Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 5, No.2, pp 103-117, 2006 jmmce.org Printed in the USA. All rights reserved 103 Enrichment of Molybdenum and Fluorite by Flotation of Fluorite Ore Containing Molybdenum Abuzer Akgün, İbrahim Teğin, Recep Ziyadanoğulları* Department of Chemistry, Faculty of Science and Art, Dicle University, 21280 Diyarbakir, TURKEY *For correspondence: Fax: +90-412-2488039 Tel : +90-412-2488550/3159 E-mail: recepz@dicle.edu.tr ABSTRACT The molybdenum and fluorite were obtained in different phases with the flotation of fluorite ore containing molybdenum obtained from Karaamağaradere-Keban-Elazığ district in Turkey. It has been found that the original ore contains 1.08 % Mo, 0.05 %Cu, 1.20 % Pb, 1.12 % Zn and 12.0 % F. It was determined that molybdenum and fluorite could not be enriched in different phases with satisfactory yields by flotation of original ore at the defined size. Thus, the ore was sulfurized before the flotation. Concentrates containing molybdenum and the other concentrates containing fluorite were collected with fairly high yields in different phases by the flotation of the sulfurized ore. In the optimum flotation conditions, the Mo, Pb, Cu and Zn were obtained over 95 % yields and approximately 6 % fluorite passed into concentrate phase. Mo and Cu containing phase were enriched with high yield by selective flotation of concentrate obtained from the concentrate. On the other hand, the most of Pb and Zn remained in tailing. In order to gain molybdenum, the appropriate concentrate was obtained by this process with hydrometallurgical method. It was also determined that the trace amounts of Ta, Nb and V in ore were collected in the concentrate phase. In the first step of flotation, the fluorite left in the tailing contained 29.6 % of CaF2. After flotation of this tailing, concentrate grade of CaF2 raised to 96 %. 0.5 % of sulfur was found in this concentrate. It was concluded that this concentrate contained fluorite with suitable particle size (-100 mesh) and at sufficient concentration for metallurgical processes. Key Words: Molybdenum, fluorite, sulfurization, flotation 104 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 1. INTRODUCTION Fluorite (CaF2) is an important fluorine mineral, which is mostly used for the production of hydrofluoric acid and as a flux in steel making. Other uses are the manufacture of glass, fiberglass, pottery and enamel, etc. Recently, there have been numerous reports on the modification of the chemical scheme in fluorite flotation, in order to increase the separation efficiency and lower the operation costs. It was [1] reported that methanoic acid could selectively adsorb onto fluorite surfaces in preference to calcite surfaces at ambient temperature. This means that fluorite flotation to remove calcite might be achieved at a much lower temperature if methanoic acid is used as collector, resulting in a large reduction in energy consumption. It was [2, 3] found that sodium N-dodecanoyl sarcosine (anionic collector) in combination with dodecylammonium chloride (cationic collector) as co-collectors could greatly enhance the floatability of fluorite, compared with the use of the anionic collector alone. Also, fluorite flotation could be improved by using effective depressants when fatty acid is used as collector. For example, acidized sodium silicate