Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 1, No.2, pp79-96,2002 Printed in the USA. All rights reserved 79 Unburned Carbon from Fly Ash for Mercury Adsorption: II. Adsorption Isotherms and Mechanisms Z. Li, X. Sun, J. Luo and J. Y. Hwang Institute of Materials Processing J. C. Crittenden Department of Environmental Engineering Michigan Technological University, Houghton, Michigan 49931 Abstract Adsorption behavior of unburned carbons from fly ash has been investigated in this study. Batch tests and column test were carried out for several unburned carbon samples from various ash sources and processing schemes. Adsorption isotherms have been obtained from these tests. Results show that the unburned carbons have equal or better adsorption capacity for elemental mercury comparing with some general purpose commercial activated carbons at low gas phase mercury concentration that is in the range of power plant emissions. Also it has been found that heat treatment of unburned carbon in the presence of air at 400 o C enhanced the adsorption capacity, and the adsorption capacity decreased with the increase of the adsorption temperature. The mechanism of mercury adsorption on the unburned carbon was explained by the physical and chemical interaction between mercury and primary sites on the carbon surface.  80 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Introduction As mentioned in the previous paper, the mercury emissions from coal-fired utility boilers has become a great concern due to the potential environmental threat to human health. [1] In this regard, several technologies have been developed to remove mercury emitted during combustion of coals, and, among them, adsorption with activated carbon is a promising technology. [2-9] As early as 1920s, Coolidge and Shiels proposed the use of activated carbon as the sorbent for mercury. [12,13] With the evolution of the technology, carbon-type materials have become the primary sorbent in the field. Currently, there are two major types of process configuration, i.e. fixed bed and carbon injection methods. [14] In a fixed bed practice, the sorbent (e.g. activated carbon granulates) is packed in a column reactor and almost no flow or movement of sorbent is taking place inside the column. For the carbon injection method, fine carbon particles are spread into the flue gas stream, and adsorption is achieved during the flight of the carbon particles. Then the particles are collected