Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 1, No.2, pp69-78, 2002 Printed in the USA. All rights reserved 69 RECYCLING PRACTICES OF SPENT MgO-C REFRACTORIES Kyei-Sing Kwong and James P. Bennett Albany Research Center, USDOE 1450 Queen Ave. SW. Albany, OR 97321 USA Phone (541) 967-5825 ABSTRACT The recycling options of spent MgO-C refractories from an electrical arc furnace (EAF) have been evaluated. The economic, quality of spent refractories and products made from it, the ease of implementation of a recycling practice and the interest of steel melt shops were considered. It was decided that the best option of most EAF shops would be to recycle spent MgO-C refractory as a foaming slag conditioner because of their MgO content. Crushed MgO-C spent refractories can be reused directly back into an EAF without complex and costly beneficiation. Even though this practice is simple, it is critical to know the optimum amount of MgO in the slag to achieve the best foaming quality. A computer model was designed to find the optimum MgO amount. This modeling also helps the melt shop extend refractory service life, increase the energy efficiency, increase productivity, and decrease the amount of slag. Issues related to the refractory recycling will be discussed.  70 Kyei-Sing Kwong and James P. Bennett Vol. 1, No. 2 Introduction Refractories are made from abundant industrial minerals such as SiO 2 , Al 2 O 3 , MgO, dolomite and chromite for usage as insulation or molten metal/slag containers. These refractories have been engineered to meet different challenging specifications such as high temperature resistance, long service life, creep or spalling resistance, and economic savings. Most refractories are consumable materials which may last from few weeks to several years depending on service conditions and material performance. In general, spent refractories do not have much value after service, creating disposal issues. In some locations in the USA, companies may pay high fees to landfill spent refractories. Over half of refractory products are used by steel producers. These steel producers are both integrated mills (BOF) and mini mills (EAF). There are about 80 EAF companies with 193 EAF furnaces in the United States producing iron steel (1). Their total annual steel production is about equal to the integrated mills (BOF). EAF companies have a wide variety of interests recycling their spent refractories. Some EAF companies are located in developing urban areas and do not have space to landfill spent refractories or they may face high disposal costs. A few companies see this as part of their quest to be a “green” company or to gain a competitive advantage of business. The processes to recycle spent refractories may be simple, however, engineering the spent refractory applications may require a strong technical background, a team approach and company commitments. This paper will emphasize the importance of a technical background and a team approach to recycling. MgO+C and MgO are widely used refractory materials in the EAF. The low value of the spent refractories makes them difficult to recycle. Only simple beneficiation of spent refractories is