on IMCNTS. Pb(II) 10 μg·mL−1, V 25 mL, pH 4.0. 3.6. Cycling and Reproducibility of Pb(II)-IMCNTs Pb(II)-IMCNTs, were subjected to adsorption-desorption cycles in a batch process to test their reproducibility and cycling behavior. 6 M HCl (10 mL) acted as a desorption agent. The recycled Pb(II)-IMCNTs presented a recovery not less than 95% at the 10th cycle, suggesting an ad- sorption capacity loss only by about 4%. Therefore, it can be concluded that the IMCNTs can be used repeat- edly without compromising their adsorption capacities, noticeably. 3.7. Selectivity of the IMCNTs The selectivity of an imprinted material for a given ion plays the key role in its practical use. This paper has es- timated the selectivity of the imprinted MCNTs for Pb(II) relative to Cd(II), Cu(II) and Ni(II), separately. Each metal ion in its solution had the concentration of 10 μg·mL−1. As shown in Table 1, with IMCNTs, the rela- tive selectivity coefficients () of Pb(II)/Cd(II), Pb(II)/ Cu(II) and Pb(II)/Ni(II), were 21.6, 5.5 and 22, respec- tively, greater than one, indicating IMCNTs displayed higher selectivity for Pb(II) relative to Cd(II), Cu(II) and Ni(II). Therefore, the above IMCNTs could be applied to selectively separating Pb(II) from the solution containing Cd(II), Cu(II) and Ni(II). Two possible factors contri- buted to the higher selectivity for Pb(II) in the presence of the above competitive ions. One was the holes size selectivity. The size of Pb(II) ion cut out for the cavity of the IMCNTs. The other was the coordination geometry selectivity. IMCNTs could provide ligand groups with preferred selectivity for Pb(II) ions. 'k 3.8. Accuracy and Precision of the Analytical Method The accuracy and precision of the analytical method were evaluated by repeating the experiment for eight times under the optimal experimental conditions. The results indicated that the precision of the method, evalu- ated as the relative standard deviation (RSD, n = 8), was 1.16%. The limit of detection (LOD), calculated based on the three times of the blank standard deviation of eight runs the blank solution, was 0.47 μg·L−1. This indicated that the method had good precision for the analysis of trace Pb(II). 3.9. Application of the Proposed Method The accuracy of the method was evaluated by determin- ing the amount of Pb2+ ions in the certified reference material and three environmental water samples (River water, Tap water, Waste water). For water samples, the standard addition method was adopted. From Table 2, it could be seen that the estimated values agreed well with the certified values. The recoveries of Pb(II) ions were in Table 1. Competitive sorption of Pb(II) and metal ions on IMCNTs and NIMCNTs at pH 4.0. Mixture of ions (10 μg·mL−1, 250 mL) Kd-IMCNTs (mL·g−1) Kd-NIMCNTs (mL·g−1) kIMCNTs kNIMCNTs k' Pb(II)/Cd(II) 1658.3/64 21.7/18.1 25.9 1.2 21.6 Pb(II)/Cu(II) 1646/71 33/7.9 23.2 4.2 5.5 Pb(II)/Ni(II) 1613/6720.1/18.7 24.1 1.1 22 Table 2. Determination of Pb(II) in environmental water samples. Sample Pb(II) added (μg·L−1) Pb(II) founded (μg·L−1) Recovery (%) 0 BQLb 5.0a 4.85 ± 0.02 97.0 GBW08619 10.0a 9.75 ± 0.03 97.5 0 0.59 ± 0.01 - 5.0 5.76 ± 0.06 103.0 River water 10.0 10.57 ± 0.12 99.8 0 BQLb - 5.0 4.98 ± 0.07 99.6 Tap water 10.0 10.01 ± 0.11 100.1 0 1.17 ± 0.02 - 5.0 6.24 ± 0.08 101.1 Waste water 10.0 11.08 ± 0.14 99.2 aReference value. The certified sample solution was accurately diluted to 5 and 10 μg·L−1, respectively; bBelow the quantification