was reported to depress calcite strongly in preference of fluorite at ambient temperature, leading the fluorite flotation to be achieved at a lower temperature [4]. Molybdenum and fluorite are important materials for modern industry. Therefore, production of fluorite and molybdenum from fluorite ore containing molybdenum are significant. The most important minerals of molybdenum are molybdenite (MoS2), wulpherite (PbMoO4), and molybdite (MoO3). However, for molybdenum production from ores containing molybdenum in the first step, enrichment is required. Therefore, the flotation method was applied. Molybdenum usually occurs as molybdenite in nature and enrichment of molybdenum is much easier in comparison to other ores with high yield. Molybdenum has different constituent in minerals. Therefore, it was determined that flotation yields are low. Moreover, recovery of concentrate of molybdenum and fluorite separately reduce flotation yield [5]. Flotation tests on fluorite ores with various carbonate content (CaCO3 contents from 4.43% to 74.40%) were carried out using acidized sodium silicate as modifier and oleic acid as collector. A high purity fluorite concentrate (99% CaF2) was obtained. It was demonstrated that acidized sodium silicate has an activating effect on fluorite and a selective depressing effect on calcite in rougher flotation, at the same time effectively removing the floated calcite from the rougher concentrate [6]. In this study, we aimed to collect molybdenum and fluorite in separate phases and to increase flotation yield. Therefore, before the flotation, the sulfurization process was made in order Vol.5, No.2 Enrichment of Molybdenum and Fluorite 105 to change the ore structure and surface properties [7, 8]. Sulfurized sample were subjected to flotation and results were evaluated [9]. 2. MATERIALS AND METHOD 2.1. Materials The fluorite ore containing molybdenum used in this study was obtained from Karamağaradere district of Keban-Elazığ, which is in eastern part of Turkey. About 50 kg of mine sample was taken. Analyses showed that the sample contained 1.08 % Mo, 0.05 % Cu, 1.20 % Pb, 1.12 % Zn, 12.0 % F, 0.016 % Ta, 0.20 % Nb and 0.14 % V 98% (w/v) H2SO4, 37% (w/v) HCl, 65% (w/v) HNO3 and KClO3 were purchased from Merck. The K-Amyl xanthate and Aeroflot 65 used in flotation were provided from Cyanamid Co. in the USA. A flame atomic absorption spectrometer (Unicam 929 Model AAS) was used for determination of Mo, Cu, Pb, Zn, Ta, Nb and V concentrations in the solution. The fluor analysis was carried out titrimetrically [10]. Denver Mark and 890 Model pH meter were used for flotation and determination of pH of samples, respectively. The sulfurization process was conducted in an autoclave of 1.3 liter internal volume, resistant to 250 atm pressure and to 350 oC 2.2. Method The mine sample was first crushed and then ground to -100 mesh size, sieved and dried at 110 oC. For sulfurization, 1000 g of sample was sulfurized with gas containing different amounts H2S + H2O in an autoclave for 1 hour at 120 oC. Then, samples obtained from autoclave were floated with potassium amyl xanthate. 3. RESULTS AND DISCUSSION 3.1. Separation of Molybdenum from Fluorite Ore: 3.1.1. Flotation of Original Ore 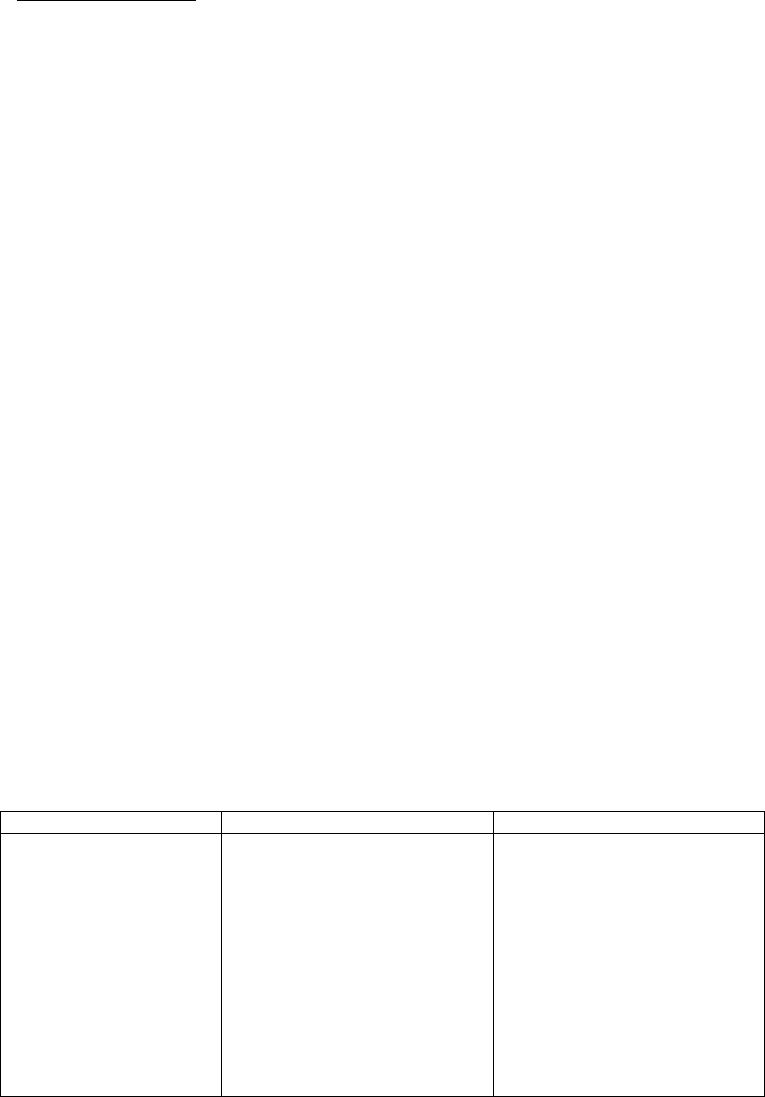 106 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 After sieved to -100 mesh size of the original ore, flotation process was conducted under the conditions indicated blow. Flotation condition: Particle size : - 100 mesh Solid / Liquid Rate : 100 g / L Collector : 0. 