 Open Journal of Soil Science, 2012, 2, 100-110 http://dx.doi.org/10.4236/ojss.2012.22015 Published Online June 2012 (http://www.SciRP.org/journal/ojss) Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs* Oskars Purmalis, Maris Klavins Department of Environmental Sciences, University of Latvia, Riga, Latvia. Email: maris.klavins@lu.lv Received March 12th, 2012; revised April 13th, 2012, accepted April 30th, 2012 ABSTRACT Studies of the living organic matter humification process are essential for understanding the carbon biogeochemical cycle. The aim of this study is to analyze relations between the properties of peat, peat humic acids and peat humifica- tion degree. The analysis has been done on samples of humic substances extracted from peat profiles in two ombrotro- phic bogs and relations between peat age, decomposition and humification degree, botanical composition and properties of peat humic acids (elemental, functional composition) were studied. The found variability of peat properties is less significant than differences in the properties of peat-forming living matter, thus revealing the dominant impact of humi- fication process on the properties of peat. Correspondingly, composition of peat humic acids is little affected by differ- ences in the composition of precursor living organic material. Keywords: Peat; Humic Substances; Humic Acids; Humification 1. Introduction In the carbon biogeochemical cycle, the transformation of living organic matter into refractory part of organic matter (humic substances, such as humic acids, fulvic acids, and humin) or humification is of key importance. Humification can be defined as the transformation of numerous groups of substances (proteins, carbohydrates, lipids etc.) and individual molecules present in living organic matter into groups of substances with similar properties (humic substances) [1]. Humification plays an important role in the diagenesis of fossil carbon deposits [2]. Humification is a sum of very complex processes in- cluding degradation and synthetic reactions, but also con- sidering the high variability of environmental conditions under which living organic matter decays, slow pace of humification reactions and large number of structural di- fferences of the organic molecules composing living or- ganic matter. It can be supposed that humification condi- tions may have an impact on the structure and properties of refractory intermediate transformation products of liv- ing organic matter—humic substances. From this per- spective, it is important to study humification processes in a relatively homogeneous and stable environment, for example, bogs to reduce the impact of natural environ- mental variability. Peat is a light brown to black organic material, which is formed under waterlogged conditions from the partial decomposition of mosses and other bryophytes, sedges, grasses, shrubs, or trees [3]. The interest in peat proper- ties is growing, as peat is a substance that supports and influences bog and wetland ecosystems, while peat pro- files can serve as “archives” indicating conditions in past environments [4,5]. Significant amounts of organic car- bon are stored in the form of peat. Therefore, peat re- serves play a major role in the carbon biogeochemical cycling, which is of key importance in the context of the ongoing process of climate change [6,7]. Industrial and agricultural uses of peat are growing [8,9], and signifi- cant amounts of peat are mined industrially. Considering this, there is an increasing interest into studies of peat properties and their diagenesis. Humification in peat has been taking place in very different conditions both geo- graphically (from tropical regions to Arctic environment) and temporally (historically peat development can last for many thousands of years). During peat formation even at one particular site, significant changes in vegeta- tion, temperature, amount of precipitation and, corre- spondingly, in the bog hydrological conditions and land use in the basin of wetland [10-12] could have happened. As a result, we can expect not only to understand changes in the properties of peat humic substances but also to identify molecular descriptors of organic matter diagene- sis process. Notwithstanding the importance of this sub- *The research was financially supported by ERAF, the project “Innova- tion in Peat Studies for Development of New Applications”. o: 210/0264/2DP2/2.1.1.1.0/10/APIA/VIAA/037 Copyright © 2012 SciRes. OJSS  Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 101 ject, relations between the properties of peat (especially in full peat profiles) and those of peat humic substances have been studied comparatively little, just in a few studies [12-14]. The aim of this study is to analyze relations between the properties of peat, peat humic acid and peat humifi- cation degree. 