by electrostatic precipitator (ESP) for regeneration or disposal. More recently, novel adsorbents such as sulfur impregnated activated carbon have emerged to enhance the efficiency of mercury removal from flue gas. [15,16] However, due to the extremely high cost of these types of sorbent materials, full-scale industrial applications of these technologies have been impeded. Therefore, low cost adsorbent with sufficient adsorption capacity needs to be developed for the implementation of the mercury removal control technology. Bergstrom studied the possibility of using fly ash to remove mercury from flue gas, and it has been found that 91% of the total mercury was removed with a fabric filter when additional fly ash was injected into the flue gas upstream of the filter. [17] Recently, there are also several studies related to the adsorption of mercury by fly ash. [18-23] They claimed that the mercury partitioning is directly related to the carbon content among individual ash samples. Our previous study showed that the mercury content in the unburned carbon separated from fly ash was significantly higher than that of the raw fly ash and the clean ash. [1] One possibility for the high mercury content in the unburned carbon is due to its adsorbability. To understand the adsorption behaviors of the unburned carbon, this paper presents the results of adsorption tests for different carbons from fly ashes. Experimental Methods Material. Unburned carbons from six different fly ash sources were studied in this project. C1, C2 and C3 were the +100 mesh carbons from FA1, FA2, and FA3 fly ashes separated by the gravity separation followed by the electrostatic separation as described in the previous paper of the series. C1-F-L and C1-F-H were the –100 mesh carbons of FA1 fly ash separated by froth flotation. C1-F-L was dried in the air at 105 o C, whereas C1-F-H was heated in the air at 400 o C for 4 hours. Properties of these materials have been presented earlier. [1] For comparison, two commercial activated carbons, BPL and F400 from Calgon Carbon Co. were also tested.  Unburned Carbon from Fly Ash for Mercury Adsorption: II 81 Mercury source. A U-shaped mercury permeation tube (VICI Metronics) was used as the elemental mercury source. A thermal bath was used to maintain a constant temperature of the source. The carrier gas was nitrogen of P.P. grade (Interstate Welding Sales Co.). The VICI tube included a mercury-containing permeation tube in one