2 g Z5 (potassium amyl xanthate), 3 minute mix Frother : 0, 5 mL Aeroflot 65 (1%) 2 minute mix pH : 6.5-9.0 Mix Speed : 900 rpm At the end of flotation process, results aimed were not attained, and flotation yield was low. Approximately 12 % of fluorite passed into the concentrate phase. Since an efficient separation could not be carried out, the experimental results were not given in detail. Thus, original ore was sulfurized in an autoclave before flotation. Then, these samples were floated. 3.1.2. Flotation of Sulfurized Samples Firstly, samples of original fluorite ore containing molybdenum were ground, sieved to - 100 mesh size, dried at 110 oC and then reacted with gas mixtures containing different amounts of H2S + H2O for 1 hour at 120 oC. For this purpose, the six samples each of which was 1000 g, were reacted with mixtures of H2S and H2O separately as seen in Table 1. Table 1. The used amount of gas mixtures of H2S and H2O. Experimental No Amount of H2S (g) Amount of H2O (g) 1 2 3 4 5 6 7.66 11.49 12.44 13.40 15.32 19.44 100.0 60.0 65.0 70.0 80.0 100. 0 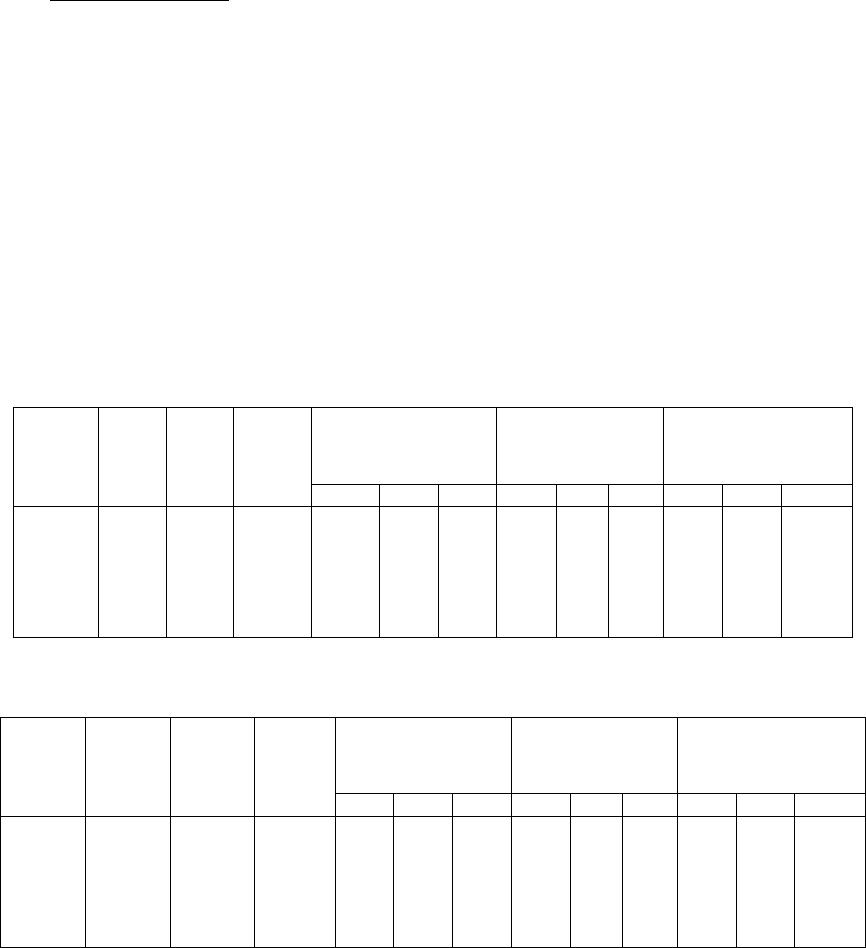 Vol.5, No.2 Enrichment of Molybdenum and Fluorite 107 After sulfurization, the samples were floated under conditions stated below. Flotation condition: Particle size : - 100 mesh Solid / Liquid Rate : 100 g / L Collector : 0. 2 g Z5 (potassium amyl xanthate) 3 Minute mix Frother : 0, 5 mL Aeroflot 65 (1%) 2 Minute mix Mix Speed : 900 rpm The sixth different sulfurized samples were floated under the same conditions. The results are given in Table 2-7. Table 2: The values obtained by flotation of the first sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Mo Pb Cu Mo Pb Cu 3.0 2.0 2.3 2.1 1.5 1.3 6.50 7.05 7.51 8.01 8.53 8.97 33.60 22.19 27.95 23.48 22.33 20.15 65.50 77.40 71.55 75.48 77.16 79.96 96.0 91.7 95.0 92.5 95.2 94.3 93.6 79.6 83.2 74.2 81.0 69.0 53.0 63.2 69.2 85.0 77.0 75.0 3.05 4.45 3.65 4.24 2.60 5.03 3.30 4.29 3.57 3.77 4.33 4.10 0.08 0.14 0.12 0.18 0.17 0.18 0.06 0.11 0.08 0.11 0.07 0.07 0.11 0.16 0.29 0.41 0.30 0.46 0.035 0.030 0.020 0.009 0.014 0.015 Table 3: The values obtained by flotation of the second sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Mo Pb Cu Mo Pb Cu 1.50 1.50 1.15 1.04 1.10 1.10 6.54 6.97 7.54 8.00 8.52 9.02 29.76 19.83 26.59 20.25 20.83 18.49 69.56 79.88 72.93 79.30 78.38 81.34 94.9 91.2 92.3 95.4 91.7 92.2 88.6 68.0 78.0 78.0 69.4 52.0 67.4 74.0 68.5 75.0 65.6 66.0 3.42 4.95 3.72 5.06 4.73 5.38 3.56 4.10 3.50 4.61 3.98 3.36 0.11 0.18 0.12 0.18 0.15 0.18 0.08 0.12 0.11 0.06 0.11 0.10 0.19 0.49 0.36 0.34 0.47 0.71 0.025 0.016 0.022 0.047 0.022 0.021 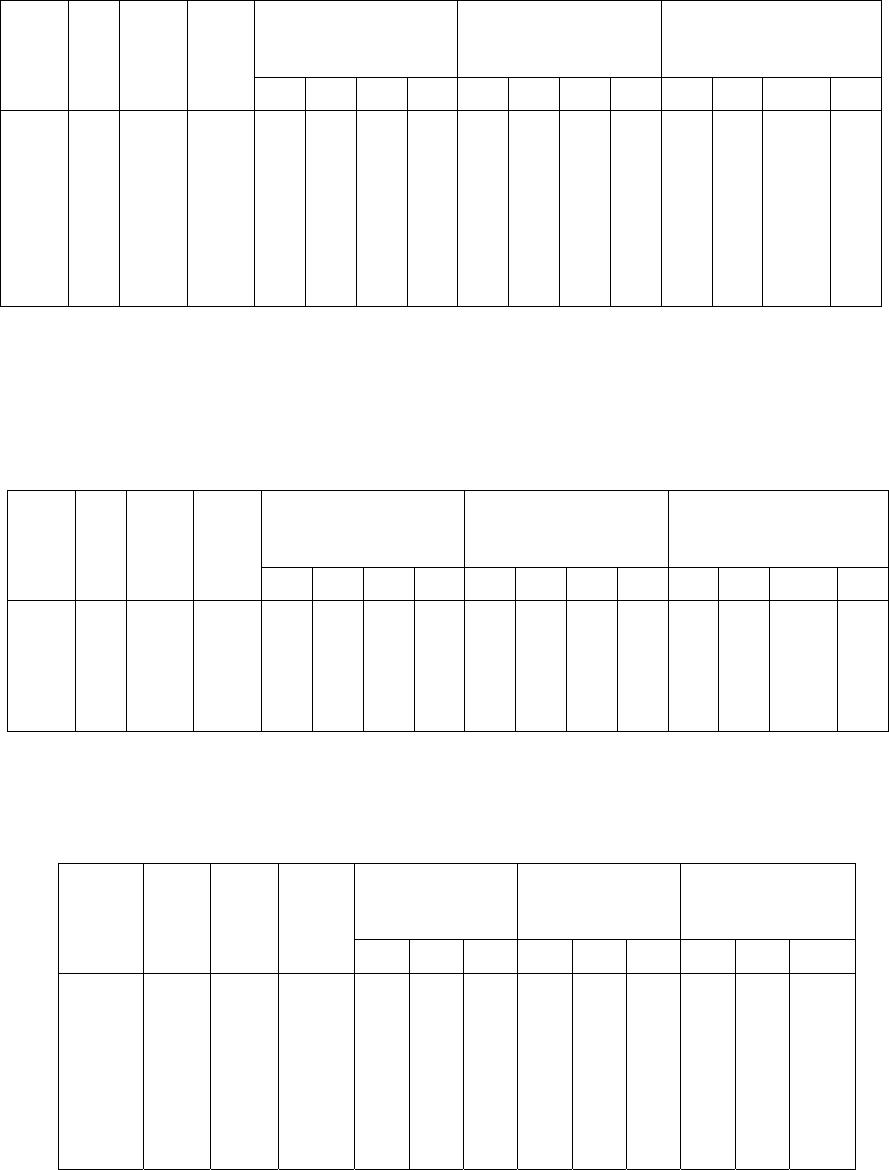 108 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 Table 4: The values obtained by flotation of the third sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 1.20 1.30 1.30 1.30 1.45 1..30 6.51 7.00 7.50 8.00 8.50 9.01 26.27 27.19 26.74 24.80 24.63 20.49 73.16 71.29 72.53 74.49 75.54 79.23 100 98.2 100 88.7 96.3 92.0 87.0 88.4 95.9 79.0 86.0 66.2 87.0 92.3 95.0 88.9 91.5 87.6 52.0 79.0 85.8 43.0 52.0 37.0 4.10 3.90 4.03 3.86 4.21 4.89 3.96 3.90 4.29 3.81 4.17 3.47 0.16 0.17 0.18 0.18 0.19 0.21 2.22 3.24 3.58 1.93 2.35 2.02 - 0.03 - 0.16 0.05 0.11 0.21 0.20 0.06 0.34 0.23 0.66 0.008 0.005 0.003 0.007 0.006 0.007 0.80 0.17 0.22 0.86 0.71 0.88 Table 5: The values obtained by flotation of the fourth sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 1.15 1.20 1.15 1..20 2.00 2.05 6.53 6.97 7.55 8.00 8.55 9.02 28.31 24.84 29.79 27.79 31.74 30.77 71.12 75.24 70.63 71.72 67.70 69.50 95.0 95.3 96.0 99.0 94.4 100 78.0 79.0 80.0 90.0 81.0 95.0 86.5 88.0 90. 