justified so they may compete with abundant natural materials. Two applications were selected at the Albany Research Center (ARC) to reuse these spent refractory materials. The first one is to reuse spent refractories as repair materials. However, the quality, availability, consistency, and the transportation cost are some of the major concerns for the application of spent refractories as repair materials. The second application is to reuse spent refractories as an EAF foamy slag additive. Foamy slags are widely practiced in electric arc furnaces and provide for electrical energy savings, longer refractory service life, productivity improvements, lower noise level, and lower nitrogen pick up in the steel. Foamy slags shield the electrical arcs, preventing radiation energy loss, eliminating arc flares, saving overall energy and extending refractory service life. It has been reported (2) that a foamy slag practice saves 3-10% in energy and decreases 25-63% of refractory consumption. The reuse of spent refractories as a slag additive will not change any slag quantity and will save recycling associated costs as well as operating costs. Foamy slags are obtained by the reaction of FeO with C to generate gas bubbles. “Optimum” slag chemistry is required to sustain these gas bubbles. If the slag viscosity is too  Vol. 1, No. 2 RECYCLING PRACTICES OF SPENT MgO-C REFRACTORIES 71 thin, the gas bubbles can not be sustained. If the slag viscosity is too thick, it is difficult to form a foam. The “optimum” slag is a molten saturated in MgO to the point of having suspended second phase particles (MgO C FeO magnesium wustite (MW)) at operating temperature (3). The goal of this program is to reuse much of the basic MgO containing refractories as a slag additive and satisfy the MgO requirement. To melt one ton steel takes a total energy input of 560 to 680 KWh for most modern EAF operations (4), making EAF steelmaking an energy consumption industry. The Office of Industrial Technology (OIT) , USDOE funded this program along with the Steel Manufacturer Association (SMA). Its goals are to save energy and eliminate landfill disposal by reusing spent refractories as slag conditioners for foamy slag. In order to implement the recycling of spent refractories as a slag additive, the spent refractories have been characterized and a model to predict MgO saturated EAF slag chemistry has been developed. Based on the model, the amount of MgO additive can be predicted. Several issues in implementing reuse of spent refractories as slag additives will be also discussed in this paper. The characterization of MgO+C spent refractories MgO refractories are generally used for EAF linings because MgO has a high melting point, slag compatibility, and good service life. Carbon was added to the refractories to enhance their non-wetting properties. Some antioxidants such as aluminum (Al) and silicon (Si) were also added to the refractories to prevent carbon oxidation. Table I shows the chemical composition of new and spent MgO+C refractories from a EAF. The chemistry indicates that this spent refractory is fairly clean. Table I New and spent MgO+C refractory chemical composition MgO+C