leg and glass beads in the other leg to ensure a uniform heating of the carrier gas stream. The permeation rate of unit length of the tube is dependent on the temperature. By controlling the temperature of the thermal bath (-2 o C ~ 70 o C), various mercury concentrations of carrier gas can be obtained at a certain gas flow rate (100 ml/min ~ 800 ml/min). The permeation tube for this study was 4.0 cm long and had a releasing rate of approximately 300 ng/min at 70 o C. Mercury analysis. Mercury concentration in gas phase was analyzed with a gold film mercury vapor analyzer (JEROME 431-X, Arizona Instrument Corp.). The measuring range of the analyzer is 0.000 to 0.999 mg/m 3 , with a resolution of 0.001 mg/m 3 and a sensitivity of 0.003 mg/m 3 . The precision is 5% relative standard deviation at 0.100 mg/m 3 and the accuracy is +5% at 0.100 mg/m 3 . The maximum gas temperature is 40°C. A measurement cycle takes 12 seconds with a gas flow rate of 750 ml/min. Setups and procedure. Batch tests were carried out with Tedlar sampling bags (231 series) purchased from SKC Co. According to the supplier, these Tedlar bags were made from chemically inert film. They had a low memory effect of previous samples and can be used in a wide temperature range (-72 o C to 107 o C). They are strong, flexible and resistant to fatigue. In order to test the mercury permeability of these sample bags, the bags were filled with 9 liters of nitrogen gas with an initial mercury concentration of 0.418 mg/m 3 and sealed right after filling. The mercury concentration was measured after 7 days at 0.409 mg/m 3 and 0.399 after 14 days, corresponding to a reduction of 2% and 4.5% respectively. Therefore, the mercury permeability of such bags was very low. The 231 Series sampling bags had two fittings, a hose/valve and a septum fitting. The hose/valve fitting was used for bag flushing, filling and sealing. The septum fitting had a syringe port on the top and was used for small amount sampling. The capacity of the bags was 10 liters. Representative carbon samples were taken from the bulk materials and ground to pass a 200-mesh sieve. The fine particles were dried in an oven at 105 o C for 24 hours and stored in a desiccator for future use. The Tedlar bags were evacuated with a vacuum pump, and the desired amount of the dried carbon sample was put into the bag through the septum fitting. The carbon samples were weighed with a balance with an accuracy of 0.1 mg. The bag was