88.5 86,0 95.0 52.5 76.5 59.0 65.0 48.0 52.3 3.61 4.13 3.47 3.83 3.20 3.50 3.31 3.80 3.21 3.87 3.05 3.69 0.15 0.18 0.15 0.16 0.14 0.15 2.06 3.44 3.38 2.60 1.69 1.90 0.08 0.07 0.06 0.02 0.09 - 0.38 0.35 0.34 0.17 0.34 0.01 0.009 0.007 0.007 0.008 0.010 0.003 0.75 0.35 0.64 0.55 0.86 0.77 Table 6: The values obtained by flotation of the fifth sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Mo Pb Cu Mo Pb Cu 3.0 2.0 2.10 1.5 2.1 2.36 6.51 7.01 7.51 8.00 8.49 9.0 12.04 12.23 12.45 13.33 12.21 13.69 86.45 87.44 87.11 86.78 87.31 86.28 88.5 91.0 90.7 94.4 84.0 89.8 72.0 74.0 74.4 76.0 78.0 81.0 71.0 83.0 81.6 79.6 72.0 74.8 7.92 8.02 7.85 7.63 7.41 6.79 7.16 7.25 7.16 6.83 7.65 7.09 0.28 0.33 0.32 0.29 0.30 0.26 0.15 0.12 0.10 0.07 0.20 0.13 0.39 0.36 0.35 0.33 0.31 0.25 0.017 0.010 0.011 0.012 0.016 0.014 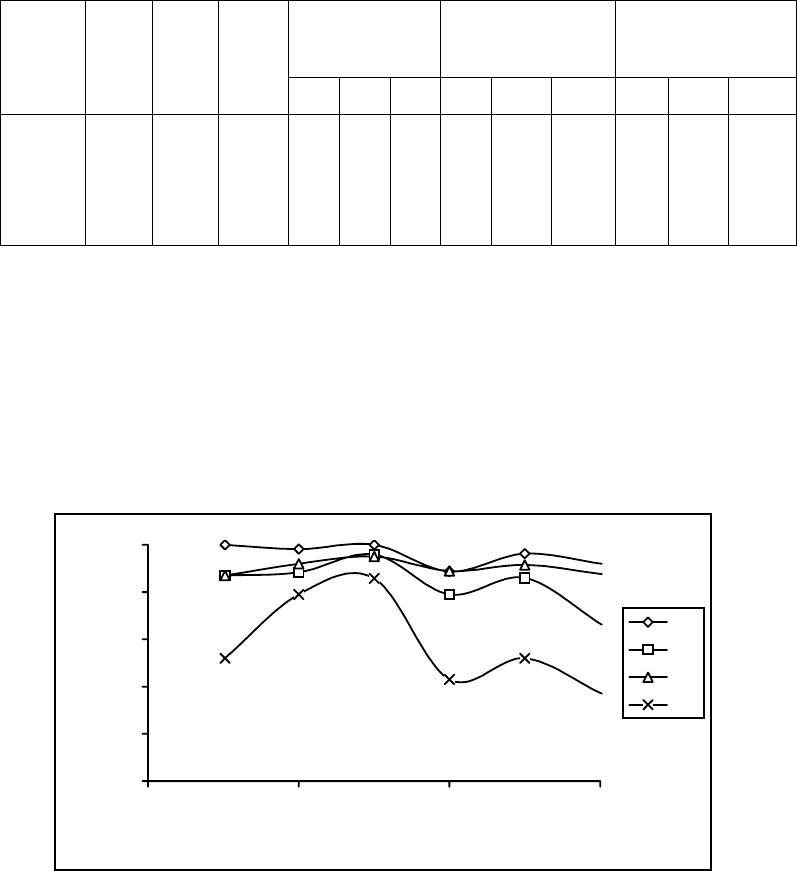 Vol.5, No.2 Enrichment of Molybdenum and Fluorite 109 Table 7: The values obtained by flotation of the sixth sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Mo Pb Cu Mo Pb Cu 2.0 4.0 5.0 7.2 7.00 7.02 7.00 7.01 29.21 33.33 70.40 90.66 69.69 215.34 228.43 257.22 89.3 91.4 89.8 90.6 73,0 82.1 91.7 95.0 80.0 88.4 83.5 85.7 3.29 7.40 4.13 3.78 2.98 7.37 4.68 4.40 0.13 0.33 0.17 0.16 0.16 0.10 0.15 0.14 0.46 0.25 0.13 0.08 0.014 0.007 0.011 0.010 As seen in tables, the best result was obtained from third sulfurized sample by flotation. In order to understand these results better, the results were plotted in Figure 1 with respect to different pH values. 0 20 40 60 80 100 6789 pH Flotation yield (%) Mo Pb Cu Zn Figure 1: The effect of pH on the flotation yield As seen in Figure 1, the best of flotation yield was at pH 7.5. Thus, six different sulfurized samples were floated at this pH and the results are given in Figure 2. 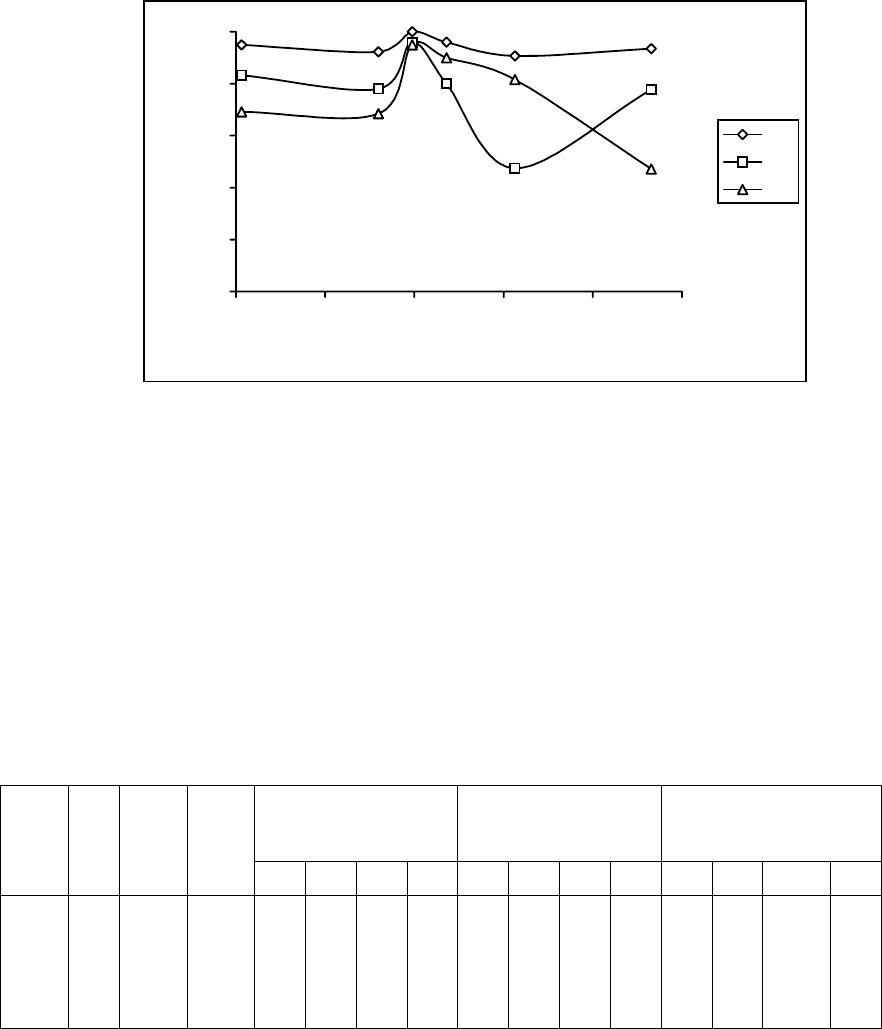 110 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 0 20 40 60 80 100 7,510 12,5 15 17,5 20 H2S (g) Flotation yield (%) Mo Pb Cu Figure 2: The effect of sulfurization at pH 7.5 on the flotation yield of sulfurized samples When Figure 2 is examined, it can be seen that the highest flotation yield was achieved by flotation of third sulfurized samples. In the next step, flotation process was conducted to test the effect of pulp density and results are given in Table 8. Table 8: The effect of pulp density on the floatability of results from third sulfurized sample Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 2.40 3.50 3.60 3.75 7.50 7.51 7.52 7.50 25.73 61.84 67.04 83.33 73.52 188.73 232.23 266.20 97.4 96.4 97.6 94.6 94.6 93.0 94.0 92.0 94.0 94.0 .95.4 85.6 86.0 85.5 89.0 82.0 4.08 4.20 4.71 4.29 4.41 4.50 5.03 4.62 0.18 0.19 0.21 0.17 3.74 3.86 4.45 3.86 0.04 0.05 0.03 0.08 0.06 0.11 0.09 0.13 0.004 0.003 0.004 0.003 0.21 0.22 0.16 0.27 When Table 8 is examined, it can be seen that there is not a significant difference in flotation yield as pulp density changes. However, the