Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.1, pp 33-39, 2004 jmmce.org P rin ted in th e U SA. Al l r igh ts res erv ed Column Leaching for Simulating Heap and In-situ Soil Remediation with Metallic Fenton Reaction. H. K. Lin Mineral Industry Research Laboratory, University of Alaska Fairbanks and H. V. Luong Chemistry Department, Seattle University Metallic Fenton reaction was found to be more effective than classical Fenton reaction in decomposing Pentachlorophenol (PCP) in contaminated soil. The combination of metallic iron and hydrogen peroxide was used in column leaching experiments to simulate heap and in-situ soil remediation. PCP in the contaminated soil was effectively decomposed by 32% in 24 hours leaching tests, and by 41% in 48 hours leaching experiments. PCP destruction was further increased to 52% in the 48 hours leaching by lowering the solution pH to 1.8. Other than ferric oxide and carbon dioxide, no byproducts were found at the end of the reaction. Keywords: Soil remediation, PCP, PCB, iron, hydrogen peroxide, in-situ remediation, heap remediation, metallic Fenton reaction INTRODUCTION Soil contaminated with hazardous organic compounds is currently one of the greatest environmental problems facing many countries around the world. Many of these compounds, especially the chlorinated organics, are toxic to humans and some are known carcinogens. One of the most effective methods of remediation for contaminated soils, approved by the United States Environmental Protection Agency, is based on high temperature incineration. High temperature incineration requires high energy consumptions and thus is a costly solution. In addition, incineration facilities are usually non-mobile, which results in additional expenses, mainly due to soil excavation and transportation. Alternative methods of remediation have been developed over the years. One such method is utilizing elemental metal and oxidant in an agitating reactor. This method was shown to be effective in decomposing pentachlorophenol (PCP) and polychlorinated biphenyls (PCBs) in contaminated soil (Luong and Lin, 1999; Luong and Lin, 2000). This paper will present the possibility of combining the metallic Fenton reaction technology with heap and in-situ leaching, two methods widely used around the world for extracting minerals. By combining mineral engineering methods with chemical remediation technology, we hope to enhance this technology further. This type of idea was suggested and investigated for soil remediation earlier in the latter part of the 20th century (Heil et al., 1996, York and Aamodt, 1990). However, with the exception of bioremediation, the idea of combining these two technologies has not been widely adopted. Heap leaching and in-situ leaching have been employed in the mineral industry for many years. In the mid-sixteenth century, mines in Hungary recycled copper-bearing solutions through waste heaps to recover copper (Hiskey, 1985). In Spain, miners circulated acid solutions through large heaps of oxide copper ore on the banks of Rio Tinto around 1752 (Dorey et al., 1988). Currently, leaching of copper dumps is practiced worldwide for low grade ores. Heap leaching 34 H. K. Lin and H. V. Luong Vol. 3, No. 1 of gold and silver also has been done in the U.S. since 1967, with the U. S. Bureau of