then filled with the nitrogen gas with a mercury content of 450 µg/m 3 through the hose/valve fitting of the bag at a flow rate of 1 l/min. The total volume of gas was controlled with a flow meter and a timer. The filled bags were allowed to set for 7 days in order to reach equilibrium, and the equilibrium concentration of mercury was measured at the end of the 7 days. The amount of mercury being adsorbed was calculated according to the following equation:  82 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Q = V(C 0 -C eq )/m, where Q is the amount of mercury adsorbed on the carbon (mg/g), V is the gas volume filled in the bag (m 3 ), C 0 is the initial mercury concentration of the gas (mg/m 3 ), C eq is the equilibrium mercury concentration of the gas (mg/m 3 ), and m is the weight (g) of the carbon sample put into the bag. During the process of adsorption, the bags were periodically shaken to ensure uniform adsorption. For each test two concentration points were measured and the average value was reported. The measurements are within 5% of difference. Column Tests. In order to investigate the kinetics of the adsorption process, column tests were carried out for the unburned carbon samples at various mercury concentrations of the carrier gas. The experimental apparatus is illustrated in Figure 1. Carbon samples were packed in a U-shaped tube with a diameter of 4 mm to form an adsorption bed. During the experiment, the carbon bed was placed in a thermal bath filled with antifreeze to maintain a constant temperature. The feed carrier gas line and the carbon bed were connected with a three-way valve, which allows the measurement of the mercury concentration of the feed gas prior to entering the bed. The mercury concentration analysis was carried out by sampling the carrier gas (either feed gas or exhaust gas) in a one-liter Tedlar bag and analyzed with the Jerome mercury vapor analyzer. For each packed bed, about 0.3 gram of unburned carbon sample was loaded, and glass wool was used as the supporting material. The column parameters are summarized in Table 1. The total adsorption of mercury at equilibrium q was calculated by integration as shown in the following equation: qCCdQ Q t =− ∫ () 0 0 , where C 0 and C are the influent and effluent concentrations of mercury, and Q is the gas volume flowing into the bed at time t. Results and discussion Adsorption of unburned carbons and commercial activated carbons. The batch adsorption