limit. Copyright © 2012 SciRes. JASMI  Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS Copyright © 2012 SciRes. JASMI 66 Table 3. Comparisons of maximum adsorption capacity, analytical precision and accuracy of different SPE materials for pre-concentration of Pb(II). SPE material coupled with analytical technique The maximum adsorption capacity (mg·g−1) LOD (ng·mL−1) R.S.D (%) Ref. P2AT modified MWCNTs/AAS 186.4 1 3.2 [25] PANI modified MWCNTs/UV 22.2 ND ND [26] Column SPE on MWCNTs/AAS ND* 8.0 <2.5 [27] IDA modified MWCNTs/ICP-MS 8.98 0.00070 1.0 [28] IMCNTs/AAS 115.5 0.47 1.16 Present work *ND: not detected. the range of 97.0% - 103.0%. The comparisons of maxi- mum adsorption capacity, analytical precision and accu- racy of different SPE materials for Pb(II) was given in Tables 3. As could be seen, the present analytical method was comparable with that obtained by other re- ported different SPE materials in recent years. These results clearly demonstrated that the IMCNTs prepared were highly efficient, suitable and satisfactory for ex- tracting and determining Pb(II) ions. 4. Conclusion The newly IMCNTs was synthesized using a complex of PDABMBAPOL with Pb(II) as functional monomer and template ion. The IMCNTs exhibited high affinity, selec- tivity and fast kinetics for Pb(II) ions. Under optimal conditions, the maximum adsorption quantity of IMCNTs was 115.5 mg·g−1 much higher than that of Non- IMCNTs (84.4 mg·g−1). The presence of other metal ions such as Cd(II), Cu(II), or Ni(II) did not affect IMCNTs selectivity for Pb(II). Separation and pre-concentration by solidphase extraction with IMCNTs particles results in a limit of detection of 0.47 μg·L−1 (3σ) and RSD (n = 8) of 1.16% by using AAS. In addition, the IMCNTs prepared had high reproducibility and were efficient, sensitive and reliable for the enrichment and determina- tion of trace lead. 5. Acknowledgements This research was supported by the National Natural Science Foundation of China (No. 11175080) and by the Nature Science Fund of the Hunan Province (No. 10JJ6025). REFERENCES [1] C. Esen, M. Andac, N. Bereli, R. Say, E. Henden and A. Denizli, “Highly Selective Ion-Imprinted Particles for Solid-Phase Extraction of Pb2+ Ions,” Materials Science and Engineering C, Vol. 29, No. 8, 2009, pp. 2464-2470. doi:10.1016/j.msec.2009.07.012 [2] L. M. Wang, M. H. Zhou, Z. J. Jing and A. F. Zhong, “Selective Separation of Lead from Aqueous Solution with a Novel Pb(II) Surface Ion-Imprinted Sol-Gel Sor- bent,” Microchimica Acta, Vol. 165, No. 3-4, 2009, pp. 367-372. doi:10.1007/s00604-009-0146-2 [3] F. Q. An, B. J. Gao and X. Q. Feng, “Adsorption and Recognition Properties of Ionic Imprinted Polyamine IIP-PEI/SiO2 towards Pb2+ Ion,” Journal of Applied Polymer Science, Vol. 112, No. 4, 2009, pp. 2241-2246. doi:10.1002/app.29751 [4] H. X. Shan, Z. J. Li and M. Li, “Ionic Liquid 1-Octyl- 3-methylimidazolium Hexafluorophosphate as a Solvent for Extraction of Lead in Environmental Water Samples with Detection by Graphite Furnace Atomic Absorption Spectrometry,” Microchimica Acta, Vol. 159, No. 1-2, 2007, pp. 95-100. doi:10.1007/s00604-006-0720-9 [5] J. Otero-Romaní, A. Moreda-Pineiro, P. Bermejo-Barrera and A. Martin-Esteban, “Inductively Coupled Plasma- Optical Emission Spectrometry/Mass Spectrometry for the Determination of Cu, Ni, Pb and Zn in Seawater after Ionic Imprinted Polymer Based Solid Phase Extraction,” Talanta, Vol. 79, No. 3, 2009, pp. 723-729. doi:10.1016/j.talanta.2009.04.066 [6] X. B. Zhu, Y. M. Cui, X. J. Chang, X. J. Zou and Z. H. Li, “Selective Solid-Phase Extraction of lead(II) from Bio- logical and Natural Water Samples Using Surface- Grafted Lead(II)-Imprinted Polymers,” Microchimica Acta, Vol. 164, No. 1-2, 2009, pp. 125-132. doi:10.1007/s00604-008-0045-y [7] S. Daniel, P. P. Rao and T. P. Rao, “Investigation of Dif- ferent Polymerization Methods on the Analytical Per- formance of Palladium(II) Ion Imprinted Polymer Mate- rials” Analytica Chimica Acta, Vol. 536, No. 1-2, 2005, pp. 197-206. doi:10.1016/j.aca.2004.12.052 [8] M. Shamsipur, J. Fasihi and K. Ashtari, “Grafting of Ion-Imprinted Polymers on the Surface of Silica Gel Par- ticles through Covalently Surface-Bound Initiators: A Selective Sorbent for Uranyl Ion,” Analytical Chemistry, Vol. 79, No. 18, 2007, pp. 7116-7123. doi:10.1021/ac070968e [9] N. Jiang, X. J. Chang, H. Zheng, Q. He and Z. Hu, “Se- lective Solid-Phase Extraction of Nickel(II) Using a Sur- face-Imprinted Silica Gel Sorbent,” Analytica Chimica Acta, Vol. 577, No. 2, 2006, pp. 225-231. doi:10.1016/j.aca.2006.06.049 [10] Z. Q. Cheng, H. Q. Wang, Y. Y. Wang, F. F. He, H. S.  Selective Separation and Analysis of Pb(II) Using a New Surface Imprinted Multi-Walled Carbon Nanotubes Combined with AAS 67 Zhang and S. Q. Yang, “Synthesis and Characterization of an Ion-Imprinted Polymer for Selective Solid Phase Extraction of Thorium(IV),” Microchimica Acta, Vol. 173, No. 3-4, 2011, pp. 423-431. doi:10.1007/s00604-011-0576-5 [11] N. García-Otero, C. Teijeiro-Valiño, J. Otero-Romaní, E. Peña-Vázquez, A. Moreda-Piñeiro and P. Bermejo- Barrera, “On-Line Ionic Imprinted Polymer Selective Solid-Phase Extraction of Nickel and Lead from Seawater and Their Determination by Inductively Coupled Plasma- Optical Emission Spectrometry,” Analytical and Bioana- lytical Chemistry, Vol. 395, No. 4, 2009, pp. 1107-1115. doi:10.1007/s00216-009-3044-x [12] H. Zheng, D. Zhang, W. Y. Wang, Y. Q. Fan, J. Li and H. P. Han, “Highly Selective Determination of Palladium(II) after Preconcentration Using Pd(II)-Imprinted Function- alized Silica Gel Sorbent Prepared by a Surface Imprint- ing Technique,” Microchimica Acta, Vol. 157, No.1-2, 2007, pp. 7-11. doi:10.1007/s00604-006-0649-z [13] Y. Li, X. Li, J. Chu, C. Dong, J. Qi and Y. Yuan, “Syn- thesis of Core-Shell Magnetic Molecular Imprinted Polymer by the Surface RAFT Polymerization for the Fast and Selective Removal of Endocrine Disrupting Chemicals from Aqueous Solutions,” Environmental Pollution, Vol. 158, No. 6, 2010, pp. 2317-2323. doi:10.1016/j.envpol.2010.02.007 [14] C. R. T. Tarley, F. N. Andrade, F. M. de Oliveira, M. Z. Corazza, L. F. M. de Azevedo and M. G. Segatelli, “Synthesis and Application of Imprinted Polyvinylimi- dazole-Silica Hybrid Copolymer for Pb2+ Determination by Flow-Injection Thermospray Flame Furnace Atomic Absorption Spectrometry,” Analytica Chimica Acta, Vol. 703, No. 2, 2011, pp. 145-151. doi:10.1016/j.aca.2011.07.029 [15] G. Z. Fang, J. Tan and X. P. Yan, “An Ion-Imprinted Functionalized Silica Gel Sorbent Prepared by a Surface Imprinting Technique Combined with a Sol-Gel Process for Selective Solid-Phase Extraction of Cadmium(II),” Analytical Chemistry, Vol. 77, No. 6, 2005, pp. 1734- 1739. doi:10.1021/ac048570v [16] X. J. Wang, Z. L. Xu, N. C. Bing and Z. G. Yang, “Preparation and Aqueous Recognition of Metal Com- plex Imprinted Polymer Using N-vinyl-2-pyrrolidone as Functional Monomer,” Chinese Journal