 Open Journal of Soil Science, 2012, 2, 196-201 http://dx.doi.org/10.4236/ojss.2012.22024 Published Online June 2012 (http://www.SciRP.org/journal/ojss) Changes in Bacterial Density, CO2 Evolution and Enzyme Activities in Poultry Dung Amended Soil Lakshmikanti Bhoi, Pramod Chandra Mishra* Department of Environmental Sciences, Sambalpur University, Orissa, India. Email: *profpcmishra@gmail.com Received March 31st, 2012; revised April 28th, 2012; accepted May 12th, 2012 ABSTRACT The utilization of cattle and poultry manure as organic fertilizer improves soil productivity, but arsenic contaminated poultry dung may interfere in soil metabolism and soil fertility. The study was conducted to assess the effects of poultry dung as well as arsenic contamination on soil properties in 1%, 3% and 5% poultry dung amended soil and 1, 5 and 10 ppm sodium arsenite contaminated soil. pH and conductivity were found to be increasing with increase in poultry dung in soil. Other chemical parameters like nitrate, phosphate and organic carbon were found higher in poultry dung amended soil than that of arsenic contaminated soil. Soil bacteria, CO2 evolution and enzymatic activities like amylase, invertase and dehydrogenase were also found higher in poultry dung amended soil suggesting the effectiveness of poul- try dung in enhancing soil productivity, even if it was contaminated by As through feed additive. Keywords: Soil; Poultry Dung; Arsenic; Soil Enzyme; CO2 Evolution; Bacteria 1. Introduction The increasing demand of chicken meat has prompted more poultry farming with consequent effects on in- creased utilization of organic wastes (e.g. chicken dung manure) as fertilizers. Organic wastes contain varying amounts of water, mineral nutrients, organic matter [1,2]. While the use of organic wastes as manure has been in practice for centuries world-wide [3,4], there still exists a need to assess the potential impacts of poultry manure on soil chemical properties and crop yield and in particular evaluating the critical application levels. Moreover, the need and utilization of poultry manure has overtaken the use of other animal manure (e.g. pig manure, kraal ma- nure) because of its high content of nitrogen, phosphorus and potassium [5,6]. Escalating prices of inorganic fer- tilizers due to the increase in the fuel prices has also prompted the use of poultry manure [7]. Similarly, or- ganic wastes are also being advocated for by different environmental organizations world-wide to preserve the sustainability of agricultural systems. Recent studies have shown a host of nutrient management practices un- dertaken by small scale African farmers [8]. While the relative adoption rates between organic and mineral nu- trients vary by location, the incidence of organic prac- tices is often more than the use of mineral fertilizers. Increase in nitrogen levels from 40% - 60% and 17% - 38% with respect to control for Norfolk sandy soils and Cecil sandy loam soils, respectively following applica- tion of manure