Al 2 O 3 CaO FeO MgO SiO 2 C N 2 New 4.40 0.99 0.37 84.74 4.47 9.49 0.20 Spent 6.07 1.99 0.56 86.06 0.93 6.61 0.41 MgO+C spent refractories have been analyzed by SEM (Scanning Electron Microscope). SEM analysis indicated that carbon was present in the form of graphite and Al/Si metallic antioxidant throughout the samples. During service, elements of Al and Si can be nitridized to form AlN and Si 3 N 4 . The nitride powders react with water, releasing N 2 gas, possibly causing cracks limiting their use as repair materials. A model to predict MgO saturated EAF slag chemistry EAF slags consist of five major oxides, CaO, MgO, SiO 2 , FeO and Al 2 O 3 . EAF slags are always characterized by an index of basicity in the steel industry. There are many different ways to calculate basicity. Based on the analysis of the SiO 2 -Al 2 O 3 -CaO-MgO phase diagram, the basicity of (B3 = (CaO/(SiO 2 +Al 2 O 3 ))) is selected in this paper. Usually, the steel companies adjust the basicity of their EAF slags between 1.5 and 2.5.  72 Kyei-Sing Kwong and James P. Bennett Vol. 1, No. 2 In general, lime and dolomite are used to provide the source of CaO and MgO for adjusting the slag basicity and to saturate a slag with MgO. SiO 2 and Al 2 O 3 may be formed from the oxidation of Si and Al in the scrap, dust and coal slags. FeO may originate from the oxidation of iron and from coal slags. EAF steel making involves an oxidation process to oxidize extra carbon in the molten steel. During this operation, the FeO concentration in the slag is generally increased and dynamic changed by the lancing amount of carbon and oxygen. In the meantime, the increased FeO in the slag also affects CaO, MgO, SiO 2 and Al 2 O 3 concentration. Based on the linear relationship finding in the SiO 2 -CaO-FeO-MgO phase digrams, an ARC slag model has been proposed to predict MgO saturated EAF slag chemistry. Hence, the amount of slag conditioner (MgO+C spent refractory) can be predicted to saturate the slags. More detailed discussions on these relationships and models can be found in an earlier paper (5). As previously mentioned, the “optimum” slag at EAF operating temperature is a molten MgO saturated slag with the presence of suspended second phase particles (MgO C FeO magnesium wustite (MW))(3). Consequently, the basicity and FeO content affect the amount of slag conditioners needed to saturate a slag. Figure 1 demonstrates the ideal foamy slag chemistry region and the ideal foamy slag chemistry path during EAF operation with MgO injection. The crossed area is an ideal foamy slag chemistry region. Line 1, 2, and 3 are examples of slag chemistry paths. In general, the EAF process is an oxidation process to produce low carbon steel. Therefore, FeO content in slags will be increased during operation. Slag chemistry path 1 will generate a viscous slag containing too much precipitated solid particles, causing little foam. Slag chemistry path 3 will cause thin molten slag which cannot sustain gas bubbles. Slag chemistry path 2 provides an ideal situation for generating an “optimum” foamy slag. Detailed information about slag foamy practice can be found elsewhere (3). FeO% MW+L C 2 S+L L C 2 S+ MW+ L 1 2 3 Figure 1. The ideal foamy slag chemistry region and foamy slag chemistry path  Vol. 1, No. 2 RECYCLING PRACTICES OF SPENT MgO-C REFRACTORIES 73 Computer programming A computer program was designed using Visual Basic to calculate a MgO saturated slag chemistry. The input factors are the CaO/(SiO 2 +Al 2 O 3 ) ratio ( C/S ratio, by weight), the Al 2 O 3 /(SiO 2 +Al 2 O 3 ) ratio ( Al 2 O 3 ratio, by weight), temperature (EC), FeO content and slag chemistry. The output values are dual saturated slag chemistry, saturated MgO slag chemistry, and solid content (MW solid contents). Model validation The MgO saturated slag chemistry prediction from the model was compared with experimental and industrial EAF slags. Table II indicates a good agreement between the data which was collected from MgO and CaO saturated phase diagrams and predictions which were calculated from the model for a dual saturated EAF slag chemistry. Table II predicted results from the ARC model and MgO and CaO saturated phase diagrams MgO FeO SiO 2 CaO C/S Model Phase Model Phase Model Phase Model Phase 2.83 6.26 6.00 34.97 34.57 15.34 15.53 43.42 43.90 2.27 7.92 8.00 30.37 30.00 18.87 18.95 43.05 43.05 1.84 9.96 10.00 24.12 24.19 23.21 23.16 42.71 42.65 Table III lists the slag chemical composition from a participating steel plant and the model’s predictions. Because the MgO content in the slag is higher than the MgO saturated content from the model’s predictions, this slag should be saturated in MgO and should contain some precipitated MW solids. A cup test has been performed using MgO crucibles fired at 1600°C for 1 hour in a box furnace. This test also indicated that the slag was MgO-saturated. Table III Slag chemical composition from a participating steel plant and the model’s predictions EAF Slag1Model EAF Slag 2Model Al 2 O 3 5.70 5.86 5.47 5.61 SiO 2 14.07 14.52 14.31 14.72 CaO 37.10 37.45 36.00 36.24 FeO+Fe 2 O 3 +MnO 33.91 33.91 34.96 34.96 MgO 9.22 8.23 9.26 8.47 Table IV lists the slag chemical composition from another participating steel plant and the model’s predictions. Samples were obtained by freezing slags on steel rods. The samples contained too much Fe 2 O 3 and FeO. It was thought iron from the steel rods oxidized and dissolved into slag samples at high temperature. No conclusions about the MgO saturation of these slags could be drawn. A better sampling procedure should be designed and adopted. 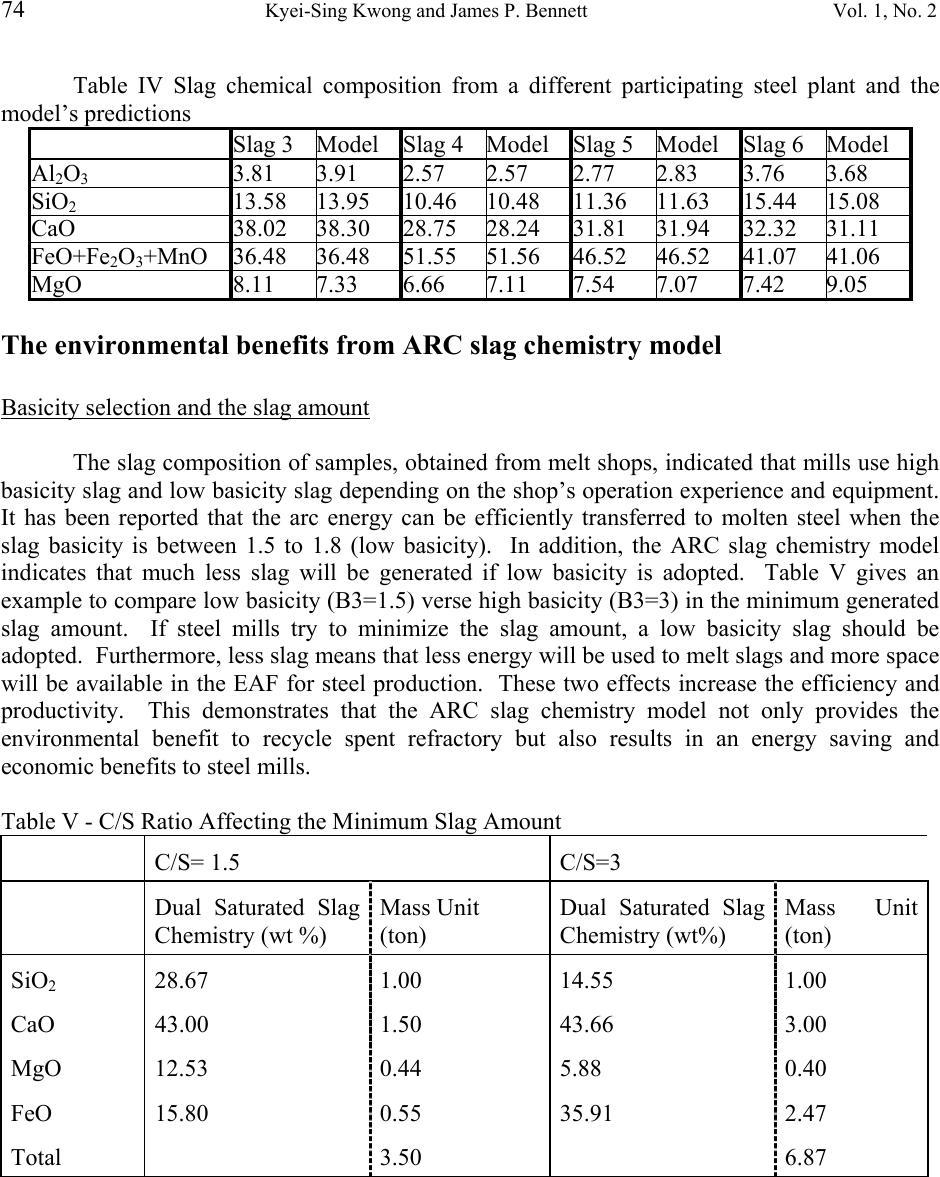 74 Kyei-Sing Kwong and James P. Bennett Vol. 1, No. 2 Table IV Slag chemical composition from a different participating steel plant and the model’s predictions Slag 3 Model Slag 4 Model Slag 5 Model Slag 6 Model Al 2 O 3 3.81 3.91 2.57 2.57 2.77 2.83 3.76 3.68 SiO 2 13.58 13.95 10.46 10.48 11.36 11.63 15.44 15.08 CaO 38.02 38.30 28.75 28.24 31.81 31.94 32.32 31.11 FeO+Fe 2 O 3 +MnO 36.48 36.48 51.55 51.56 46.52 46.52 41.07 41.06 MgO 8.11 7.33 6.66 7.11 7.54 7.07 7.42 9.05 The environmental benefits from ARC slag chemistry model Basicity selection and the slag amount The slag composition of samples, obtained from melt shops, indicated that mills use high basicity slag and low basicity slag depending on the shop’s