results are better when solid/liquid ratio was done at 300 g ore / 1 L 3.1.3. The effect of the activator and the depressant on the flotation yield 3.1.3.1. The effect of the activator:  Vol.5, No.2 Enrichment of Molybdenum and Fluorite 111 The results of the flotation in which CuSO4 was used as activator are given in Table 9. Table 9: The values obtained from flotation of third sulfurized sample using activator (CuSO4) As seen in Table 9, floated amount of sample was too much. Moreover, in order to investigate whether flotation yield could be increase more, a series of experiments were carried out by using depressant (Na2SiO3) as well as activator. 3.1.3.2. The effect of the activator and depressant together: In these series of experiments, flotation studies were conducted by using CuSO4 and Na2SiO3 and results obtained are given in Table 10. Table 10: The values obtained from flotation of third sulfurized sample using the activator and the depressant together at pH 7.5 Note: 1 mL solution contains 5.10-3 g Na2SiO3. * Conditions under which the best results were obtained for Mo, Cu, Pb and Zn recovery Recovery (%) Concentrate Assay (%) Unfloated Assay (%) CuSO4 (mg) Time Min pH Froth (g) Tail (g) MoPb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 5.0 10.0 10.0 1.50 1.20 1.20 7.50 7.52 8.50 33.00 38.83 31.83 66.32 59.92 67.98 100 100 100 87.0 84.0 90.0 80.0 99.0 95.0 60.0 92.0 72.0 3.27 2.78 3.05 3.16 2.59 3.38 0.12 0.12 0.15 2.03 2.65 2.52 - - - 0.24 0.33 0.18 0.015 0.001 0.004 0.68 0.15 0.46 Recovery (%) Concentrate Assay (%) Unfloated Assay (%) CuSO4 (mg) Na2Si O3 (mL) Time (Min) Froth (g) Tail (g) Mo Pb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 5.0 15.0 20.0 20.0 20.0 25.0 30.0* 35.0 0.5 1.0 1.50 1.0 0.5 1.0 1.0 1.0 1.30 2.10 2.20 2.30 2.20 1.50 1.40 1.30 29.85 19.75 19.56 19.94 22.74 24.90 23.47 23.85 70.29 79.66 79.91 79.43 78.27 74.64 75.92 75.49 100 95.0 91.0 96.0 89.0 96.3 97.3 96.3 87.0 80.0 66.0 67.0 73.0 92.0 93.0 86.0 94.0 84.0 55.0 76.0 72.0 95.6 98.0 100 66.0 78.0 67.0 69.0 65.0 89.0 95.2 66.2 3.61 5.18 5.01 5.18 4.21 4.16 4.46 4.35 3.48 4.85 4.03 4.02 3.84 4.42 4.74 4.31 0.15 0.21 0.14 0.19 0.15 0.19 0.21 0.21 2.46 4.41 3.82 3.86 3.20 4.00 4.53 3.10 - 0.07 0.13 0.05 0.15 0.05 0.04 0.05 0.23 0.31 0.52 0.50 0.42 0.13 0.11 0.22 0.004 0.010 0.028 0.015 0.018 0.003 0.001 - 0.55 0.31 0.52 0.44 0.51 0.17 0.07 0.50  112 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 As seen in Table 10, although molybdenum yield decreased a bit, yields of other elements increased considerably. Particularly, the optimum results were obtained when 30.0 mg CuSO4 and 1.0 mL Na2SiO3 were used in the flotation. In addition, amount of sample floated by depressant fairly decreased and 94 % of fluorite was depressed (not shown in Table). The results obtained from flotation of the samples at up to 30 % solid/liquid ratio as the pulp density changed were similar (not shown in Table). Additionally, this concentrate phase (flotation carried out with 30 mg CuSO4) contained 0.05 % Ta, 0.68 % Nb and 0.41 % V and was produced with recoveries of 76 % Ta, 84.4 % Nb and 76.2 % V by flotation. Separation of Mo and Cu from Pb and Zn in the Concentrate The concentrate phase (flotation carried out with 30 mg CuSO4) containing 4.46 % Mo, 4.74 % Pb, 0.21 % Cu, 4.53 % Zn, 0.05 % Ta, 0.68 % Nb and 0.41 %V was used in this process. In