Mines as a pioneer (Thorstad, 1987). In the case of in-situ leaching, no excavation is needed, and the process of leaching is done at the site. This method has been tested for uranium recovery since 1975 (Taylor, 1979). The leaching solution is injected into the ore formation to dissolve uranium. The uranium-bearing pregnant solution can then be recovered through production wells. Uranium is subsequently extracted from the pregnant solution and the barren solution is then re-injected into the ore formation for further dissolution of uranium. In-situ leaching has also been employed in coal and sulfur industry for many years. EXPERIMENTAL PCP-contaminated soil used in this study was obtained from an abandoned fence post treatment facility at Point McKenzy, Alaska. Soil samples were air-dried in the fume hood before treatment. All chemicals used were of analytical grade. PCP analysis was performed on an HP5890 gas chromatograph equipped with a 30 meter HP- 5MS capillary GC column. An HP 5971 mass spectrometer (MS) was used as the detector to identify PCP and its byproducts. The GC injection port was set at 250oC. The initial oven temperature was at 80oC. After 1 minute, it was raised to 250oC and maintained there for one minute, at a rate of 6 oC/minute. For the mass spectrometer, the interface was set at 280oC. Both scanning method and specific ion method (SIM) were used in this study. In the case of SIM, the instrument was set to scan for ions 266, 230, 202, 165, and 132. To extract PCP from soil, solid phase microextraction (SPME) was used prior to GC/MS analysis. An 85-micrometer polyacrylate fiber assembly from Supelco was used in the procedure. For each extraction, 1 gram of dried soil and 10 ml of pH 2.5 solution were added to a vial. A magnetic stirring bar was then added, and the polyacrylate fiber of the SPME assemblies was then exposed to the liquid for 25 minutes. The fiber was then withdrawn for GC/MS injection. A two minutes time injection was employed in the injection process. For column experiments, air-dried soil was first passed through a 1.25 cm sieve . All column-leaching tests were conducted using a 3.8 cm diameter glass column, with a length of 61 cm. Solid reagents (metallic iron or ferrous sulfate powder) were mixed with the soil material before loaded into the column. The soil bed was about 50 cm in height. Sulfuric acid was used to adjust the solution pH initially. At the beginning of each test, a 23oC solution was pumped into the top of the soil bed. Effluent was discharged from the bottom of the soil bed. Percolation rate was controlled at about 0.35 liter per square meter per minute. At the completion of the leaching, the soil material was rinsed with a 2.0 % lime solution until the solution pH reached 6.5. The treated soil was air-dried followed by GC/MS analysis procedure for PCP and its byproducts. Selection of reagents for the column leaching was based on the results of the agitation leaching tests. Agitation leaching was performed in a conventional one-liter glass reactor equipped with a glass impeller. The impeller was driven by a motor and the stirring speed was controlled and monitored with a tachometer. The reactor system has been detailed elsewhere (Man, 1997). Soil particles larger than 0.15 mm were removed by sieving. Only the minus 0.15 mm soil sample was used for the leaching tests. The soil sample was found to contain a PCP concentration of 195 mg/kg. An aqueous solution of 200 ml and 50 g of the soil were used to form a slurry for the leaching tests. The tests were conducted at 23oC with the slurry agitated by the impeller 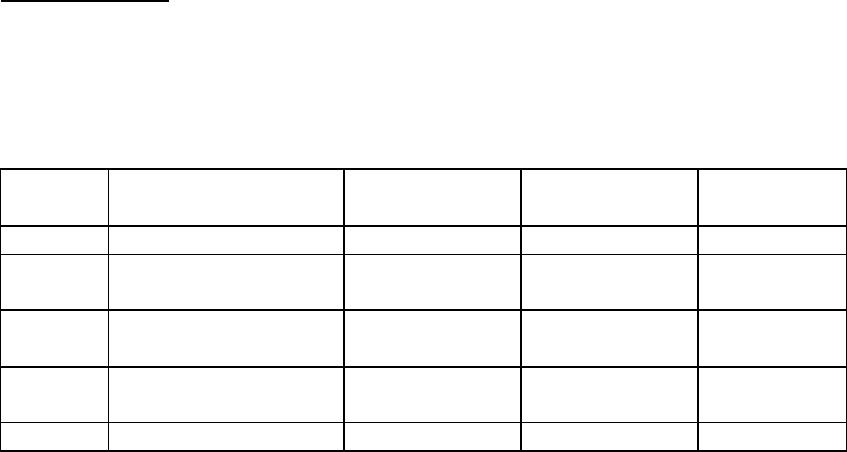 Vo1. 