has been reported [9]. In addition, appli- cation of poultry manure to soil enhances concentration of water soluble salts. Plants absorb plant nutrients in the form of soluble salts, but excessive accumulation of so- luble salts (or soil salinity) suppresses plant growth. Roxarsone is added to poultry feed at the rate of 22.7 to 45.4 grams per ton, or 0.0025 to 0.005 per cent as feed additive [10]. Most of the roxarsone passes through the birds and is excreted unchanged. Each broiler excretes about 150 milligrams of roxarsone during the 42-day growth period in which it is administered [11]. Chemical and microbial reactions readily transform roxarsone into inorganic forms of arsenic. These inorganic forms are then subject to a variety of chemical and biological reac- tions in the soil. Soil mineralogy, soil moisture, soil pH, and microbial reactions all determine arsenic mobility and its toxicity. 2. Materials and Methods The physico-chemical analysis like pH, conductivity, organic carbon, nitrate, phosphate and arsenic content of soil samples were done following standard methods. pH and conductivity were measured using digital pH meter and conductivity meter with automatic temperature com- pensation and calibrated with calibration solutions [12]. Organic Carbon was determined by Walkey-Black titra- tion method [13], Nitrate was estimated by phenol disul- *Corresponding author. Copyright © 2012 SciRes. OJSS 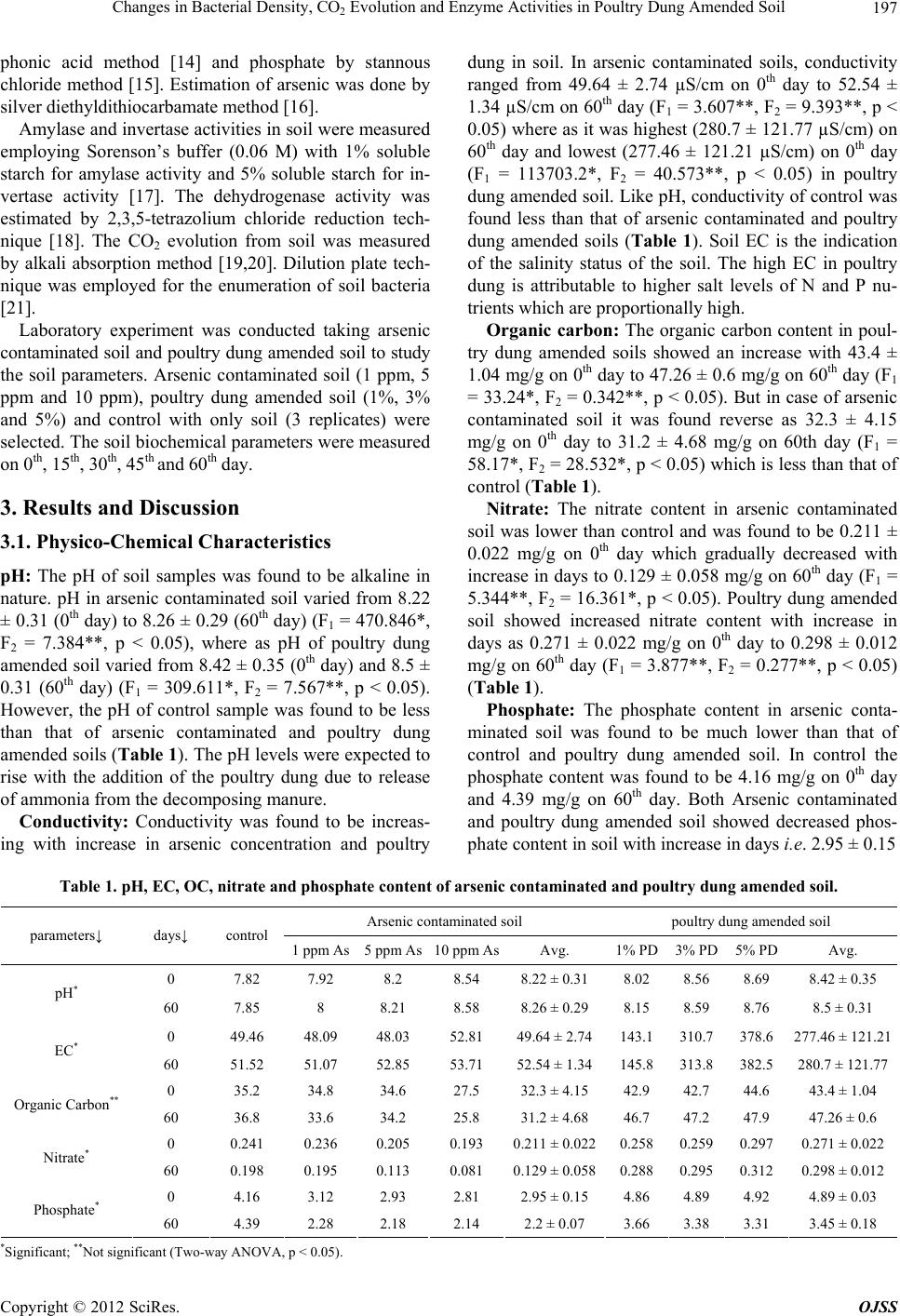 Changes in Bacterial Density, CO2 Evolution and Enzyme Activities in Poultry Dung Amended Soil 197 phonic acid method [14] and phosphate by stannous chloride method [15]. Estimation of arsenic was done by silver diethyldithiocarbamate method [16]. Amylase and invertase activities in soil were measured employing Sorenson’s buffer (0.06 M) with 1% soluble starch for amylase activity and 5% soluble starch for in- vertase activity [17]. The dehydrogenase activity was estimated by 2,3,5-tetrazolium chloride reduction tech- nique [18]. The CO2 evolution from soil was measured by alkali absorption method [19,20]. Dilution plate tech- nique was employed for the enumeration of soil bacteria [21]. Laboratory experiment was conducted taking arsenic contaminated soil and poultry dung amended soil to study the soil parameters. Arsenic contaminated soil (1 ppm, 5 ppm and 10 ppm), poultry dung amended soil (1%, 3% and 5%) and control with only soil (3 replicates) were selected. The soil biochemical parameters were measured on 0th, 15th, 30th, 45th and 60th day. 3. Results and Discussion 3.1. Physico-Chemical Characteristics pH: The pH of soil samples was found to be alkaline in nature. pH in arsenic contaminated soil varied from 8.22 ± 0.31 (0th day) to 8.26 ± 0.29 (60th day) (F1 = 470.846*, F2 = 7.384**, p < 0.05), where as pH of poultry dung amended soil varied from 8.42 ± 0.35 (0th day) and 8.5 ± 0.31 (60th day) (F1 = 309.611*, F2 = 7.567**, p < 0.05). However, the pH of control sample was found to be less than that of arsenic contaminated