of Chemical En- gineering, Vol. 15, No. 4, 2007, pp. 595-599. doi:10.1016/S1004-9541(07)60130-X [17] S. Hazra, S. Majumder, M. Fleck, R. Koner and S. Mo- hanta, “Syntheses, Structures, Absorption and Emission Properties of a Tetraiminodiphenol Macrocyclic Ligand and Its Dinuclear Zn(II) and Pb(II) Complexes,” Polyhe- dron, Vol. 28, No. 14, 2009, pp. 2871-2878. doi:10.1016/j.poly.2009.06.039 [18] S. Iihan, “Synthesis and Spectral Studies of New Macro- cyclic Schiff Base Cu(II), Ni(II), Cd(II), Zn(II), Pb(II), and La(III) Complexes Containing Pyridine Head Unit,” Russian Journal of Coordination Chemistry, Vol. 35, No. 5, 2009, pp. 347-351. doi:10.1134/S1070328409050066 [19] S. Ilhan and H. Temel, “Synthesis and Characterization of a New Macrocyclic Schiff Base Derived from 2,6-Dia- minopyridine and 1,10-Bis(2-formylphenyl)-1,4,7,10- tetraoxadecane and Its Cu(II), Ni(II), Pb(II), Co(III) and La(III) Complexes,” Transition Metal Chemistry, Vol. 32, No. 8, 2007, pp. 1039-1046. doi:10.1007/s11243-007-0276-5 [20] Y. Ying, R. K. Saini, F. Liang, A. K. Sadana and W. E. Billups, “Functionalization of Carbon Nanotubes by Free Radicals,” Organic Letters, Vol. 5, No. 9, 2003, pp. 1471-1473. doi:10.1021/ol0342453 [21] U. Dettlaff-Weglikowska, J. M. Benoit, P. W. Chiu, R. Graupner, S. Lebedkin and S. Roth, “Chemical Function- alization of Single Walled Carbon Nanotubes,” Current Applied Physics, Vol. 2, No. 6, 2002, pp. 497-501. doi:10.1016/S1567-1739(02)00164-5 [22] C. R. Lin, H. Q. Wang, Y. Y. Wang and Z. Q. Cheng, “Selective Solid-Phase Extraction of Trace Thorium(IV) Using Surface-Grafted Th(IV)-Imprinted Polymers with Pyrazole Derivative,” Talanta, Vol. 81, 2010, pp. 30-36. doi:10.1016/j.talanta.2009.11.032 [23] J. Pan, S. Wang and R. Zhang, “A Novel Pb(II)-Im- printed IPN for Selective Preconcentration of Lead from Water and Sediments,” Analytical Chemistry, Vol. 86, No. 11, 2006, pp. 855-865. [24] H. Keypour, M. Rezaeivala, L. Valencia and P. Pérez- Lourido, “Synthesis and Characterization of Two Zinc(II) Macrocyclic Schiff-Base Complexes Containing Pipera- zine Moiety, Crystal Structure of One Complex,” Poly- hedron, Vol. 28, No. 16, 2009, pp. 3415-3418. doi:10.1016/j.poly.2009.07.012 [25] M. R. Nabida, R. Sedghia, A. Bagheria, M. Behbahania, M. Taghizadeha, H. A. Oskooieb and M. M. Heravib, “Preparation and Application of Poly(2-amino thiophe- nol)/MWCNTs Nanocomposite for Adsorption and Separation of Cadmium and Lead Ions via Solid Phase Extraction,” Journal of Hazardous Materials, Vol. 203- 204, 2012, pp. 93-100. doi:10.1016/j.jhazmat.2011.11.096 [26] D. D. Shao, C. L. Chen and X. K. Wang, “Application of Polyaniline and Multiwalled Carbon Nanotube Magnetic Composites for Removal of Pb(II),” Chemical Engineer- ing Journal, Vol. 185-186, 2012, pp. 144-150. doi:10.1016/j.cej.2012.01.063 [27] S. G. Ozcan, N. Satiroglu and M. Soylak, “Column Solid Phase Extraction of Iron(III), Copper(II), Manganese(II) and Lead(II) Ions Food and Water Samples on Multi- Walled Carbon Nanotubes,” Food and Chemical Toxi- cology, Vol. 48, 2010, pp. 2401-2406. doi:10.1016/j.fct.2010.05.078 [28] J. P. Wang, X. X. Ma, G. Z. Fang, M. F. Pan, X. K. Ye and S. Wang, “Preparation of Iminodiacetic Acid Func- tionalized Multi-Walled Carbon Nanotubes and Its Ap- plication as Sorbent for Separation and Preconcentration of Heavy Metal Ions,” Journal of Hazardous Materials, Vol. 186, 2011, pp. 1985-1992. doi:10.1016/j.jhazmat.2010.12.087 Copyright © 2012 SciRes. JASMI
|