operation experience and equipment. It has been reported that the arc energy can be efficiently transferred to molten steel when the slag basicity is between 1.5 to 1.8 (low basicity). In addition, the ARC slag chemistry model indicates that much less slag will be generated if low basicity is adopted. Table V gives an example to compare low basicity (B3=1.5) verse high basicity (B3=3) in the minimum generated slag amount. If steel mills try to minimize the slag amount, a low basicity slag should be adopted. Furthermore, less slag means that less energy will be used to melt slags and more space will be available in the EAF for steel production. These two effects increase the efficiency and productivity. This demonstrates that the ARC slag chemistry model not only provides the environmental benefit to recycle spent refractory but also results in an energy saving and economic benefits to steel mills. Table V - C/S Ratio Affecting the Minimum Slag Amount C/S= 1.5 C/S=3 Dual Saturated Slag Chemistry (wt %) Mass Unit (ton) Dual Saturated Slag Chemistry (wt%) Mass Unit (ton) SiO 2 28.67 1.00 14.55 1.00 CaO 43.00 1.50 43.66 3.00 MgO 12.53 0.44 5.88 0.40 FeO 15.80 0.55 35.91 2.47 Total 3.50 6.87  Vol. 1, No. 2 RECYCLING PRACTICES OF SPENT MgO-C REFRACTORIES 75 Slag conditioner amount Some steel mills do not add enough dolomite to condition slags and have high gunning mix consumption or have shorter refractory service life and delay the onset of slag foaming. The ARC slag chemistry model can help steel mills to design the targeted slag composition early to minimize the loss of refractory and extend the refractory service life. FeO content Most FeO in the slag is from the iron oxidation during decarbonization. FeO is a major oxide component in slag and has a pronounced effect on viscosity, foaming ability, slag volume, productivity, and power consumption. Too little FeO in a slag causes a viscous slag that is hard to foam. Too much FeO causes a thin slag with gas bubbles that can not be sustained. Slag samples from steel mills indicated excessive FeO content in the most slags. Excessive FeO increased slag volume, decreased productivity, shortened refractory service life and wasted expensive chemical energy. Implementation issues The current situation of foamy slag practices A survey was conducted of several EAF shops to understand foamy slag practices. The survey showed that each EAF shop has its own practice and formulas to melt steel. In general, steel can be melted by heat treatment in an EAF during a 45 to 60 minute furnace cycle. Scrap steel/iron and slag may be added from 1 to 3 times per heat or added to a furnace continuously. During operation, real foaming time varies from 5 to 20 minutes. There is wide recognition of a need to improve the slag chemistry and foamy slag practice. Team approach and company commitments The major driving forces of recycling spent refractories are economic and regulatory. The company policy and commitments are critical to implement a recycling program. In addition, the enthusiasm and willingness of employees and managers are crucial to achieve recycling goals. The results of recycling should benefit the company, different departments, and individuals in order to keep commitments and enthusiasm. The more people commitmented and involved in recycling will keep recycling programs active. Many successful recycling programs ended because one individual changed his job. Consequently, team approach and good recycling application are very important in any recycling program implementation. Foamy slag quality measurements. 