order to separate Mo and Cu from Pb and Zn, the experiments were conducted with respect to pH. Thus, these experiments were performed with -100 mesh size and -160 mesh sizes, and pH 11 to pH 13. Results obtained are given in Table 11. Table 11: The values obtained by flotation of the collective concentrate -100 mesh size Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tail (g) Mo Pb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 2.50 2.30 2.50 2.50 2.50 3.00 11.0 11.50 12.00 12.50 12.75 13.00 78.20 62.34 50.79 45.26 38.59 31.31 21.55 37.71 48.97 53.43 61.04 69.87 98.0 95.5 90.0 98.0 93.0 97.3 97.6 94.0 77.6 57.0 47.5 31.6 98.0 96.7 93.9 65.8 74.0 84.0 87.7 75.8 60.5 55.0 25.4 30.0 5.58 6.82 7.90 9.65 10.74 13.85 5.90 7.14 7.23 5.96 5.82 4.77 0.26 0.32 0.38 0.30 0.40 0.56 5.07 5.50 5.39 5.49 2.98 4.33 0.42 0.53 0.91 0.17 0.51 0.17 0.53 0.75 3.14 3.82 4.08 4.64 0.019 0.018 0.026 0.134 0.089 0.048 2.59 2.90 3.65 3.82 5.54 4.54 -160 mesh size 3.00 13.0 33.46 66.98 84.6 31.368.434.411.27 4.410.434.65 1.03 4.86 0.1244.97 As seen in Table 11, the best separation was performed at pH 13 and with -100 mesh particle size. However, in order to obtain better flotation results, -100 mesh size samples were sulfurized again. For resulfurization, two samples were prepared in two different autoclave mediums: Sample A containing 0.96 g H2S + 5 g H2O steam for 500 g of sample and Sample B containing 1.92 g H2S+ 10 g H2O for 500 g of sample. Additionally, -200 mesh size samples were  Vol.5, No.2 Enrichment of Molybdenum and Fluorite 113 sulfurized under the same conditions. The best results were obtained from the flotation of the samples of -100 mesh size sulfurized in a medium containing 0.96 g H2S + 5 g H2O steam for 500 g of sample and are given in Table 12. Table 12: The values obtained by flotation of resulfurized collective concentrate Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) pH Froth (g) Tailing (g) Mo Pb Cu Zn Mo Pb Cu Zn Mo Pb Cu Zn 3.0 2.5 2.30 12.00 12.50 13.00 74.06 36.05 22.45 25.88 64.68 77.69 97.6 96.0 98.0 94.0 64.0 12.6 97.0 88.0 94.0 83.0 44.0 22.5 5.87 11.87 19.46 6.01 8.40 2.65 0.27 0.51 0.87 5.06 5.51 4.54 0.41 0.28 0.11 1.10 2.64 5.33 0.024 0.038 0.016 2.98 3.92 4.52 As seen in Table 12, most of Mo and Cu were floated at pH 13, while most of Pb and Zn were depressed. Then, Mo, Cu, Ta, Nb and V were recovered from this concentrate. For this process, these elements were separated by a hydrometallurgical method (9). Moreover, the flotation yields of Ta, Nb and V, which were not given in Table 12, were found as 86 %, 91 % and 89 %, respectively and their concentrate grades of Ta, Nb and V were ascertained as 0.16 % Ta, 2.43 % Nb and 1.49 % V. In the next step, the sample was floated to test the effects of pulp density. As results given in Table 12, the best of yield was obtained with 10 % solid/liquid ratio. Flotation of Fluorite in the Tailing Tailing of first step flotation (collective flotation) contained 14.4 % F (29.6 % CaF2). It was tried to enrich fluorite by flotation of this sample. For this aim, the effect of some parameters such as pH, amount of the collector, the activator and the depressant were examined. Results obtained under flotation conditions stated below are given in Table 13. Flotation condition: Particle size : - 100 mesh Solid / Liquid Rate : 100 g / L Collector : Na-Oleate (5.10-2 g/mL) 