and poultry dung amended soils (Table 1). The pH levels were expected to rise with the addition of the poultry dung due to release of ammonia from the decomposing manure. Conductivity: Conductivity was found to be increas- ing with increase in arsenic concentration and poultry dung in soil. In arsenic contaminated soils, conductivity ranged from 49.64 ± 2.74 µS/cm on 0th day to 52.54 ± 1.34 µS/cm on 60th day (F1 = 3.607**, F2 = 9.393**, p < 0.05) where as it was highest (280.7 ± 121.77 µS/cm) on 60th day and lowest (277.46 ± 121.21 µS/cm) on 0th day (F1 = 113703.2*, F2 = 40.573**, p < 0.05) in poultry dung amended soil. Like pH, conductivity of control was found less than that of arsenic contaminated and poultry dung amended soils (Table 1). Soil EC is the indication of the salinity status of the soil. The high EC in poultry dung is attributable to higher salt levels of N and P nu- trients which are proportionally high. Organic carbon: The organic carbon content in poul- try dung amended soils showed an increase with 43.4 ± 1.04 mg/g on 0th day to 47.26 ± 0.6 mg/g on 60th day (F1 = 33.24*, F2 = 0.342**, p < 0.05). But in case of arsenic contaminated soil it was found reverse as 32.3 ± 4.15 mg/g on 0th day to 31.2 ± 4.68 mg/g on 60th day (F1 = 58.17*, F2 = 28.532*, p < 0.05) which is less than that of control (Table 1). Nitrate: The nitrate content in arsenic contaminated soil was lower than control and was found to be 0.211 ± 0.022 mg/g on 0th day which gradually decreased with increase in days to 0.129 ± 0.058 mg/g on 60th day (F1 = 5.344**, F2 = 16.361*, p < 0.05). Poultry dung amended soil showed increased nitrate content with increase in days as 0.271 ± 0.022 mg/g on 0th day to 0.298 ± 0.012 mg/g on 60th day (F1 = 3.877**, F2 = 0.277**, p < 0.05) (Table 1). Phosphate: The phosphate content in arsenic conta- minated soil was found to be much lower than that of control and poultry dung amended soil. In control the phosphate content was found to be 4.16 mg/g on 0th day and 4.39 mg/g on 60th day. Both Arsenic contaminated and poultry dung amended soil showed decreased phos- phate content in soil with increase in days i.e. 2.95 ± 0.15 Table 1. pH, EC, OC, nitrate and phosphate conte nt of ar senic contaminated and poultry dung amended soil. Arsenic contaminated soil poultry dung amended soil parameters↓ days↓ control 1 ppm As 5 ppm As10 ppm AsAvg. 1% PD3% PD 5% PD Avg. 0 7.82 7.92 8.2 8.54 8.22 ± 0.31 8.02 8.56 8.69 8.42 ± 0.35 pH* 60 7.85 8 8.21 8.58 8.26 ± 0.29 8.15 8.59 8.76 8.5 ± 0.31 0 49.46 48.09 48.03 52.81 49.64 ± 2.74143.1310.7 378.6 277.46 ± 121.21 EC* 60 51.52 51.07 52.85 53.71 52.54 ± 1.34145.8313.8 382.5 280.7 ± 121.77 0 35.2 34.8 34.6 27.5 32.3 ± 4.15 42.9 42.7 44.6 43.4 ± 1.04 Organic Carbon** 60 36.8 33.6 34.2 25.8 31.2 ± 4.68 46.7 47.2 47.9 47.26 ± 0.6 0 0.241 0.236 0.205 0.193 0.211 ± 0.0220.2580.259 0.297 0.271 ± 0.022 Nitrate* 60 0.198 0.195 0.113 0.081 0.129 ± 0.0580.2880.295 0.312 0.298 ± 0.012 0 4.16 3.12 2.93 2.81 2.95 ± 0.15 4.86 4.89 4.92 4.89 ± 