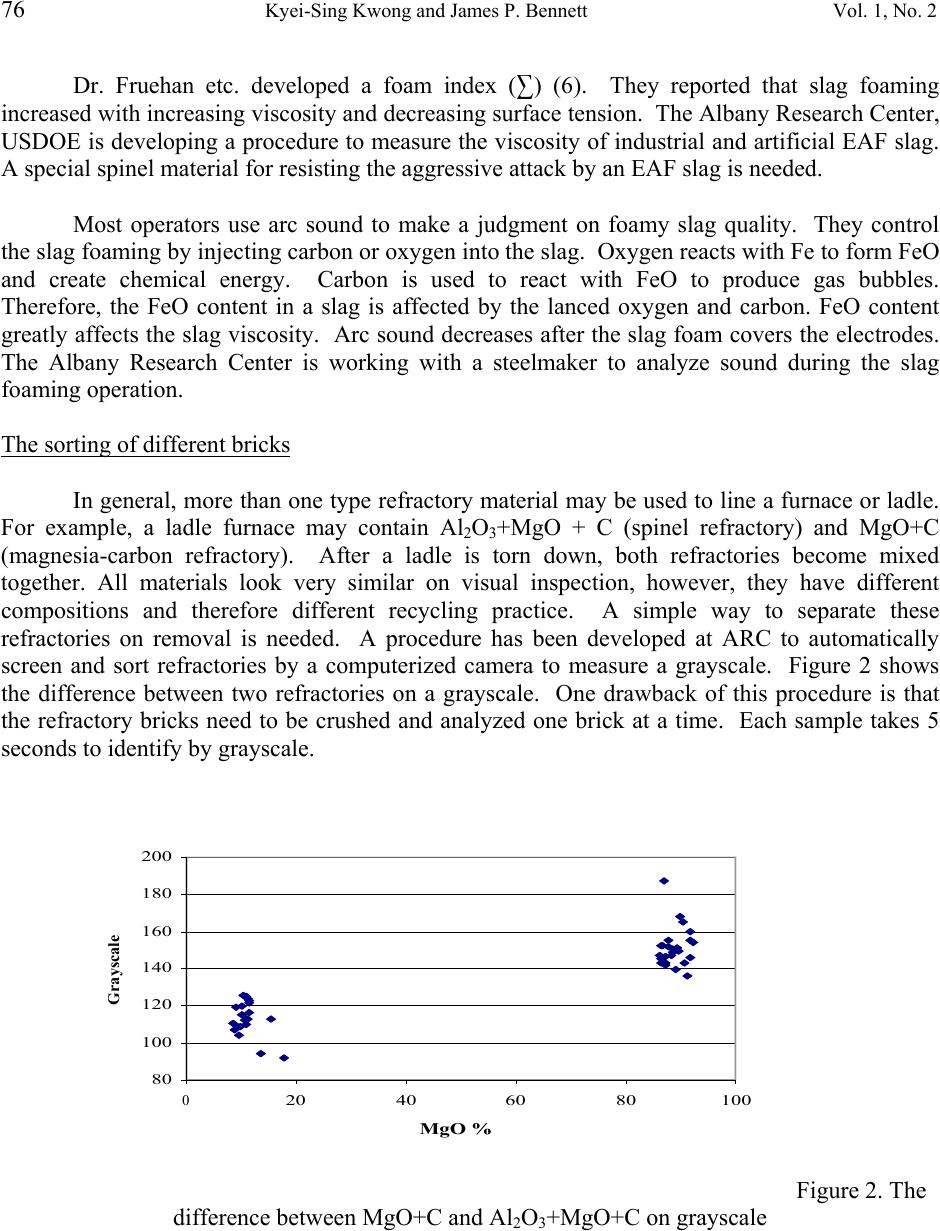 76 Kyei-Sing Kwong and James P. Bennett Vol. 1, No. 2 Dr. Fruehan etc. developed a foam index (3) (6). They reported that slag foaming increased with increasing viscosity and decreasing surface tension. The Albany Research Center, USDOE is developing a procedure to measure the viscosity of industrial and artificial EAF slag. A special spinel material for resisting the aggressive attack by an EAF slag is needed. Most operators use arc sound to make a judgment on foamy slag quality. They control the slag foaming by injecting carbon or oxygen into the slag. Oxygen reacts with Fe to form FeO and create chemical energy. Carbon is used to react with FeO to produce gas bubbles. Therefore, the FeO content in a slag is affected by the lanced oxygen and carbon. FeO content greatly affects the slag viscosity. Arc sound decreases after the slag foam covers the electrodes. The Albany Research Center is working with a steelmaker to analyze sound during the slag foaming operation. The sorting of different bricks In general, more than one type refractory material may be used to line a furnace or ladle. For example, a ladle furnace may contain Al 2 O 3 +MgO + C (spinel refractory) and MgO+C (magnesia-carbon refractory). After a ladle is torn down, both refractories become mixed together. All materials look very similar on visual inspection, however, they have different compositions and therefore different recycling practice. A simple way to separate these refractories on removal is needed. A procedure has been developed at ARC to automatically screen and sort refractories by a computerized camera to measure a grayscale. Figure 2 shows the difference between two refractories on a grayscale. One drawback of this procedure is that the refractory bricks need to be crushed and analyzed one brick at a time. Each sample takes 5 seconds to identify by grayscale. Figure 2. The difference between MgO+C and Al 2 O 3 +MgO+C on grayscale 80 100 120 140 160 180 200 0 20406080100 MgO % G r a y s c a l e  Vol. 1, No. 2 RECYCLING PRACTICES OF SPENT MgO-C REFRACTORIES 77 The dissolution of spent MgO particles Concern exists about the slow dissolution rate of spent MgO refractory aggregates in an EAF slag. Laboratory tests were performed using spent MgO refractory aggregates which are about 4-5 mm in size to verify this concern. No evidence of undissolved particles was found by visual examination. Field tests about this issue will be conducted at a participating steel company to study the dissolving of MgO particles. The issue related to low basicity slag practice The recycling implementation of spent refractory should consider the steel making process control. If the recycling program is based only on the environmental consideration, then