F. A. ALAZABI, M. A. ZOHDY
38
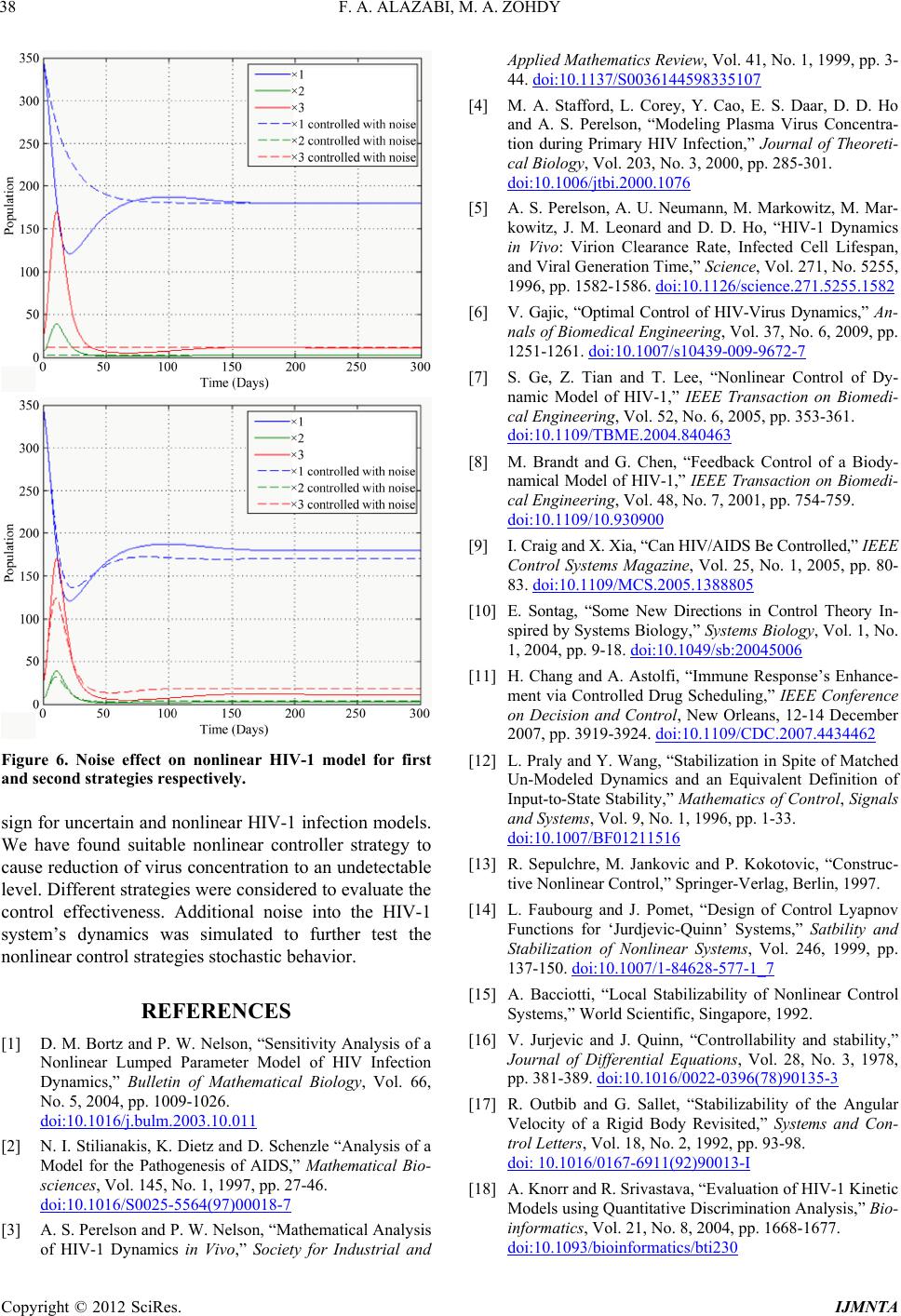
Figure 6. Noise effect on nonlinear HIV-1 model for firs
sign for uncertain and nonlinear HIV-1 infection models.
REFERENCES
[1] D. M. Bortz ativity Analysis of
1
t
and second strategies respectively.