0.03 Phosphate* 60 4.39 2.28 2.18 2.14 2.2 ± 0.07 3.66 3.38 3.31 3.45 ± 0.18 *Significant; **Not significant (Two-way ANOVA, p < 0.05). Copyright © 2012 SciRes. OJSS 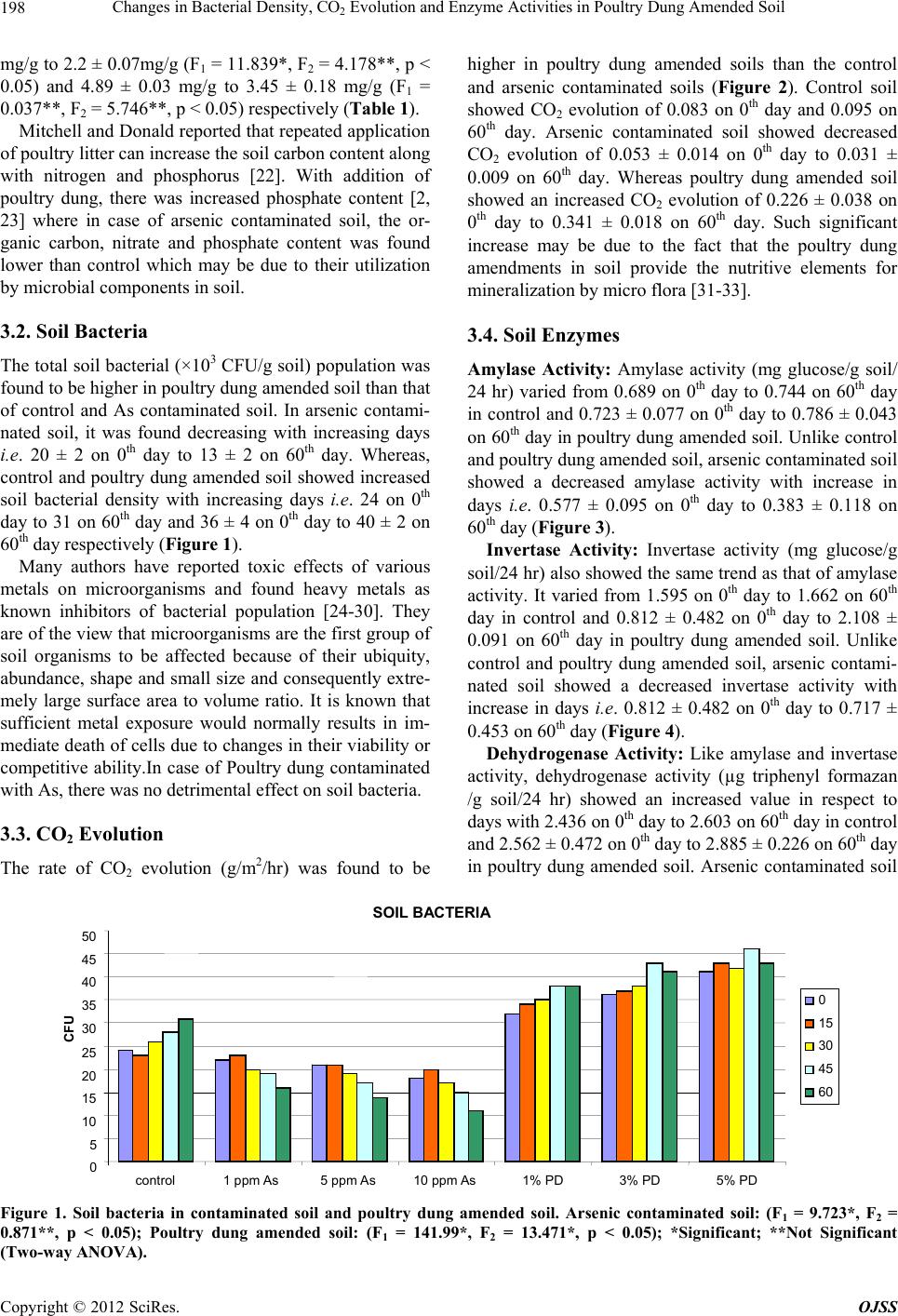 Changes in Bacterial Density, CO2 Evolution and Enzyme Activities in Poultry Dung Amended Soil 198 mg/g to 2.2 ± 0.07mg/g (F1 = 11.839*, F2 = 4.178**, p < 0.05) and 4.89 ± 0.03 mg/g to 3.45 ± 0.18 mg/g (F1 = 0.037**, F2 = 5.746**, p < 0.05) respectively (Table 1). Mitchell and Donald reported that repeated application of poultry litter can increase the soil carbon content along with nitrogen and phosphorus [22]. With addition of poultry dung, there was increased phosphate content [2, 23] where in case of arsenic contaminated soil, the or- ganic carbon, nitrate and phosphate content was found lower than control which may be due to their utilization by microbial components in soil. 3.2. Soil Bacteria The total soil bacterial (×103 CFU/g soil) population was found to be higher in poultry dung amended soil than that of control and As contaminated soil. In arsenic contami- nated soil, it was found decreasing with increasing days i.e. 20 ± 2 on 0th day to 13 ± 2 on 60th day. Whereas, control and poultry dung amended soil showed increased soil bacterial density with increasing days i.e. 24 on 0th day to 31 on 60th day and 36 ± 4 on 0th day to 40 ± 2 on 60th day respectively (Figure 1). Many authors have reported toxic effects of various metals on microorganisms and found heavy metals as known inhibitors of bacterial population [24-30]. They are of the view that microorganisms are the first group of soil organisms to be affected because of their ubiquity, abundance, shape and small size and consequently extre- mely large surface area to volume ratio. It is known that sufficient metal exposure would normally results in im- mediate death of cells due to changes in their viability or competitive ability.In case of Poultry dung contaminated with As, there was no detrimental effect on soil bacteria. 3.3. CO2 Evolution The rate of CO2 evolution (g/m2/hr) was found to be higher in poultry dung amended soils than the control and arsenic contaminated soils (Figure 2). Control soil showed CO2 evolution of 0.083 on 0th day and 0.095 on 60th day. Arsenic contaminated soil showed decreased CO2 evolution of 0.053 ± 0.014 on 0th day to 0.031 ± 0.009 on 60th day. Whereas poultry dung amended soil showed an increased CO2 evolution of 0.226 ± 0.038 on 0th day to 0.341 ± 0.018 on 60th day. Such significant increase may be due to the fact that the poultry dung amendments in soil provide the nutritive elements for mineralization by micro flora [31-33]. 