Simple Method for Preparing Glucose Biosensor Based on Glucose Oxidase in Nanocomposite
Material of Single-Wall Carbon Nanotubes/Ionic Liquid
58
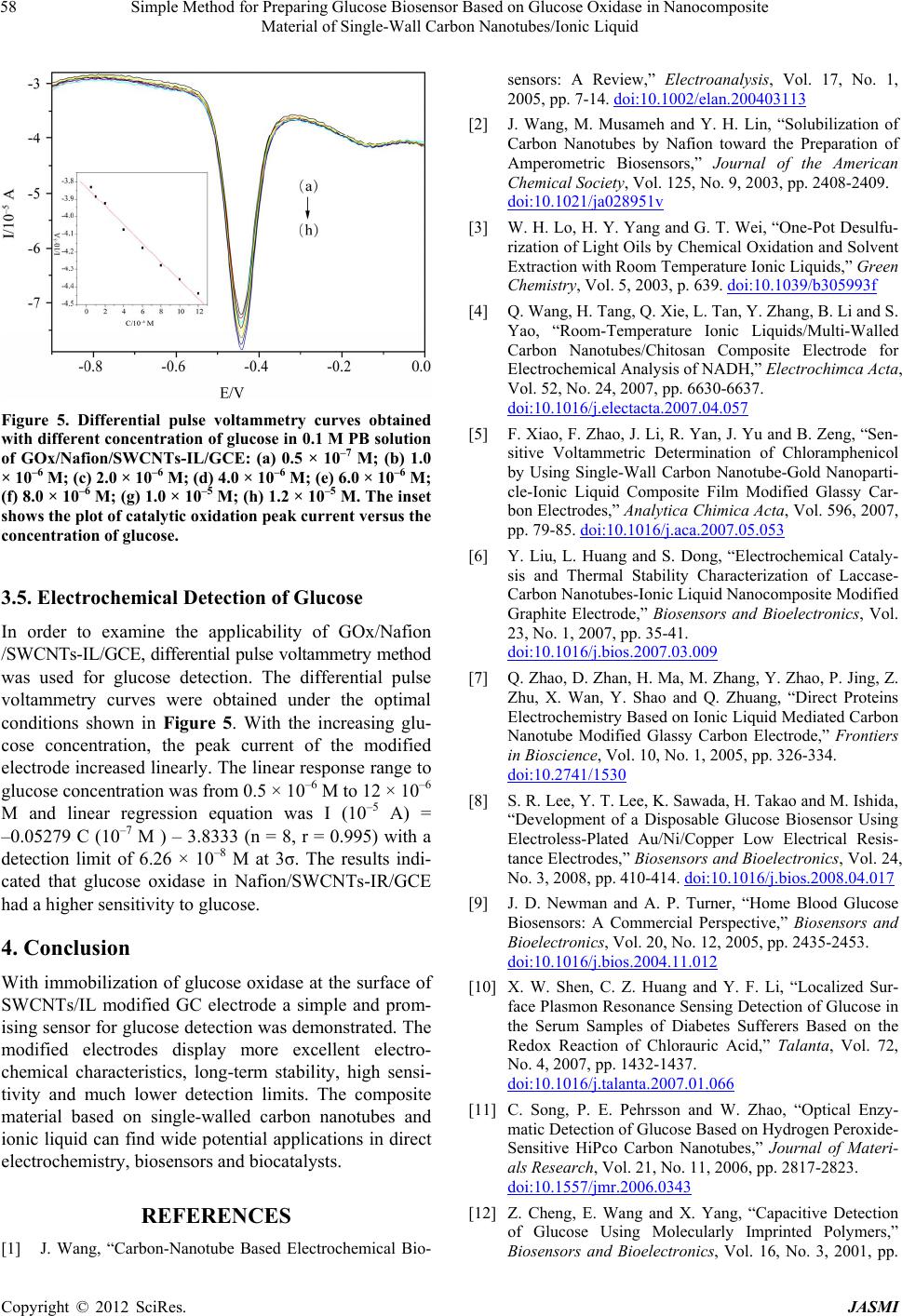
Figure 5. Differential pulse voltammetry curves obtained
with different concentration of glucose in 0.1 M PB solution
of GOx/Nafion/SWCNTs-IL/GCE: (a) 0.5 × 10–7 M; (b) 1.0
× 10–6 M; (c) 2.0 × 10–6 M; (d) 4.0 × 10–6 M; (e) 6.0 × 10–6 M;
(f) 8.0 × 10–6 M; (g) 1.0 × 10–5 M; (h) 1.2 × 10–5 M. The inset
shows the plot of catalytic oxidation peak current versus the
concentration of glucose.
3.5. Electrochemical Detection of Glucose
In order to examine the applicability of GOx/Nafion
/SWCNTs-IL/GCE, differential pulse voltammetry method
was used for glucose detection. The differential pulse
voltammetry curves were obtained under the optimal
conditions shown in Figure 5. With the increasing glu-
cose concentration, the peak current of the modified
electrode increased linearly. The linear response range to
glucose concentration was from 0.5 × 10–6 M to 12 × 10–6
M and linear regression equation was I (10–5 A) =
–0.05279 C (10–7 M ) – 3.8333 (n = 8, r = 0.995) with a
detection limit of 6.26 × 10–8 M at 3σ. The results indi-
cated that glucose oxidase in Nafion/SWCNTs-IR/GCE
had a higher sensitivity to glucose.