tests indicated that the unburned carbons from fly ash had significant adsorption capacities for elemental mercury. Adsorption isotherms of these materials are presented in Figures 2-5. For the purpose of comparison, the isotherm of BPL activated carbon (Calgon Carbon Co.) is also shown in the figures. It can be seen from the plots that a concave type of isotherm is apparent for all the carbon samples. At low mercury concentration of the gas phase, the mercury adsorbed on carbon increased linearly with the increase of the gas phase mercury concentrations. When the gas phase mercury concentration reached a certain level (>0.28~0.33 mg/m 3 ), the mercury adsorption increased rapidly with the gas phase mercury concentrations. It is noticed that the adsorption capacity of C1 and C2 carbons is higher than that of F-400 and BPL activated carbons at low gas phase mercury concentrations (<0.3 mg/m 3 ). The order of mercury  Unburned Carbon from Fly Ash for Mercury Adsorption: II 83 adsorption capacity from high to low is C1, C2, F-400, BPL, C3. For these unburned carbons the mercury adsorption capacity could be as high as 60 µg/g. However, BPL activated carbon had a much higher capacity, up to 380 µg/g, at a gas phase mercury concentration of 0.32 mg/m 3 . Since the mercury concentration in flue gas is in the range of 0.01 to 0.3 mg/m 3 , it can be seen from the above results that the unburned carbon is better suited for the mercury removal from flue gas. Comparison of C1 carbons from different processes. Figure 6 presents the isotherms of C1 and C1-F carbon samples derived from different processing schemes. The C1 carbon was obtained by gravity separation followed by electrostatic separation as described in the previous paper, and it had a particle size of +100 mesh. The C1-F was the concentrate from froth flotation and had a size of –100 mesh. It can be seen that the isotherms of these two materials are basically the same with adsorption capacities very close to each other. This result indicated that the residual flotation reagents on the carbon surface had no or very little influence on the adsorption capacity of the unburned carbon. Also, it has been shown that the unburned carbon can be used as the adsorbent for elemental mercury, no matter the particle size and processing routes. Effect of heat treatment on the adsorption capacity of C1 carbon. Figure 7 shows the isotherms of C1 carbon samples with or without heat treatment. Both samples