3 minute mix  114 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 Activator : Al2(SO4)3 (5.10-2 g/mL) Suppressor : Na2SiO3 (5.10-3 g /mL) Mix Speed : 900 rpm Table 13: The values obtained from the flotation performed for enrichment of fluorite Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Time (Min) Al2(SO4)3 (mL) Na2SiO3 (mL) Na oleat (mL) pH Froth (g) Tailing (g) F F CaF2 F CaF2 1.0 1.0 1.10 1.20 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 7.5 8.0 8.5 9.0 24.77 16.81 19.20 24.39 74.49 82.48 80.53 75.03 78.0 44.4 53.2 71.3 45.35 38.03 39.90 42.10 93.21 78.18 82.02 86.53 4.2 9.7 7.3 5.5 8.7 19.9 15.7 11.3 *1.0 mL Al2(SO4)3 = 5.10-2 g , 1.0 mL Na2SiO3 =5.10-3 g, 1.0 mL Na-Oleate = 5.10-2 g As seen in Table 13, the highest flotation yield was at pH 7.5. However, since these results were not satisfactory, the sample was floated with respect to the amounts of the activator, the depressant and the collector and the results obtained are given in Table 14. Table 14: The values obtained from the flotation performed with respect to the activator, depressant and collector Recovery (%) Concentrate Assay (%) Unfloated Assay (%) Al2(SO4)3 Na2SiO3 Na oleate (mL) Time (Min) pH Froth (g) Tailing (g) F F CaF2 F CaF2 - 1.0 1.0 1.50 7.5 29.03 70.40 94.0 46.63 95.85 1.2 2.5 1.0 1.0 2.0 2.0 7.50 32.39 66.48 95.0 42.24 86.82 1.1 2.2 1.0 1.0 1.5 1.5 8.0 15.77 83.57 48.0 43.83 90.10 8.9 18.4 1.0 1.0 2.0 2.0 8.0 35.61 63.76 94.5 38.21 78.55 1.2 2.6 1.0 0.5 1.0 1.45 7.5 23.51 76.90 73.4 44.96 92.41 5.0 10.2 1.0 0.5 1.0 2.0 8.0 30.23 68.96 94.0 44.78 92.04 1.3 2.6 Vol.5, No.2 Enrichment of Molybdenum and Fluorite 115 As seen in Table 14, the highest flotation yield and in concentrate grade of CaF2 were obtained by the flotation conducted in a medium containing 1.0 mL Na2SiO3 and 1.0 mL Na- oleate. Thus, these experiments were repeated three times and the similar results were obtained. Then, the flotation studies were performed by changing the amounts of Na2SiO3 and Na- oleate. The best result was obtained from the flotation in which 1.0 mL Na2SiO3 and 1.0 mL Na- oleate was used at pH 7.5 and it was determined that grade of sulfur was 0.5 %. CONCLUSIONS The finding of processing of fluorite ore containing molybdenum are as follows. • Collecting molybdenum and fluorite in different phases by direct flotation was not possible, and the flotation yield was low • Since molybdenum and other minerals bound to gang minerals, phase separation was not possible. Liberation with respect to particle size was unsatisfactory and this resulted in low flotation yield. • In order to achieve an efficient separation by flotation, fluorite ore containing molybdenum was sulfurized to change its structure and surface. Thus, the sample was sulfurized in a medium containing different amounts of H2S and H2O steam. 12.44 g H2S + 65 g H2O gas mixture was enough for sulfurization one kg of sieved ore. The sulfurization was finished in 1 hour in the autoclave for at 120 oC. • The minerals containing fluorite and molybdenum were separated with high yields by flotation of sulfurized sample. At the end of procedure, 94 % of fluorite was remained at the tailing. Beside of Molybdenum, Pb, Zn, Cu, Ta, Nb and V also passed into concentrate phase with high yields. After the collective flotation, concentrate phase contained 4.46 % Mo, 4.74 % Pb, 4.53 % Zn, 0.21 % Cu, 