3, No. 1 Simulating Heap and In-situ Soil Remediation with Metallic Fenton Reaction 35 operated at 200 revolutions per minute. Sulfuric acid was used to adjust solution pH. Carbon dioxide analyses were conducted on a MicroGC (Model P200H, from MTI Analytical Co.). RESULTS AND DISCUSSION Agitation Leaching Agitation leaching tests were conducted to examine the effectiveness of various reagents on the destruction of PCP in contaminated soil. Table 1. Destruction of PCP in the contaminated soil by agitation leaching. Test No. Reagents Reaction Time Initial solution pH % PCP Destruction 1 H2O2: 2.5 mol/l 1.0 hr 2.7 <2 2 H2O2: 2.5 mol/l FeCl3: 0.5mol/l 1.0 hr 1.4 <2 3 H2O2: 2.5 mol/l FeCl2: 0.5 mol/l 1.0 hr 2.7 <2 4 H2O2:2.5 mol/l Fe: 0.5 mol/l 1.0 hr 2.7 >98 5 Fe: 0.5 mol/l 1.0 hr 2.7 <2 From Table 1, it is clear that metallic iron alone or hydrogen peroxide alone is ineffective in destroying PCP. It is also clear that a combination of hydrogen peroxide with either Fe+2 or Fe+3 provides negligible PCP destruction. However, hydrogen peroxide and metallic iron (Test No. 4) is a very effective combination for PCP destruction. The Fenton reaction (Test No. 3, H 2O2 with Fe+2) has long been proposed to produce reactive hydroxyl radical, which is a very effective agent for breaking down organic compounds (Voelker and Sulzberger, 1996; Chen and Pignatello, 1997; Barbeni et al., 1987). It has a very rapid and non-selective mechanism for organic destruction (Pignatello, 1992). However, the characteristics of this rapid reaction may render it ineffective in the degradation of some organic compounds. For example, PCP and PCB were shown to be resistant to the Fenton reaction (Luong and Lin 1999). In many situations, it is more advantageous to delay the reaction, to allow more time for effective interaction between all the chemical species involved. One way of delaying the reaction is to effectively introduce ferrous ions into the matrix. In this sense, the combination of metallic iron and hydrogen peroxide (Test No. 4) serves that purpose. The dissolution of metallic iron provides the mechanism for the slow but continuous release of ferrous ions. In the presence of hydrogen peroxide, a long-lasting Fenton reaction is achieved. In Test No. 4, no hydrocarbon byproducts were detected in the soil or in the end solution. Carbon dioxide was suspected to be the predominant carbon product of the reaction. To confirm this, a solution of PCP was subjected to a metallic Fenton reaction. The reactor was connected to an evacuated Teflon gas sampling bag. Gas analysis of the bag indicated a significant amount of carbon dioxide was released during the reaction. However, no satisfactory mass balance of the carbon was obtained. A further study in this aspect has been considered. 