We have found suitable nonlinear controller strategy to
cause reduction of virus concentration to an undetectable
level. Different strategies were considered to evaluate the
control effectiveness. Additional noise into the HIV-1
system’s dynamics was simulated to further test the
nonlinear control strategies stochastic behavior.
nd P. W. Nelson, “Sensi a
Nonlinear Lumped Parameter Model of HIV Infection
Dynamics,” Bulletin of Mathematical Biology, Vol. 66,
No. 5, 2004, pp. 1009-1026.
doi:10.1016/j.bulm.2003.10.01
chenzle “Analysis of a [2] N. I. Stilianakis, K. Dietz and D. S
Model for the Pathogenesis of AIDS,” Mathematical Bio-
sciences, Vol. 145, No. 1, 1997, pp. 27-46.
doi:10.1016/S0025-5564(97)00018-7
[3] A. S. Perelson and P. W. Nelson, “Mathematical Analysis
of HIV-1 Dynamics in Vivo,” Society for Industrial and
Applied Mathematics Review, Vol. 41, No. 1, 1999, pp. 3-
44. doi:10.1137/S0036144598335107
[4] M. A. Stafford, L. Corey, Y. Cao, E. S. Daar, D. D. Ho
and A. S. Perelson, “Modeling Plasma Virus Concentra-
tion during Primary HIV Infection,” Journal of Theoreti-
cal Biology, Vol. 203, No. 3, 2000, pp. 285-301.
doi:10.1006/jtbi.2000.1076
[5] A. S. Perelson, A. U. Neumann, M. Markowitz, M. Mar-
kowitz, J. M. Leonard and D. D. Ho, “HIV-1 Dynamics
in Vivo: Virion Clearance Rate, Infected Cell Lifespan,
and Viral Generation Time,” Science, Vol. 271, No. 5255,
1996, pp. 1582-1586. doi:10.1126/science.271.5255.1582
[6] V. Gajic, “Optimal Control of HIV-Virus Dynamics,” An-
nals of Biomedical Engineering, Vol. 37, No. 6, 2009, pp.
1251-1261. doi:10.1007/s10439-009-9672-7
[7] S. Ge, Z. Tian and T. Lee, “Nonlinear Control of Dy-
namic Model of HIV-1,” IEEE Transaction on Biomedi-
cal Engineering, Vol. 52, No. 6, 2005, pp. 353-361.
doi:10.1109/TBME.2004.840463
[8] M. Brandt and G. Chen, “Feedback Control of a Biody-
namical Model of HIV-1,” IEEE Transaction on Biomedi-
cal Engineering, Vol. 48, No. 7, 2001, pp. 754-759.
doi:10.1109/10.930900
[9] I. Craig and X. Xia, “Can HIV/AIDS Be Controlled,” IEEE
Control Systems Magazine, Vol. 25, No. 1, 2005, pp. 80-
83. doi:10.1109/MCS.2005.1388805
[10] E. Sontag, “Some New Directions in Control Theory In-
spired by Systems Biology,” Systems Biology, Vol. 1, No.
1, 2004, pp. 9-18. doi:10.1049/sb:20045006
[11] H. Chang and A. Astolfi, “Immune Response’s Enhance-
ment via Controlled Drug Scheduling,” IEEE Conference
on Decision and Control, New Orleans, 12-14 December
2007, pp. 3919-3924. doi:10.1109/CDC.2007.4434462
[12] L. Praly and Y. Wang, “Stabilization in Spite of Matched
Un-Modeled Dynamics and an Equivalent Definition of
Input-to-State Stability,” Mathematics of Control, Signals
and Systems, Vol. 9, No. 1, 1996, pp. 1-33.
doi:10.1007/BF01211516
[13] R. Sepulchre, M. Jankovic and P. Kokotovic, “Construc-
v
tive Nonlinear Control,” Springer-Verlag, Berlin, 1997.
[14] L. Faubourg and J. Pomet, “Design of Control Lyapno
Functions for ‘Jurdjevic-Quinn’ Systems,” Satbility and
Stabilization of Nonlinear Systems, Vol. 246, 1999, pp.
137-150. doi:10.1007/1-84628-577-1_7
[15] A. Bacciotti, “Local Stabilizability of Nonlinear Control
stability,”
Systems,” World Scientific, Singapore, 1992.
[16] V. Jurjevic and J. Quinn, “Controllability and
Journal of Differential Equations, Vol. 28, No. 3, 1978,
pp. 381-389. doi:10.1016/0022-0396(78)90135-3
[17] R. Outbib and G. Sallet, “Stabilizability of the Angular
Velocity of a Rigid Body Revisited,” Systems and Con-
trol Letters, Vol. 18, No. 2, 1992, pp. 93-98.
doi: 10.1016/0167-6911(92)90013-I
[18] A. Knorr and R. Srivastava, “Evaluation of HIV-1 Kinetic
Models using Quantitative Discrimination Analysis,” Bio-
informatics, Vol. 21, No. 8, 2004, pp. 1668-1677.
doi:10.1093/bioinformatics/bti230
Copyright © 2012 SciRes. IJMNTA