3.4. Soil Enzymes Amylase Activity: Amylase activity (mg glucose/g soil/ 24 hr) varied from 0.689 on 0th day to 0.744 on 60th day in control and 0.723 ± 0.077 on 0th day to 0.786 ± 0.043 on 60th day in poultry dung amended soil. Unlike control and poultry dung amended soil, arsenic contaminated soil showed a decreased amylase activity with increase in days i.e. 0.577 ± 0.095 on 0th day to 0.383 ± 0.118 on 60th day (Figure 3). Invertase Activity: Invertase activity (mg glucose/g soil/24 hr) also showed the same trend as that of amylase activity. It varied from 1.595 on 0th day to 1.662 on 60th day in control and 0.812 ± 0.482 on 0th day to 2.108 ± 0.091 on 60th day in poultry dung amended soil. Unlike control and poultry dung amended soil, arsenic contami- nated soil showed a decreased invertase activity with increase in days i.e. 0.812 ± 0.482 on 0th day to 0.717 ± 0.453 on 60th day (Figure 4). Dehydrogenase Activity: Like amylase and invertase activity, dehydrogenase activity (µg triphenyl formazan /g soil/24 hr) showed an increased value in respect to days with 2.436 on 0th day to 2.603 on 60th day in control and 2.562 ± 0.472 on 0th day to 2.885 ± 0.226 on 60th day in poultry dung amended soil. Arsenic contaminated soil SOIL BACTERIA 0 5 10 15 20 25 30 35 40 45 50 control 1 ppm As 5 ppm As10 ppm As1% PD3% PD5% PD CFU 0 15 30 45 60 Figure 1. Soil bacteria in contaminated soil and poultry dung amended soil. Arsenic contaminated soil: (F1 = 9.723*, F2 = 0.871**, p < 0.05); Poultry dung amended soil: (F1 = 141.99*, F2 = 13.471*, p < 0.05); *Significant; **Not Significant (Two-w ay ANOVA). Copyright © 2012 SciRes. OJSS 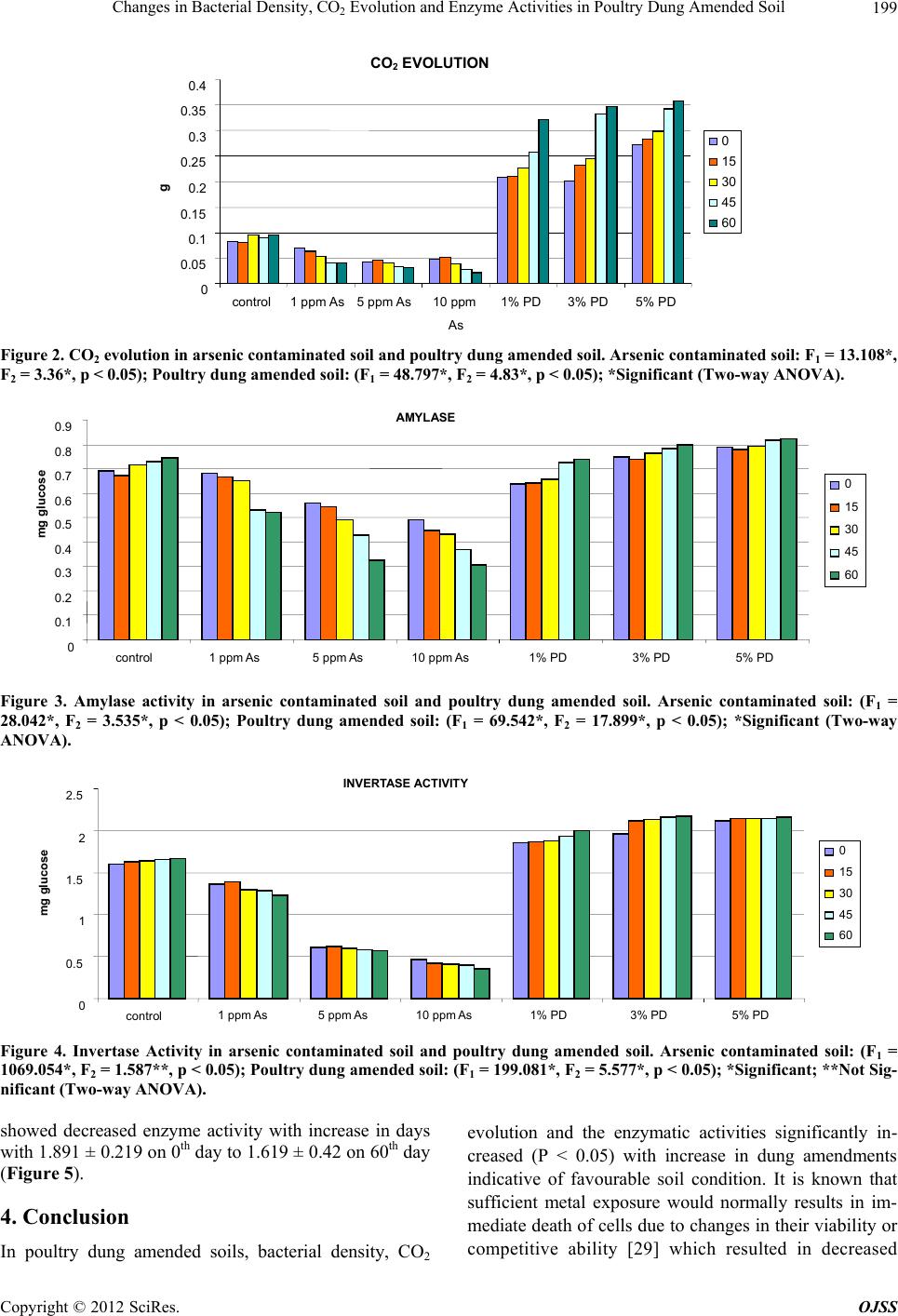 Changes in Bacterial Density, CO2 Evolution and Enzyme Activities in Poultry Dung Amended Soil 199 CO 2 EVOLUTION 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 control 1 ppm As 5 ppm As10 ppm s 1% PD3% PD5% PD g 0 15 30 45 60 Figure 2. CO2 evolution in arsenic contaminated soil and poultry dung amended so il. Ar senic contaminated soil: F1 = 13.108*, F2 = 3.36*, p < 0.05); Poultry dung amended soil: (F1 = 48.797*, F2 = 4.83*, p < 0.05); *Significant (Two-way ANOVA). AMYLASE 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 contr ol 1 ppm As 5 ppm As10 ppm As1% PD3% PD5% PD 0 15 30 45 60 mg glucose Figure 3. Amylase activity in arsenic contaminated soil and poultry dung amended soil. Arsenic contaminated soil: (F1 = 28.042*, F2 = 3.535*, p < 0.05); Poultry dung amended soil: (F1 = 69.542*, F2 = 17.899*, p < 0.05); *Significant (Two-way ANOVA). IN VE R TASE ACTI V ITY 0 0.5 1 1.5 2 2.5 control 1 ppm As 5 ppm As10 ppm As1% PD3% PD5% PD mg glucose 0 15 30 45 60 Figure 4. Invertase Activity in arsenic contaminated soil and poultry dung amended soil. Arsenic contaminated soil: (F1 = 1069.054*, F2 = 1.587**, p < 0.05); Poultry dung amended soil: (F1 = 199.081*, F2 = 5.577*, p < 0.05); *Significant; **Not Sig- nificant (Two-way ANOVA). showed decreased enzyme activity with increase in days with 1.891 ± 0.219 on 0th day to 1.619 ± 0.42 on 60th day (Figure 5). 