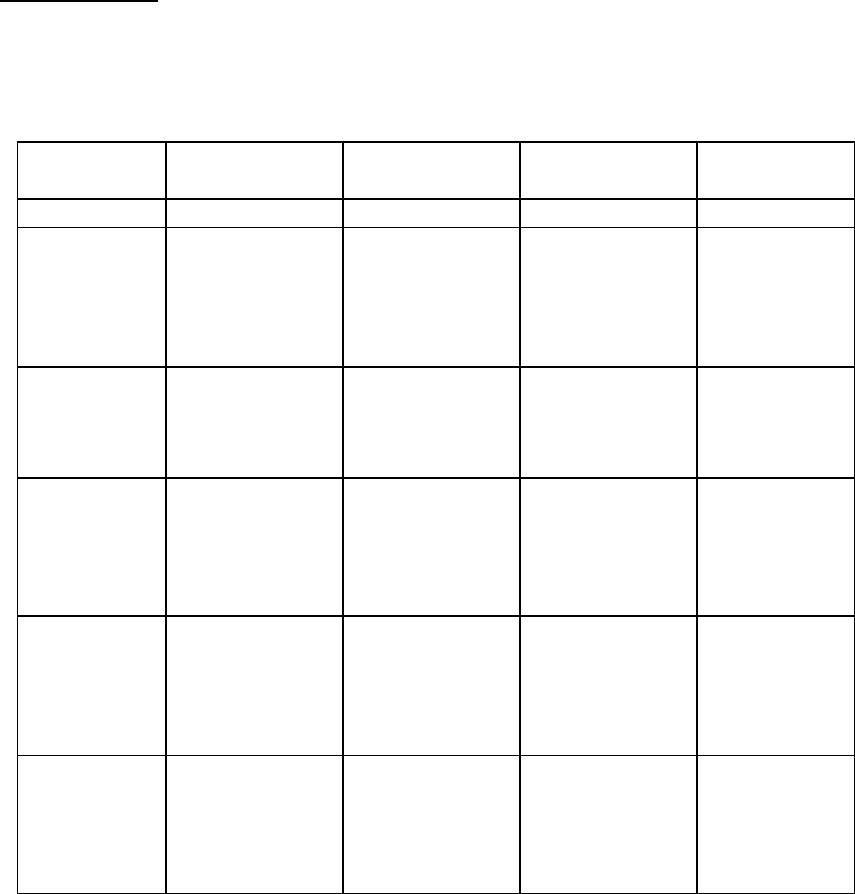 36 H. K. Lin and H. V. Luong Vol. 3, No. 1 Column Leaching Column leaching was done on minus 1.25 cm soil material, which has a PCP concentration of 132 mg/kg. Table 2. PCP destruction in the contaminated soil by column leaching. Test No. Reagents Solution pH Reaction Time % PCP Destruction 1 3.0% H2O2 2.0 24 hr Less than 2 2 2.8% of ferrous sulfate powder in the soil, 3.0% H 2O2 solution 2.0 24 hr Less than 2 3 1.0% of metallic iron powder in the soil 2.0 24 hr Less than 2 4 1.0% of metallic iron powder in the soil, 3.0% H 2O2 solution 2.0 24 hr 32 5 1.0% of metallic iron powder in the soil, 3.0% H 2O2 solution 2.0 48 hr 41 6 1.0% of metallic iron powder in the soil, 3.0% H 2O2 solution 1.8 48 hr 52 The results in Table 2 show that for 24 hours experiments, reagents used individually only provided negligible PCP destruction. The same result was found for the classical Fenton system (Test No. 2). The best result comes from the combination of metallic iron and hydrogen peroxide. Test No 4 shows that this system destroyed 32% of the PCP in 24 hours period. When the test was extended to 48 hours, PCP destruction increased to 41%. To optimize the system further, we lowered the pH to 1.8. At this pH, the PCP destruction increased to 52%. This result supports the idea that metallic iron dissolves in low pH solution and provides ferrous ions to the system (Luong and Lin 2000). In this sense, the use of metallic iron as a source of ferrous ions is advantageous, because it can be controlled by adjusting the pH of the leaching solution.  Vo1. 3, No. 1 Simulating Heap and In-situ Soil Remediation with Metallic Fenton Reaction 37 Soil Properties It is important to stress that while the physical properties of the soil are less critical in the agitating leaching, they are important factors for a successful application of heap and in situ leaching techniques. For example, an excessive amount of clay minerals in the soil can cause the soil bed to be less permeable and reduces the percolation of the leaching solution. Without proper percolation, the over all remediation process may not be successful. Another point of caution is the formation of iron hydroxide, which depends heavily on the pH of the leaching solution. This formation is a characteristic of the Fenton reaction, since ferric ion is continuously formed during the process. Iron hydroxide precipitate usually is very fine, and like clay minerals, can cause percolation problems. As a result, soil containing high amount of acid consuming matrix such as limestone or calcite, requires the use of lower pH leaching solution than normal soil. Heap Remediation In the mineral industry, agglomeration is often employed during the heap construction to prevent the percolation problems. It can also be applied to soil heap remediation to ensure the effectiveness of the heap leaching. The schematic diagram of soil heap remediation is shown in Figure 1. pad Figure 1. Schematic diagram of heap remediation. pH neutralizing solution Contaminant-removed soil h ea p Remediated and ne utraliz ed so il he ap Soil heap Acidic solution with hydrogen peroxide Sol u t i o n c o l l e c t i o n p o n d pH a d j us t m e n t solution Hydrogen peroxide 38 H. K. Lin and H. V. Luong Vol. 3, No. 1 Shredded iron scrap can be blended into the soil heap during heap construction. This will provide the necessary metallic iron source for the process. Acidic solution of hydrogen peroxide can be sprayed on the top of the heap. The solution