were obtained from the same froth flotation process, but treated differently afterwards. The C1- F-L sample was dried in air at 100°C for 4 hours while the C1-F-H was heated in air at 400°C for 4 hours. It can be seen from the figure that the heat treatment had a significant impact on the adsorption capacity of the unburned carbon. The capacity was increased by about 4 times in the concentration range of 30 µg/m 3 to 320 µg/m 3 , with a capacity up to 380 µg/g. This improvement in adsorption capacity may be explained by the possibility that the heat treatment in air caused oxidation of the carbon surface; however, further investigation is needed to confirm the conjecture. Effect of temperature on the adsorption capacity of C1 carbon. In order to investigate the effect of temperature, column tests have been carried out at two temperatures i.e. 20 o C and 40 o C. The carbon sample used for the tests was obtained from gravity separation followed by electrostatic separation, with a particle size of 48x100 mesh. The isotherms were derived by integration of a series of breakthrough curves (Figures 8-10). It can be seen from Figure 8 that the adsorption capacity at 40 o C is lower than that at 20 o C. Compared with the capacity obtained in batch tests, the capacity in the column tests is much lower, less than 10% of that from the batch tests. This may be due to the difference in particle size and in adsorption time for the column and batch tests. Mechanisms of mercury adsorption by unburned carbon. The adsorption of mercury on carbon can be explained by the physical and chemical interactions taking place between the carbon surface and mercury. According to the theory proposed by Dubinin, the carbon surface contains some adsorption centers, called primary sites. When a molecule of the adsorbate adsorbs on a primary site, the adsorbed molecule can then act as a secondary center for the adsorption of more molecules. [24]  84 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 The primary sites on the carbon surfaces investigated in this study could be any spots that have a high affinity for mercury molecules. By combination of the isotherms with the characterization data, these primary sites may be those enriched with oxygen-containing functional groups, minor/trace elements such as sulfur, and selenium and mercury, and catalyzing components. The enhancement of mercury adsorption after oxidizing unburned carbon at 400 o C in air shows that oxygen-containing functional groups may have an important role, which is also suggested by Hall et al. [25] References 1. Hwang, J.; Sun, X.; Li, Z. submitted to Environ. Sci. Technol. 