4. Conclusion In poultry dung amended soils, bacterial density, CO2 evolution and the enzymatic activities significantly in- creased (P < 0.05) with increase in dung amendments indicative of favourable soil condition. It is known that sufficient metal exposure would normally results in im- mediate death of cells due to changes in their viability or competitive ability [29] which resulted in decreased Copyright © 2012 SciRes. OJSS 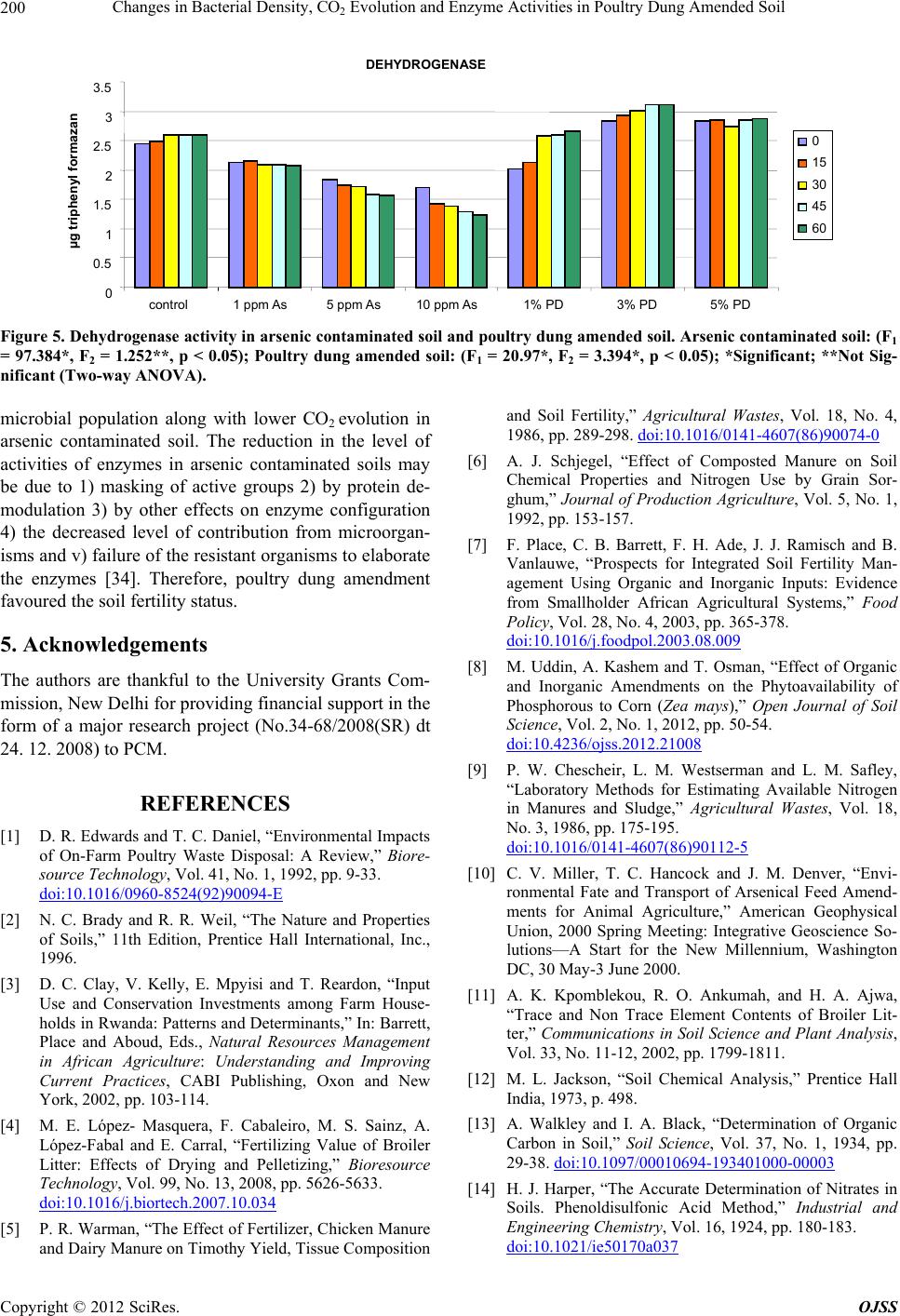 Changes in Bacterial Density, CO2 Evolution and Enzyme Activities in Poultry Dung Amended Soil 200 DEHYDROGENASE 0 0.5 1 1.5 2 2.5 3 3.5 control 1 ppm As 5 ppm As10 ppm As1% PD3% PD5% PD µg triphenyl formazan 0 15 30 45 60 Figure 5. Dehydrogenase activity in arsenic contaminated soil and poultry dung amende d soil. Arsenic contaminated soil: (F1 = 97.384*, F2 = 1.252**, p < 0.05); Poultry dung amended soil: (F1 = 20.97*, F2 = 3.394*, p < 0.05); *Significant; **Not Sig- nificant (Two-way ANOVA). microbial population along with lower CO2 evolution in arsenic contaminated soil. The reduction in the level of activities of enzymes in arsenic contaminated soils may be due to 1) masking of active groups 2) by protein de- modulation 3) by other effects on enzyme configuration 4) the decreased level of contribution from microorgan- isms and v) failure of the resistant organisms to elaborate the enzymes [34]. Therefore, poultry dung amendment favoured the soil fertility status. 5. Acknowledgements The authors are thankful to the University Grants Com- mission, New Delhi for providing financial support in the form of a major research project (No.34-68/2008(SR) dt 24. 12. 2008) to PCM. REFERENCES [1] D. R. Edwards and T. C. Daniel, “Environmental Impacts of On-Farm Poultry Waste Disposal: A Review,” Biore- source Technology, Vol. 41, No. 1, 1992, pp. 9-33. doi:10.1016/0960-8524(92)90094-E [2] N. C. Brady and R. R. Weil, “The Nature and Properties of Soils,” 11th Edition, Prentice Hall International, Inc., 1996. [3] D. C. Clay, V. Kelly, E. Mpyisi and T. Reardon, “Input Use and Conservation Investments among Farm House- holds in Rwanda: Patterns and Determinants,” In: Barrett, Place and Aboud, Eds., Natural Resources Management in African Agriculture: Understanding and Improving Current Practices, CABI Publishing, Oxon and New York, 2002, pp. 103-114. [4] M. E. López- Masquera, F. Cabaleiro, M. S. Sainz, A. López-Fabal and E. Carral, “Fertilizing Value of Broiler Litter: Effects of Drying and Pelletizing,” Bioresource Technology, Vol. 99, No. 13, 2008, pp. 5626-5633. doi:10.1016/j.biortech.2007.10.034 [5] P. R. Warman, “The Effect of Fertilizer, Chicken Manure and Dairy Manure on Timothy Yield, Tissue Composition and Soil Fertility,” Agricultural Wastes, Vol. 18, No. 4, 1986, pp. 289-298. doi:10.1016/0141-4607(86)90074-0 [6] A. J. Schjegel, “Effect of Composted Manure on Soil Chemical Properties and Nitrogen Use by Grain Sor- ghum,” Journal of Production Agriculture, Vol. 5, No. 1, 1992, pp. 153-157. [7] F. Place, C. B. Barrett, F. H. Ade, J. J. Ramisch and B. Vanlauwe, “Prospects for Integrated Soil Fertility Man- agement Using Organic and Inorganic Inputs: Evidence from Smallholder African Agricultural Systems,” Food Policy, Vol. 28, No. 4, 2003, pp. 365-378. doi:10.1016/j.foodpol.2003.08.009 [8] M. Uddin, A. Kashem