percolating through the bottom of the soil heap will be collected in a solution pond. The solution will be reused for spraying on the top of the soil bed after pH and hydrogen peroxide adjustments. In the case of in-situ remediation, recovery wells may be needed to recover leaching solution from the bottom of the soil beds. CONCLUSION The combination of metallic iron with hydrogen peroxide works well for degrading hazardous organic compounds. The process may be considered as a controlled Fenton reaction. Column leaching experiments have shown that this system is effective in degrading PCP, and it may be feasible in heap soil remediation. Given the fact that percolation is an important process in heap leaching, soil matrix is an important part in the design of the overall heap remediation set up. REFERENCES Barbeni, M., Minero, C. and Pelizzetti, E., 1987, “Chemical degradation of chlorophenols with Fenton’s reagent”, Chemosphere, vol. 16, no.(10-12), pp2225-2237. Chen, R. and Pignatello, J. J., 1997, “Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidations of aromatic compounds”, Environmental Science and Technology, vol. 31, pp2399-2406. Dorey, R., van Zyl, D. and Kiel, J., 1988, “Overview of heap leaching technology”, in Introduction to Evaluation, Design and Operation of Precious Metal Heap Leaching Projects, edited by D. J. A. van Zyl, I. P. G. Hutchison and J. E. Kiel, Society of Mining Engineers Inc., pp3-22. Heil, D., Hanson A. and Samani, Z., 1996, “Competitive binding of lead by EDTA in soils and implications for heap leaching remediation”, Radioactive Waste Management and Environmental Restoration, vol. 20, no.(2-3), pp111-127. Hiskey, J. B., 1985, “Gold and silver extraction: The application of heap leaching cyanidation”, Field Notes, Arizona Bureau of Geology and Mineral Technology, Tucson, vol. 15, no.(4), pp1-5. Luong H. V. and Lin, H. K., 1999, “Remediation of soil contaminated with organic compounds”, U. S. Patent 5,855,791. Luong, H. V. and Lin, H. K., 2000, “Controlling Fenton reaction for soil remediation”, Analytical Letters, vol. 33, no.(14), pp3051-3065. Man, X., 1997, “Removal of lead from contaminated soils by gravity concentration, oxidation leaching and sulfide precipitation”, M. S. thesis, University of Alaska Fairbanks. Pignatello, J. J., 1992, “Dark and photoassisted Fe+3-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide”, Environmental Science and Technology, vol. 26, pp944-951. Taylor, W. R., 1979, “Ground water quality protection in in situ uranium mines”, in In- situ Uranium Mining and Ground Water Restoration, edited by W. J. Schlitt and D. A. Shock, Society of Mining Engineers, pp1-6. Thorsad, L. E., 1987, “How heap leaching changed the west”, World Investment News, A Pacific Regency Publication, Vancouver, B. C., February, pp31, 33. Voelker, B. M. and Sulzberger, B., 1996, “Effects of fulvic acid on Fe(II) oxidation by Vo1. 3, No. 1 Simulating Heap and In-situ Soil Remediation with Metallic Fenton Reaction 39 hydrogen peroxide”, Environmental Science and Technology, vol. 30, pp1106-1114. York, D. A. and Aamodt, P. L., 1990, “Remediation of contaminated soil using heap leaching mining technology”, Proceedings of Western Regional Symposium on Mining and Mineral Processing Wastes, May 30-June 1, 1990, Berkeley, CA, pp255-259. |