2. Chang, R.; Offen, G. R. Power Eng. 1995, 99, 51-57. 3. Young, B. C.; Miller, S. J.; Laudal, D. L. Presented at the 1994 Pittsburgh Coal Conference, Pittsburgh, PA, Sep 1994. 4. Sinha, R. K.; Walker, P. L. Carbon 1972, 10, 754-756. 5. Matsumura, Y. Atoms. Environ. 1974, 8, 1321-1327. 6. Otani, Y.; Kanaoka, C.; Usui, C.; Matsui, S.; Emi, H. Environ. Sci. Technol. 1986, 20, 735. 7. Otani, Y.; Emi, H.; Kanaoka, C.; Uchijima, I.; Nishino, H. Environ. Sci. Technol. 1988, 22, 708. 8. Meij, R. Water, Air, Soil Polut. 1991, 56, 21. 9. Chang, R.; Owens, D. EPRI J. 1994, July/Aug, 46. 10. Liberti, L.; Notarnicola, M.; Amicarelli, V.; Campanaro, V.; Roethel, F.; Swanson, L. Waste Mange. Res. 1998, 16, 2, 183-189. 11. Krishnan, S. V.; Gullett, B. K.; Jozewicz, W. E. Environ. Sci. Technol. 1994, 28, 1506-1512. 12. Coolidge, A. S. J. Am. Chem. Soc. 1927, 149, 949-1952. 13. Shiels, D. O. J. Phys. Chem. 1929, 33, 1398-1402. 14. EPA, , Mercury Study Report to Congress, 1997, EPA-452/R-97-10 15. Korpiel, J. A.; Vidic, R. D. Environ. Sci. Technol. 1997, 31, 2319-2325. 16. Liu, W.; Vidic, R. D.; Brown, T. D. Environ. Sci. Technol. 1998, 32, 531-538. 17. Bergstrom, J. G. T. Waste Mange. Res. 1986, 4, 57-64. 18. Hassett, D. J.; Eylands, K. E. Fuel, 1999, 78, 243-248. 19. Shannon D. Serre* and Geoffrey D. Silcox ; Adsorption of Elemental Mercury on the Residual Carbon in Coal Fly Ash, Industrial & Engineering Chemistry Research; 2000; 39(6); 1723-1730. 20. Tanaporn Sakulpitakphon, James C. Hower,* Alan S. Trimble, William H. Schram, and Gerald A. Thomas; Mercury by Fly Ash: Study of the Combustion of a High- Mercury Coal at a Utility Boiler, Energy & Fuels; 2000; (3); 727-733. 21. James C. Hower,* M. Mercedes Maroto-Valer, Darrell N. Taulbee, and Tanaporn Sakulpitakphon ; Mercury Capture by Distinct Fly Ash Carbon Forms, Energy & Fuels; 2000; 14(1); 224-226.  Unburned Carbon from Fly Ash for Mercury Adsorption: II 85 22. James C. Hower,* Robert B. Finkelman, Robert F. Rathbone, and Jennifer Goodman; Intra- and Inter-unit Variation in Fly Ash Petrography and Mercury Adsorption: Examples from a Western Kentucky Power Station, Energy & ; 2000; 14(1); 212-216. 