and T. Osman, “Effect of Organic and Inorganic Amendments on the Phytoavailability of Phosphorous to Corn (Zea mays),” Open Journal of Soil Science, Vol. 2, No. 1, 2012, pp. 50-54. doi:10.4236/ojss.2012.21008 [9] P. W. Chescheir, L. M. Westserman and L. M. Safley, “Laboratory Methods for Estimating Available Nitrogen in Manures and Sludge,” Agricultural Wastes, Vol. 18, No. 3, 1986, pp. 175-195. doi:10.1016/0141-4607(86)90112-5 [10] C. V. Miller, T. C. Hancock and J. M. Denver, “Envi- ronmental Fate and Transport of Arsenical Feed Amend- ments for Animal Agriculture,” American Geophysical Union, 2000 Spring Meeting: Integrative Geoscience So- lutions—A Start for the New Millennium, Washington DC, 30 May-3 June 2000. [11] A. K. Kpomblekou, R. O. Ankumah, and H. A. Ajwa, “Trace and Non Trace Element Contents of Broiler Lit- ter,” Communications in Soil Science and Plant Analysis, Vol. 33, No. 11-12, 2002, pp. 1799-1811. [12] M. L. Jackson, “Soil Chemical Analysis,” Prentice Hall India, 1973, p. 498. [13] A. Walkley and I. A. Black, “Determination of Organic Carbon in Soil,” Soil Science, Vol. 37, No. 1, 1934, pp. 29-38. doi:10.1097/00010694-193401000-00003 [14] H. J. Harper, “The Accurate Determination of Nitrates in Soils. Phenoldisulfonic Acid Method,” Industrial and Engineering Chemistry, Vol. 16, 1924, pp. 180-183. doi:10.1021/ie50170a037 Copyright © 2012 SciRes. OJSS  Changes in Bacterial Density, CO2 Evolution and Enzyme Activities in Poultry Dung Amended Soil 201 [15] S. R. Olsen, C. V. Cole, F. S. Watanabe and L. A. Dean, “Estimation of Available Phosphorus in Soils by Extrac- tion with Sodium Bicarbonate,” USDA Cire, US Gov. Print. Office, Washington DC, Vol. 939, 1954, pp. 1-19. [16] D. C. Ballinger, R. J. Lishaka and M. E. Gales, “Applica- tion of Silver Diethyldithiocarbamate Method to Deter- mination of Arsenic,” Journal of the American Water Works Association, Vol. 54, 1962, p. 1424. [17] P. C. Mishra, R. K. Mohanty and M. C. Dash, “Enzyme activity in Subtropical Surface Soils under Pasture,” In- dian Journal of Agricultural Chemistry, Vol. 12, 1979, pp. 9-24. [18] B. F. Casida, “Microbial Metabolic Activity in Soil as Measured by Dehydrogenase Determination,” Applied and Environmental Microbiology, Vol. 34, 1977, pp. 630- 636. [19] M. Witkamp, “Rate of Carbon Dioxide Evolution from the Forest Floor,” Ecology, Vol. 47, 1966, pp. 492-494. doi:10.2307/1932992 [20] J. P. E. Anderson, “Soil Respiration,” In: A. L. Page, R. H. Miller and D. R. Kenney, Eds., Method of Soil Analy- sis, Part 2, Chemical and Microbial Properties, 2nd Edi- tion, 1982, pp. 837-871. [21] D. K. Jha, G. D. Sharma and R. R. Mishra, “Soil Micro- bial Population Numbers and Enzyme Activities in Rela- tion to Altitude and Forest Degradation,” Soil Biology and Biochemistry, Vol. 24, No. 8, 1992, pp. 761-767. doi:10.1016/0038-0717(92)90250-2 [22] P. Mitchell and B. Donald, “The Nature and Significance of Public Exposure to Arsenic: A Review of Its Rele- vance to Southwest England,” Environmental