the corporation or support from steel companies, departments, or colleagues may not be sufficient to drive it in the future. The most important goal is that recycling practice should not have an adverse effect on the steel making processes or products. As previous mentioned, a low basicity slag practice has several advantages, such as energy savings and less slag generating. However, this low basicity slag practice requires good FeO control which depends on the operators (quality control) and on equipment (lancing). The increased FeO may dilute CaO and affect the sulphur capacity of a slag, producing high sulphur steels. Table VI lists the FeO effect on the change of slag chemistry with three different basicity levels. It shows that CaO content in the lower basicity slag is susceptible to the increased FeO. Therefore, the suggestion of adopting lower basicity slag should consider the process control (slag chemistry quality control). Table VI the chemical composition changes after 1 ton FeO increasing in the slag. C/S = 1.84 C/S = 2.27 C/S = 2.83 original (%) change to (%) original (%) change to (%) Original (%) change to (%) SiO2 23.21 18.84 8.87 15.85 15.34 13.30 CaO 42.71 34.66 43.05 36.15 43.42 37.65 MgO 9.96 8.08 7.92 6.65 6.26 5.43 FeO 24.12 38.41 30.37 41.35 34.97 43.62 Conclusions Successful recycling of spent refractories requires a team approach, a technical understanding, and good plant practices. The low value of spent refractories and low cost of virgin raw materials mean beneficiation cost must be kept low. Knowledge of the refractories and industry processes can help in the proper selection of spent refractory applications. Team approach is crucial for recycling programs. This paper demonstrated the importance of knowing  78 Kyei-Sing Kwong and James P. Bennett Vol. 1, No. 2 refractory, ceramic, and industry processes when using spent refractories as slag conditioners to saturate slags with MgO. The designed application is not only for recycling spent refractories, but also for longer refractory service life, energy saving, less slag generation, efficiency enhancement and productivity improvement. REFERENCES 1 Iron & Steelmaker “EAF Roundup” Iron & Steelmaker, 26 [5] 16-28 (1999). 2 D. Capodilupo, P. Masucci, G. Brascugli, V. De Angelis “Operating Improvements in Electric Steel Production on Terni EAF after Introduction of Slag Foaming Practice,” Proceeding of the Sixth International Iron and Steel Congress, 98-104 (1990). Nagoya, ISIJ. 3 E. Pretorius, “Slag Fundamentals”; in An Introduction to the Theory and Practice of EF Steelmaking, Iron & Steel Society short course book, New Orleans, LA Nov. 15, 1998. 4 A. Jones, A.Gladwin “Slag Fundamentals”; in An Introduction to the Theory and Practice of EF Steelmaking, Iron & Steel Society short course book, New Orleans, LA Nov. 15, 1998. 5 K. S. Kwong, J. P. Bennett “Balancing MgO for Foamy Slag and Refractory Protection” D.L. Schroeder & Associates, Twenty Second Annual Sympsium, Process Systems for Electric Furnace Steelmaking, Orlando, Florida, November 9 &10, 2000 6 I.Kimihisa, R. Fruehan “Slag Foaming in Electric Furnace Steelmaking” Electric Furnace Procedings 345-351 (1987). |