23. P. Fermo, F. Cariati, S. Santacesaria, S. Bruni, M. Lasagni,# M. Tettamanti,# E. Collina,# and D. Pitea*# ; MSWI Fly Ash Native Carbon Thermal Degradation: A TG-FTIR Study, Environmental Science & Technology; 2000; 24. Dubini, M.M., J.Phys. Chem. 1965, 39(6), 697-704 25. Hall, B.; Schager, P.; Weesmaa, J. Chemosphere, 1995, 30, 4, 611-627 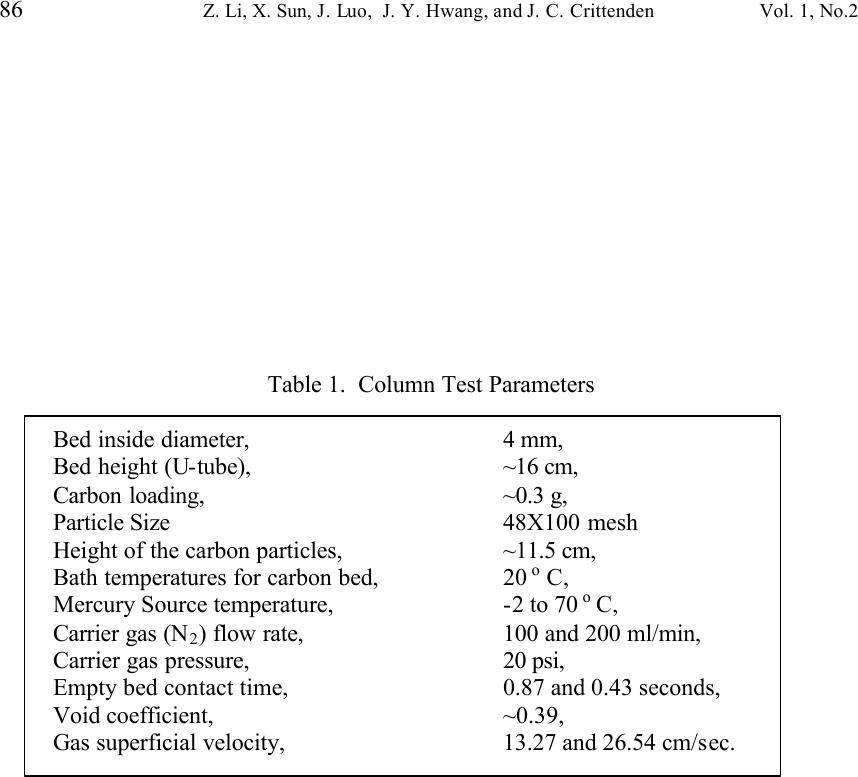 86 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Table 1. Column Test Parameters Bed inside diameter, 4 mm, Bed height (U-tube), ~16 cm, Carbon loading, ~0.3 g, Particle Size 48X100 mesh Height of the carbon particles, ~11.5 cm, Bath temperatures for carbon bed, 20 o C, Mercury Source temperature, -2 to 70 o C, Carrier gas (N 2 ) flow rate, 100 and 200 ml/min, Carrier gas pressure, 20 psi, Empty bed contact time, 0.87 and 0.43 seconds, Void coefficient, ~0.39, Gas superficial velocity, 13.27 and 26.54 cm/sec. 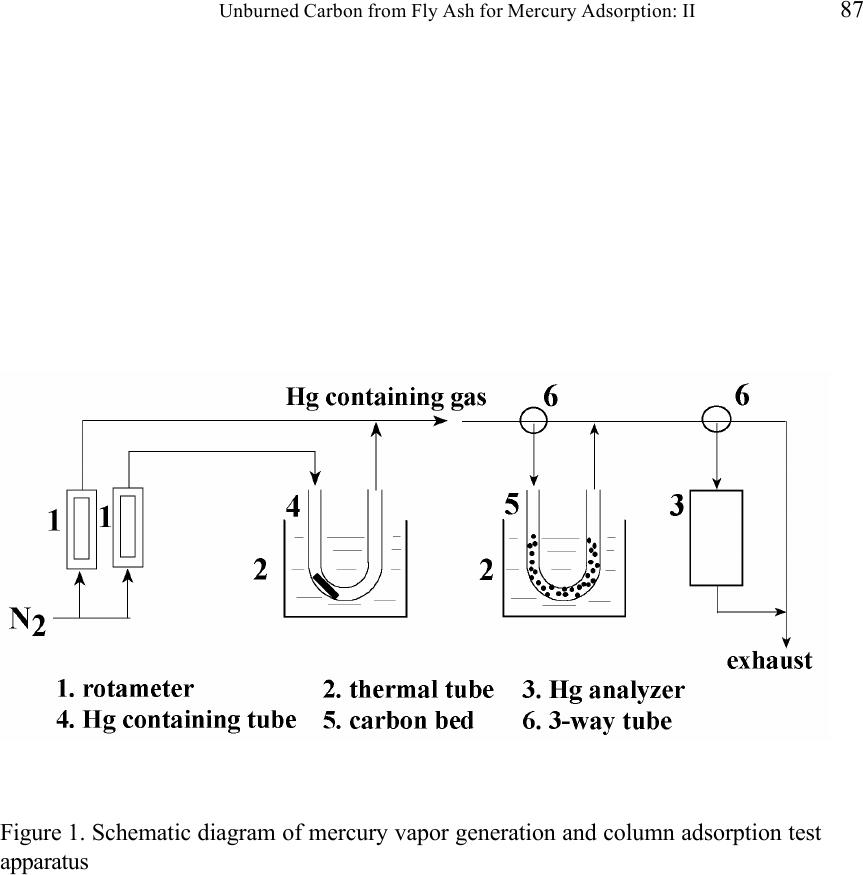 Unburned Carbon from Fly Ash for Mercury Adsorption: II 87 Figure 1. Schematic diagram of mercury vapor generation and column adsorption test apparatus 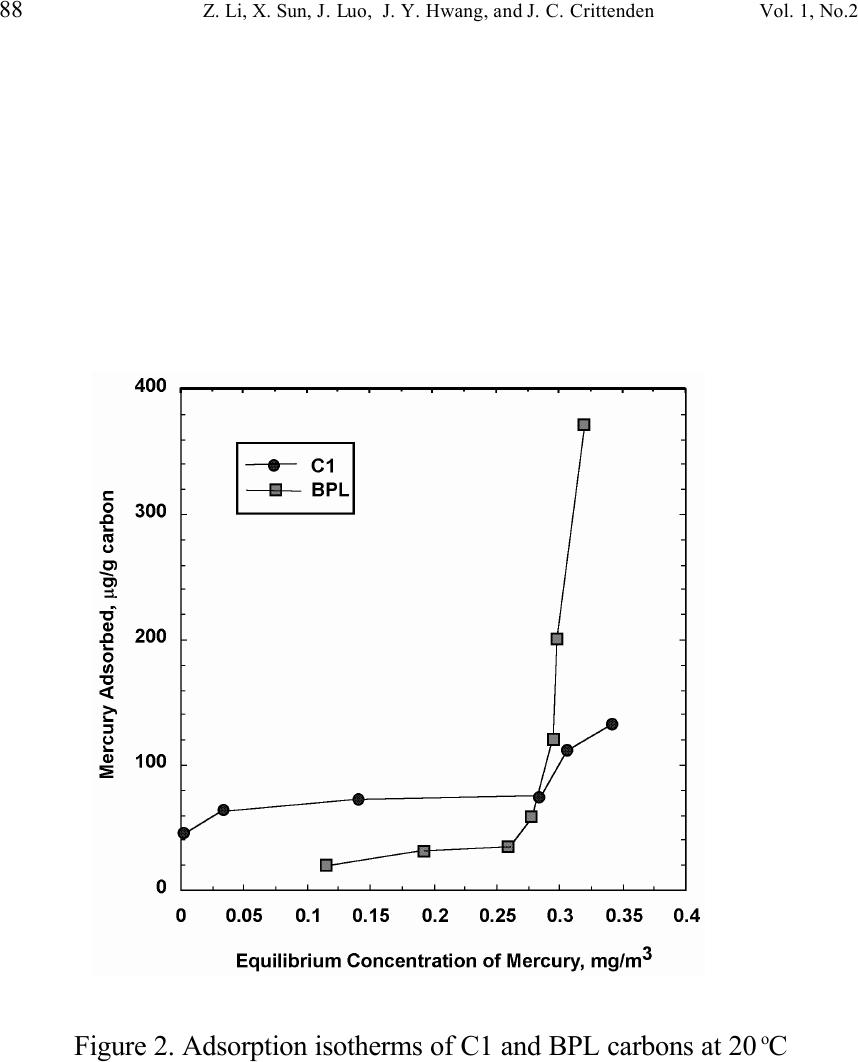 88 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Figure 2. Adsorption isotherms of C1 and BPL carbons at 20 o C 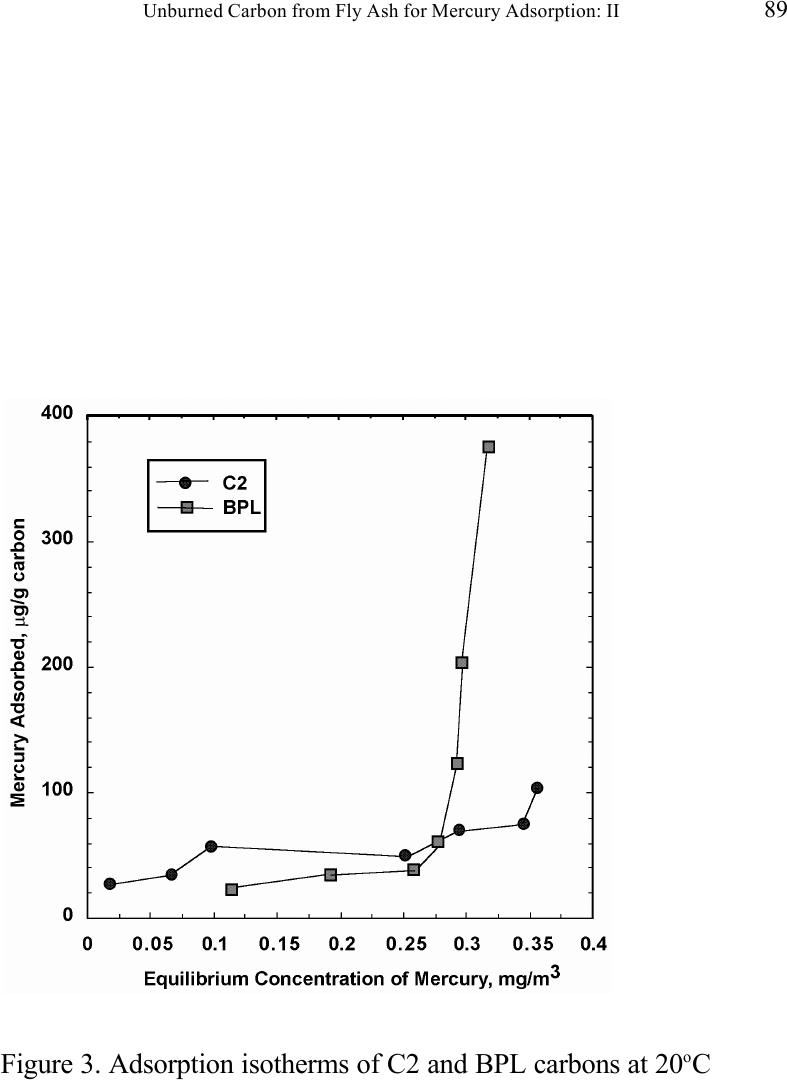 Unburned Carbon from Fly Ash for Mercury Adsorption: II 89 Figure 3. Adsorption isotherms of C2 and BPL carbons at 20 o C 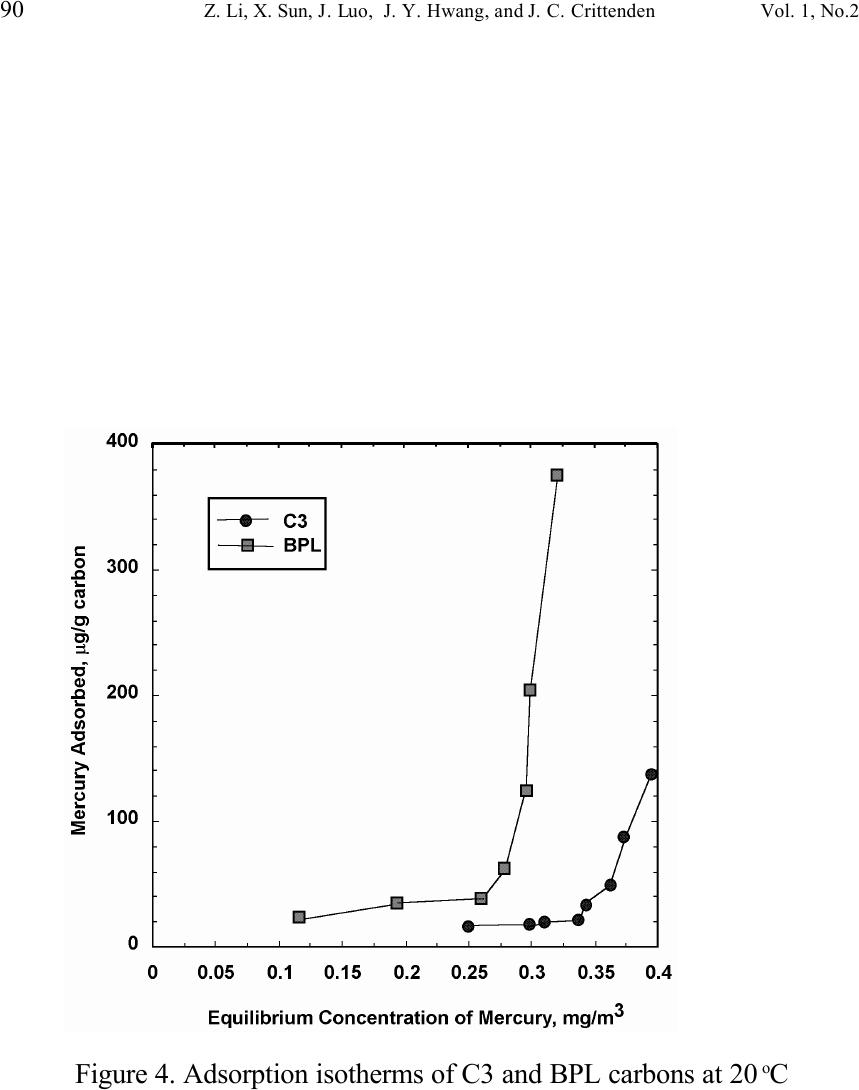 90 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Figure 4. Adsorption isotherms of C3 and BPL carbons at 20 o C 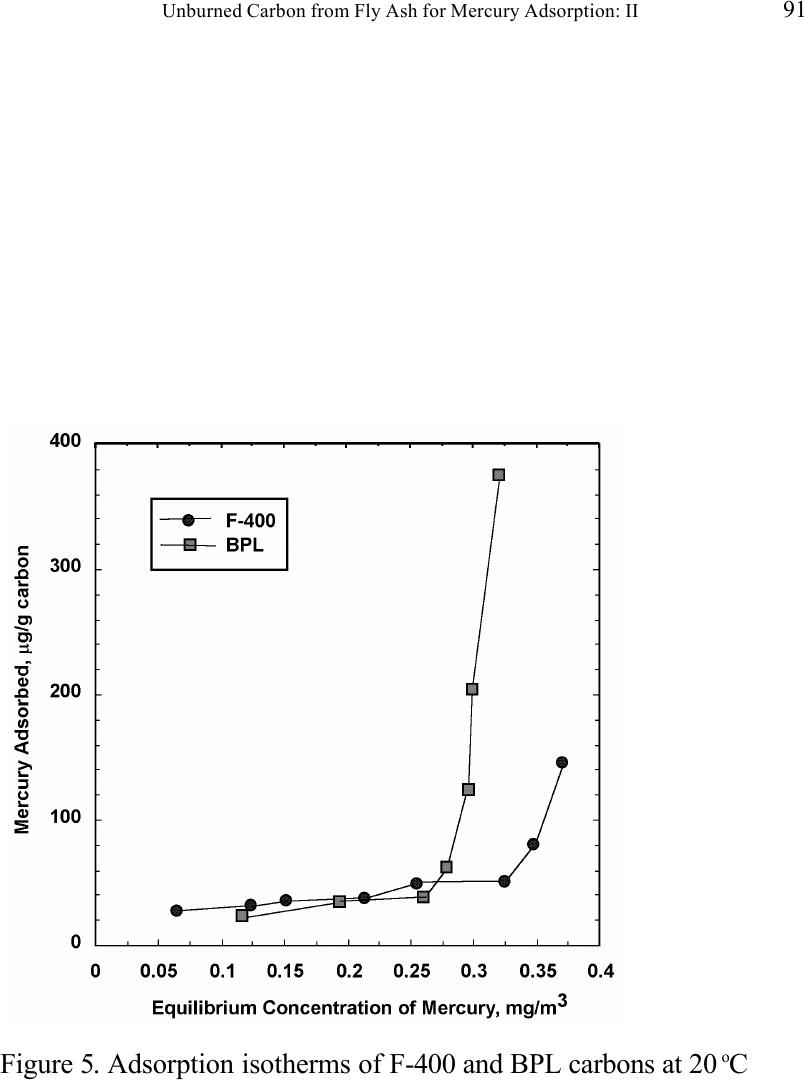 Unburned Carbon from Fly Ash for Mercury Adsorption: II 91 Figure 5. Adsorption isotherms of F-400 and BPL carbons at 20 o C 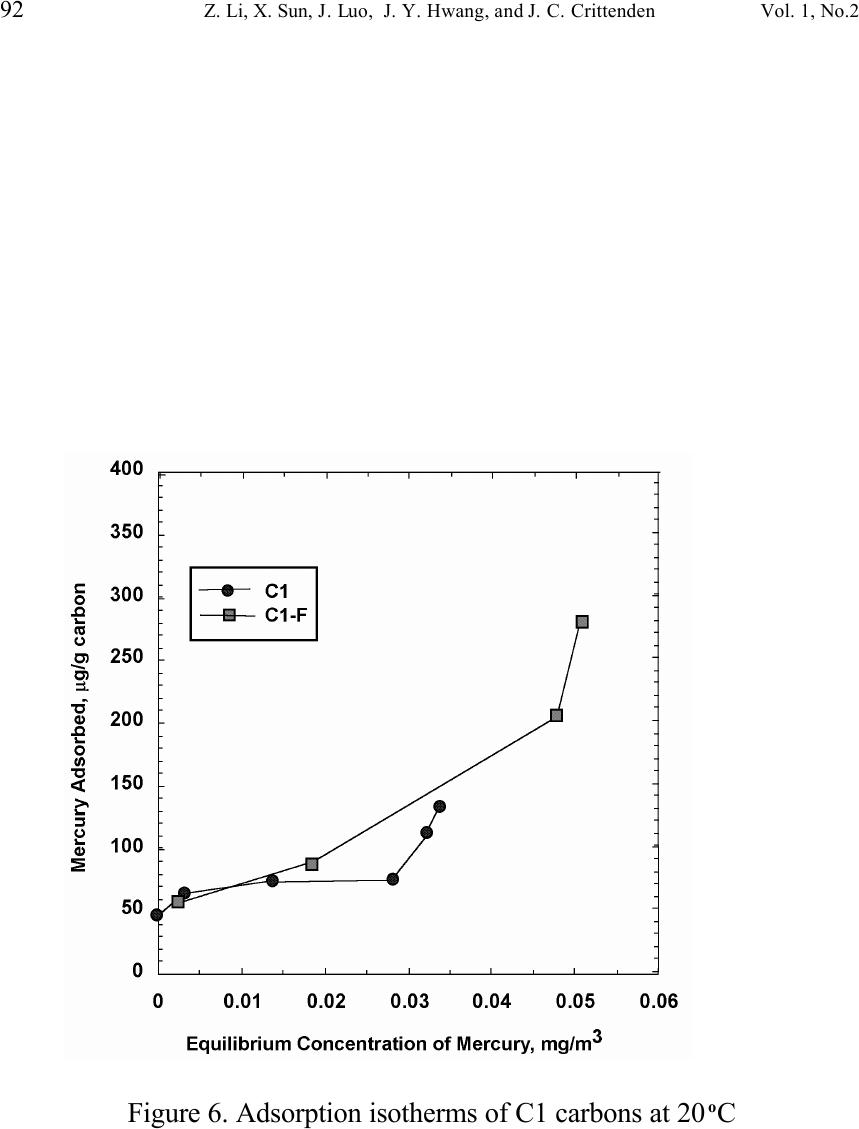 92 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Figure 6. Adsorption isotherms of C1 carbons at 20 o C 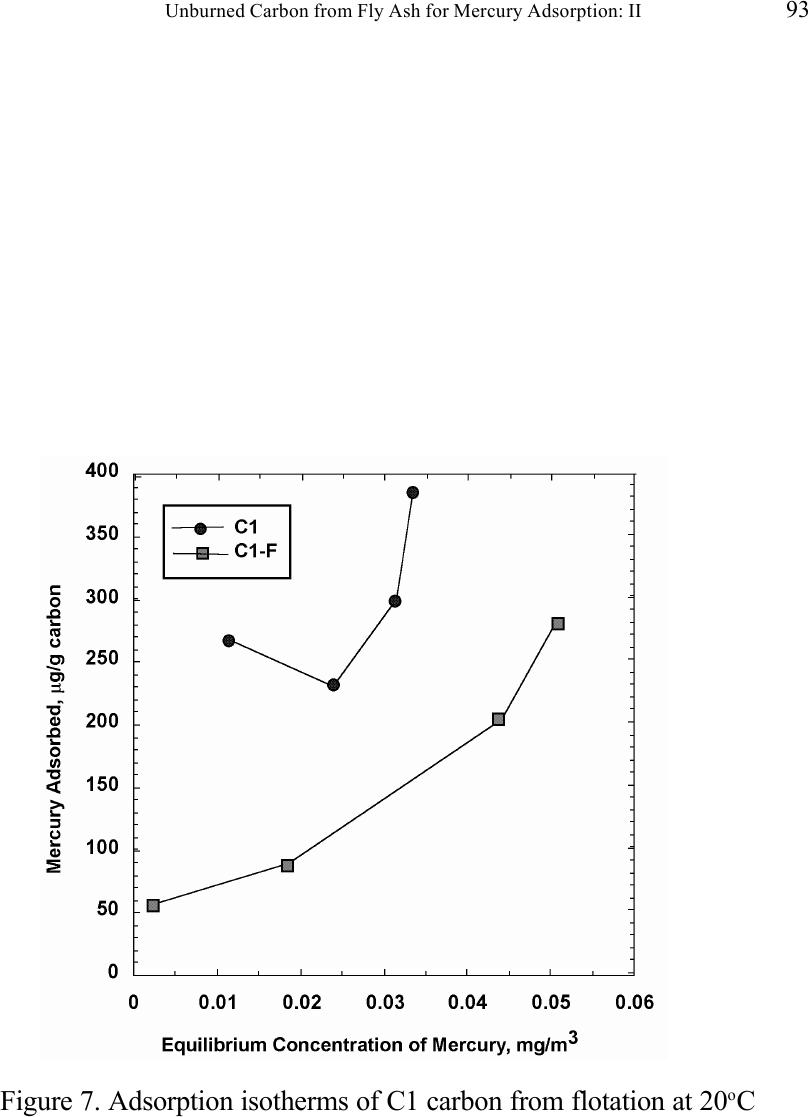 Unburned Carbon from Fly Ash for Mercury Adsorption: II 93 Figure 7. Adsorption isotherms of C1 carbon from flotation at 20 o C 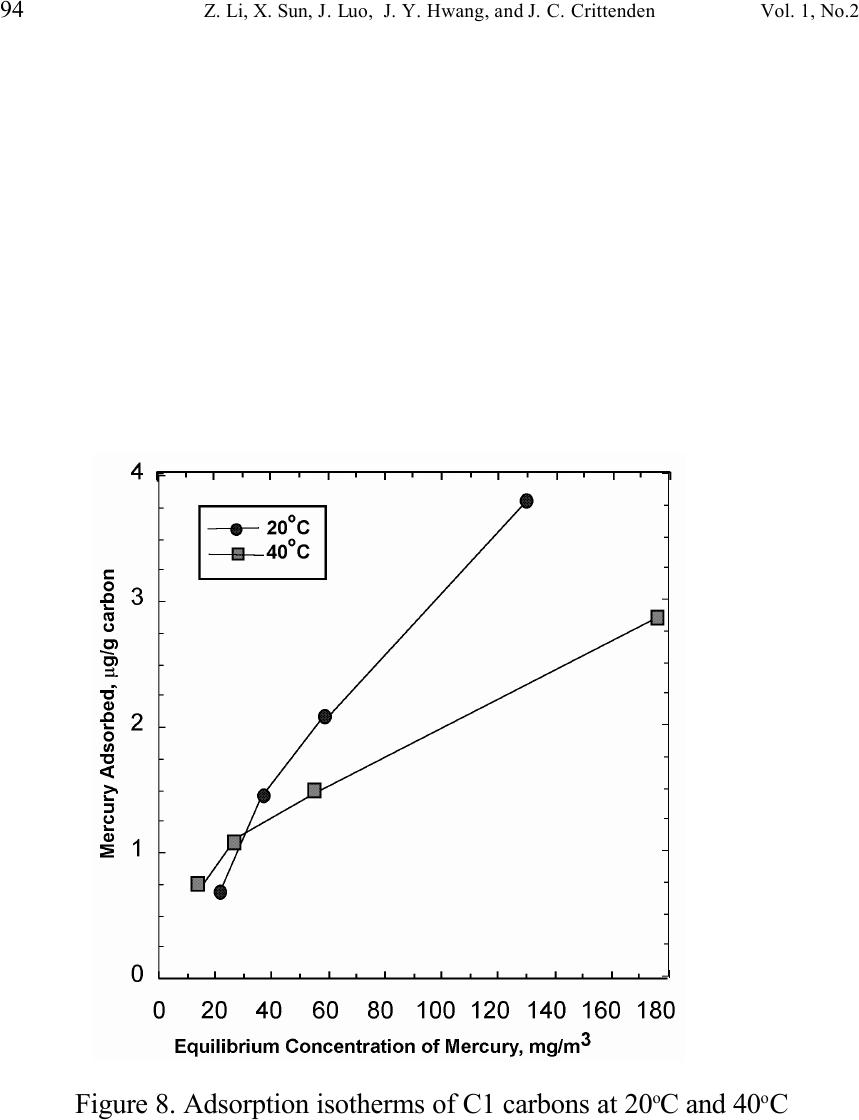 94 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Figure 8. Adsorption isotherms of C1 carbons at 20 o C and 40 o C 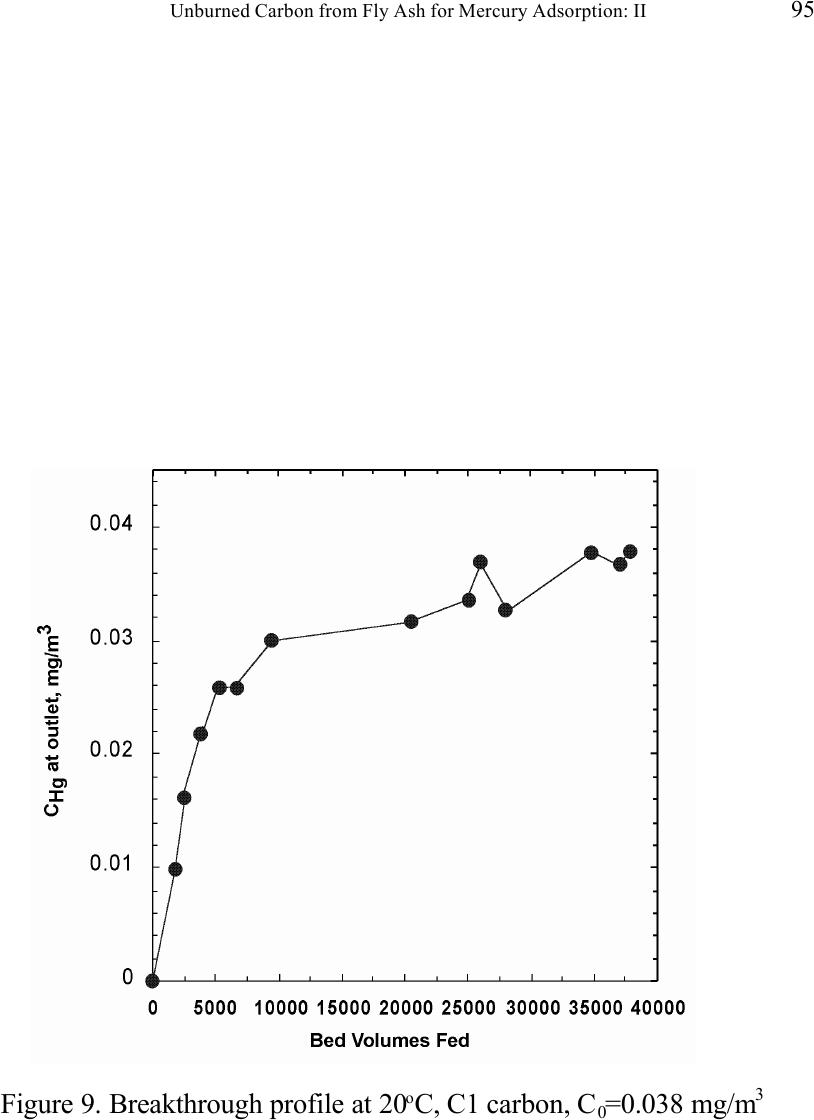 Unburned Carbon from Fly Ash for Mercury Adsorption: II 95 Figure 9. Breakthrough profile at 20 o C, C1 carbon, C 0 =0.038 mg/m 3 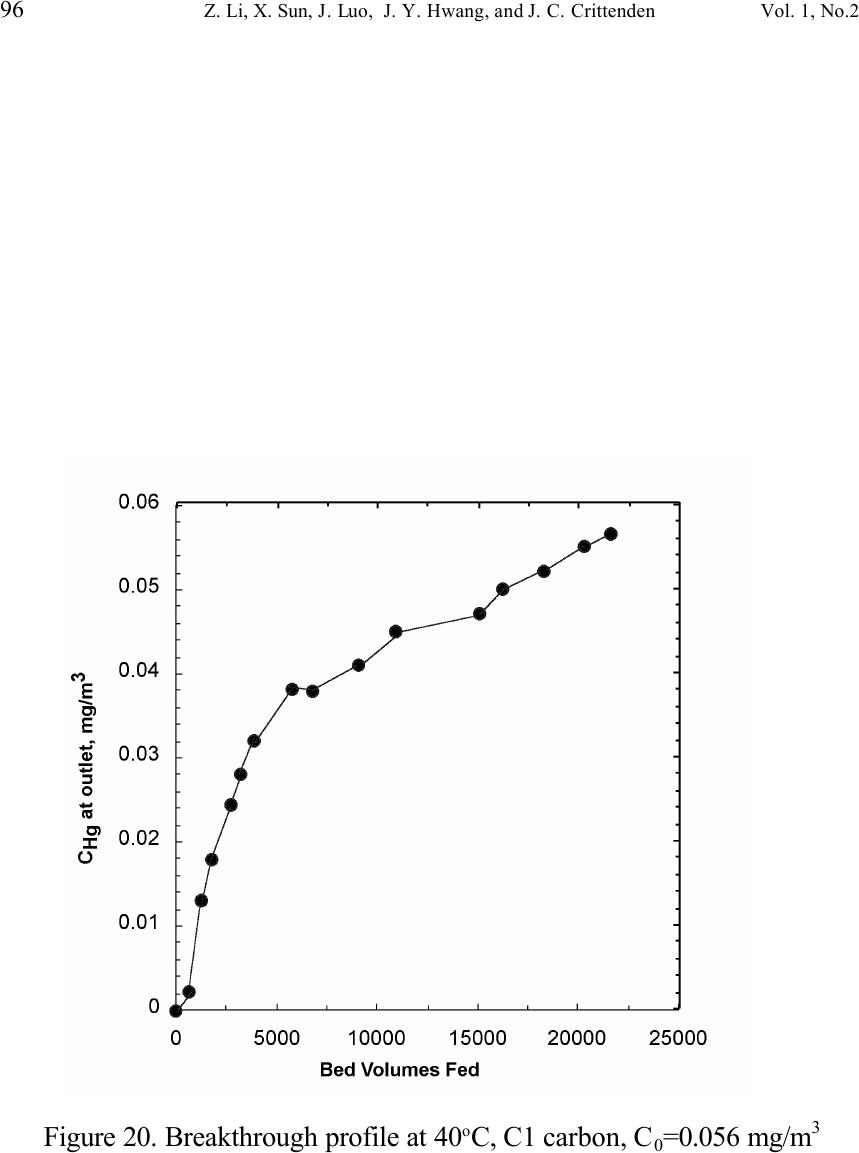 96 Z. Li, X. Sun, J. Luo, J. Y. Hwang, and J. C. Crittenden Vol. 1, No.2 Figure 20. Breakthrough profile at 40 o C, C1 carbon, C 0 =0.056 mg/m 3 |