Geochem- istry and Health, Vol. 17, No. 2, 1995, pp. 57-82. doi:10.1007/BF00146709 [23] T. M. Agbede, S. O. Ojeniyi and A. J. Adeyemo, “Effect of Poultry Manure on Soil Physical and Chemical Proper- ties, Growth and Grain Yield of Sorghum in Southwest Nigeria,” American-Eurasian Journal of Sustainable Ag- riculture, Vol. 2, No. 1, 2008, pp. 72-77. [24] M. J. Jordan and M. P. Lechevalier, “Effect of Zinc Smelter Emissions on Forest Soil Microflora,” Canadian Journal of Microbiology, Vol. 21, 1975, pp. 1855-1865. doi:10.1139/m75-269 [25] S. T. Williams, T. McNeilly and E. M. H. Wellington, “The Decomposition of Vegetation Growing on Metal Mine Waste,” Soil Biology and Biochemistry, Vol. 9, 1997, pp. 271-275. doi:10.1016/0038-0717(77)90034-7 [26] M. Wood, “A Mechanism of Aluminum Toxicity to Soil Bacteria and Ecological Implications,” In: R. A. Date, et al., Eds., Plant-Soil Interactions at Low pH, Kluwer Aca- demic Publishers, Netherlands, 1995, pp. 173-179. [27] S. Dahlin, E. Witter, A. M. Martensson, A. Turner and E. Baath, “Where’s the Limit? Changes in the Microbi- ological Properties of Agricultural Soils at Low Levels of Metal Contamination,” Soil Biology and Biochemistry, Vol. 29, 1997, pp. 1405-1415. doi:10.1016/S0038-0717(97)00048-5 [28] R. G. Kuperman and M. M. Carreiro, “Soil Heavy Metal Concentrations, Microbial Biomass and Enzyme Activi- ties in a Contaminated Grassland Ecosystem,” Soil Biol- ogy and Biochemistry, Vol. 29, 1997, pp. 179-190. doi:10.1016/S0038-0717(96)00297-0 [29] K. E. Giller, E. Witter and S. P. McGrath, “Toxicity of Heavy Metals to Microorganisms and Microbial Proc- esses in Agricultural Soils: A Review,” Soil Biology and Biochemistry, Vol. 30, No. 10-11, 1998, pp. 1389-1414. doi:10.1016/S0038-0717(97)00270-8 [30] B. Stenberg, “Monitoring Soil Quality of Arable Land: Microbiological Indicators,” Acta Agriculturae Scandi- navica Section B. Soil and Plant Science, Vol. 49, 1999, pp. 1-24. [31] T. Fan, B.A. Stewart, W. Yong, L. Junjie and Z. Guangye, “Long-Term Fertilization Effects on Grain Yield, Water- Use Efficiency and Soil Fertility in the Dryland of Loess Plateau in China,” Agriculture, Ecosystems & Environ- ment, Vol. 106, No. 4, 2005, pp. 313-329. doi:10.1016/j.agee.2004.09.003 [32] S. B. Wuest, T. C. Caesar-Ton That, S. F. Wright and J. D. Williams, “Organic Matter Addition, N, and Residue Burning Effects on Infiltration, Biological, and Physical Properties of an Intensively Tilled Silt-Loam Soil,” Soil & Tillage Research, Vol. 84, No. 2, 2005, pp. 154-167. doi:10.1016/j.still.2004.11.008 [33] P. Wang, J. T. Durkalski, W. Yu, H. A. J. Hoitink and W. A. Dick, “Agronomic and Soil Responses to Compost and Manure Amendments under Different Tillage System,” Soil Science, Vol. 171, No. 6, 2006, pp. 456-467. doi:10.1097/01.ss.0000227824.77488.ac [34] G. Tyler, “Heavy Metals in Soil Biology and Biochemis- try,” In: E. A. Paul and J. N. Ladd, Eds., Soil Biochemis- try, Moral Dekker, New York, 1991, pp. 371-414. Copyright © 2012 SciRes. OJSS
|