4. Conclusion
With immobilization of glucose oxidase at the surface of
SWCNTs/IL modified GC electrode a simple and prom-
ising sensor for glucose detection was demonstrated. The
modified electrodes display more excellent electro-
chemical characteristics, long-term stability, high sensi-
tivity and much lower detection limits. The composite
material based on single-walled carbon nanotubes and
ionic liquid can find wide potential applications in direct
electrochemistry, biosensors and biocatalysts.
REFERENCES
[1] J. Wang, “Carbon-Nanotube Based Electrochemical Bio-
sensors: A Review,” Electroanalysis, Vol. 17, No. 1,
2005, pp. 7-14. doi:10.1002/elan.200403113
[2] J. Wang, M. Musameh and Y. H. Lin, “Solubilization of
Carbon Nanotubes by Nafion toward the Preparation of
Amperometric Biosensors,” Journal of the American
Chemical Society, Vol. 125, No. 9, 2003, pp. 2408-2409.
doi:10.1021/ja028951v
[3] W. H. Lo, H. Y. Yang and G. T. Wei, “One-Pot Desulfu-
rization of Light Oils by Chemical Oxidation and Solvent
Extraction with Room Temperature Ionic Liquids,” Green
Chemistry, Vol. 5, 2003, p. 639. doi:10.1039/b305993f
[4] Q. Wang, H. Tang, Q. Xie, L. Tan, Y. Zhang, B. Li and S.
Yao, “Room-Temperature Ionic Liquids/Multi-Walled
Carbon Nanotubes/Chitosan Composite Electrode for
Electrochemical Analysis of NADH,” Electrochimca Acta,
Vol. 52, No. 24, 2007, pp. 6630-6637.
doi:10.1016/j.electacta.2007.04.057
[5] F. Xiao, F. Zhao, J. Li, R. Yan, J. Yu and B. Zeng, “Sen-
sitive Voltammetric Determination of Chloramphenicol
by Using Single-Wall Carbon Nanotube-Gold Nanoparti-
cle-Ionic Liquid Composite Film Modified Glassy Car-
bon Electrodes,” Analytica Chimica Acta, Vol. 596, 2007,
pp. 79-85. doi:10.1016/j.aca.2007.05.053
[6] Y. Liu, L. Huang and S. Dong, “Electrochemical Cataly-
sis and Thermal Stability Characterization of Laccase-
Carbon Nanotubes-Ionic Liquid Nanocomposite Modified
Graphite Electrode,” Biosensors and Bioelectronics, Vol.
23, No. 1, 2007, pp. 35-41.
doi:10.1016/j.bios.2007.03.009
[7] Q. Zhao, D. Zhan, H. Ma, M. Zhang, Y. Zhao, P. Jing, Z.
Zhu, X. Wan, Y. Shao and Q. Zhuang, “Direct Proteins
Electrochemistry Based on Ionic Liquid Mediated Carbon
Nanotube Modified Glassy Carbon Electrode,” Frontiers
in Bioscience, Vol. 10, No. 1, 2005, pp. 326-334.
doi:10.2741/1530
[8] S. R. Lee, Y. T. Lee, K. Sawada, H. Takao and M. Ishida,
“Development of a Disposable Glucose Biosensor Using
Electroless-Plated Au/Ni/Copper Low Electrical Resis-
tance Electrodes,” Biosensors and Bioelectronics, Vol. 24,
No. 3, 2008, pp. 410-414. doi:10.1016/j.bios.2008.04.017
[9] J. D. Newman and A. P. Turner, “Home Blood Glucose
Biosensors: A Commercial Perspective,” Biosensors and
Bioelectronics, Vol. 20, No. 12, 2005, pp. 2435-2453.
doi:10.1016/j.bios.2004.11.012
[10] X. W. Shen, C. Z. Huang and Y. F. Li, “Localized Sur-
face Plasmon Resonance Sensing Detection of Glucose in
the Serum Samples of Diabetes Sufferers Based on the
Redox Reaction of Chlorauric Acid,” Talanta, Vol. 72,
No. 4, 2007, pp. 1432-1437.
doi:10.1016/j.talanta.2007.01.066
[11] C. Song, P. E. Pehrsson and W. Zhao, “Optical Enzy-
matic Detection of Glucose Based on Hydrogen Peroxide-
Sensitive HiPco Carbon Nanotubes,” Journal of Materi-
als Research, Vol. 21, No. 11, 2006, pp. 2817-2823.
doi:10.1557/jmr.2006.0343
[12] Z. Cheng, E. Wang and X. Yang, “Capacitive Detection
of Glucose Using Molecularly Imprinted Polymers,”
Biosensors and Bioelectronics, Vol. 16, No. 3, 2001, pp.
Copyright © 2012 SciRes. JASMI