0.05 % Ta, 0.68 % Nb and 0.41 % V. In the flotation performed with respect to pulp density, the flotation yield was remained at the same levels with the samples consisted of up to 30 % solid/liquid ratio. Additionally, the flotation yield was increased when Na2SiO3 was used as depressant and CuSO4 was used as activator. • In order to increase the grade of Mo, Cu, Ta, Nb and V from Zn and Pb, the flotation process was repeated. Flotation was performed at higher pH and optimum pH was found as 13. However, it was decided to repeat sulfurization of concentrate since flotation yield was not satisfactory. 116 Akgün, Teğin, and Ziyadanoğulları Vol.5, No.2 • 0.96 g H2S + 5 g H2O gas mixtures were enough for the sulfurization of 500 g sample. After the flotation, the concentrate grades of Mo, Pb, Cu, Zn, Ta, Nb and V were found as 19.46 %, 2.65 %, 0.87 %, 4.54 %, 0.16 %, 2.43 %, and 1.49 % and flotation yields were determined as 98 %, 12.6 %, 94 %, 22.5 %, 86 %, 94 % and 82.2 %, respectively. So, while Mo, Cu, Ta, Nb and V was collected in the concentrate phase, Pb and Zn passed into the tailing. The concentrate was enriched approximately 10 times with respect to the original ore. • It was ascertained that the tailing of collective flotation contained 14.4 % F (29.6% CaF2). This sample was refloated for enrichment. The effect of the parameters such as pH, activator, depressant and collector were investigated. The highest flotation yield was at pH 7.5. Using Al2(SO4)3 as activator did not increased the flotation yield significantly. On the contrary, in the flotation in which Na2SiO3 and Na oleate was used, concentrate grade of CaF2 raised to 96 % and flotation yield was 94 %. Additionally, it was determined that the concentrate contained 0.5 % S. REFERENCES 1. De Leeuw, N.H., Parker, S.C., Rao, K.H., Modeling the competitive adsorption of water and methanoic acid on calcite and fluorite surfaces. Langmuir 14, 5900–5906 (1998). 2. Helbig, C., Baldauf, H., Mahnke, J., Stochelhuber, K.W., Schulze, H.J., Investigation of Langmuir monofilms and flotation experiments with anionic/cationic collector mixtures. International Journal of Mineral Processing 53, 135–144 (1998). 3. Helbig, C., Baldauf, H., Lange, T., Newmann, R., Pollex, R., Weber, E., New binary collectors with increased .otation Tenside Surfactants Detergents 36, 58–62 (1999). 4. Zhou, Q., Lu, S., Acidized sodium silicate an effective modifier in .fluorite flotation, Minerals Engineering 5, 435–444 (1992). 5. MTA Report no 76, project no 80-45, Turkey (1981) 6. Qiang Zhou and Shouci Lu, Acidized sodium silicate an effective modifier in fluorite flotation Minerals Engineering Volume 5, Issues 3-5 , March-May, Pages 435-444 (1992) 7. Aydın I., Aydın F., Ziyadanoğulları R., Enrichment of U, Mo, V, Ni and Ti from asphaltite ash, Journal of Minerals & Materials Characterization & Engineering, Vol. 4, No.1, pp 1-10 (2005) Vol.5, No.2 Enrichment of Molybdenum and Fluorite 117 8. Ziyadanoğulları R., Aydın F., A New Application For Flotation Of Oxidized Copper Ore, Journal of Minerals & Materials Characterization & Engineering, Vol. 4, No. 2, pp 67-73 (2005) 9. Akgün A., Recovery of precious elements from fluorite ore, October, pHD Thesis, Dicle University, Diyarbakir-TURKEY (2002). 10. Vogel A.I., A text book of quantitative inorganic analysis, 3 rd Edn (EIBS Longman, London), 356 (1971). |