2. Materials and Methods 2.1. Site Location In-depth study of peat composition, humification degree and peat humic acid properties was carried out in two ombrotrophic bogs located in the central part of Latvia (Figure 1). Full peat profiles were obtained and cut into 5-cm layers for analysis of peat properties and isolation of humic acids. The analysis of botanical composition was performed microscopically, using a Carl-Zeiss bino- cular microscope, thereby determining the decomposition degree [15]. 2.2. Isolation of Peat Humic Acids HAs were extracted and purified, using procedures re- commended by the International Humic Substances So- ciety (IHSS) [16]. 2.3. Analysis of Peat and Humic Acid Properties The 14C dating was done at the Institute of Geology of the Tallinn Technical University (Estonia). Carbon, hy- drogen, nitrogen and sulphur concentrations in the peat and humic acid samples (elemental analysis of C, H, N, S) were determined by combustion-gas chromatography te- chnique, using an Elemental Analyzer Model EA-1108 (Carlo Erba Instruments). Ash content was measured after heating 50 mg of each peat sample at 750˚C for 8 h. Elemental composition was corrected considering the ash content, and the oxygen amount was calculated as a di- fference. Elemental analysis was used in order to cal- culate the elemental ratios, degree of oxidation ω (1) [17] and index of hydrogen deficiency (2). H 2O 3NC (1) (2C 2) H 2 (2) Atomic ratios were calculated from elemental analysis, using the Equations (3), (4): (Mc O%) OC (MoC%) (3) H (McH%) HC (MC%) (4) where MX is the element molecular mass, and X% is percentage of the element in the sample. UV/Vis spectra were recorded on a Thermospectronic Helios γ UV (Thermoelectron Co) spectrophotometer in a 1-cm quartz cuvette. The ratio E4/E6 [18], i.e. the ratio of absorbance at 465 and 665 nm, was determined for 10 mg of humic acid solutions in 10 ml of 0.05 M NaOH. 2.4. Humification Degree (according to [19] and Modified by [20]) 1.00 g of peat sample was treated for 1(1/2) hrs with 25 ml of 8% NaOH in 25 ml plastic tube in a boiling water bath (95˚C) and filtered. 12.5 ml of the filtrate were Figure 1. Sampling sites: A—Dzelve bog; B—Eipurs bog. Copyright © 2012 SciRes. OJSS  Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 102 diluted to 100 ml and absorption was measured at 540 nm. The peat humification degree was expressed as ab- sorption at 540 nm. 2.5. Carboxylic Groups and Total Acidity An automatic titrator TitroLine easy (Schott-Geräte GmbH) was used for measuring carboxylic and phenolic acidity of each HA. The known Ca-acetate method [16], based on the formation of acetic acid, was used for determining the total number of carboxylic groups. HAs (20 mg) were weighed into a 100 ml Erlenmeyer flask, 10 ml of the 0.2 N calcium acetate solution were added and mixed under N2 for 24 hours. Samples were potentiometrically titrated to pH 9.0 with 0.1 M NaOH. To estimate the total acidity, 20 mg of humic acid, were dispersed in 10 ml 0.1 M Ba(OH)2 solution, which was then shaken overnight un- der N2 atmosphere, filtered and washed with water. The filtrate, together with the washing solution, was poten- tiometrically titrated with 0.1 M HCl down to pH 8.4 under N2 flow. 2.6. Hydrophobicity Hydrophobicity of humic substances was characterized by their distribution between water and polyethylene (PEG) phases (PEG 20000, Fluka) [21] as distribution coefficient KPEGW (analogous to octanol/water distribu- tion coefficient—Kow). The 10% PEG-10% (NH4)2SO4- HA-H2O systems were prepared by mixing 2 ml of 30% PEG solution with 2 ml of ammonium sulphate solution and 2 ml of HA (2 mg/ml in 0.05 M NaOH). The mix- tures were shaken for 10 min. After complete phase sepa- ration, 1 ml was taken from each phase and diluted by 10 times in 0.05 M NaHCO3. Then the absorbances at 465 nm were measured on a DR/2000 spectrophotometer (Hach Co). The distribution coefficients were calculated as follows: KPEGW = absorbance at 465 nm of the top (PEG-rich) phase/absorbance at 465 nm of the bottom phase. 2.7. Fluorescence Spectra Fluorescence spectra were recorded, using Perkin Elmer LS 55 fluorescence spectrometer, on aqueous solutions of each sample at a concentration of 25 mg/L, adjusted to pH 7 with 0.5 M HCl. Emission spectra were recorded (scan speed 500 nm/min, with slit 10.0 nm over the wavelength range of 380 to 650 nm) at a fixed excitation wavelength of 350 nm. The ratio of fluorescence inten- sity at 460 nm to intensity at 510 nm (I460/I510) was used, as previously suggested by [22], as a humification indi- cator. 2.8. Data Treatment Statistical analyses were performed using SPSS 16 Soft- ware. The correspondence of the obtained data to the normal distribution was checked with the Kolmogorov- Smirnov tests. In further analysis, non-parametric me- thods were used. Relationships between different chara- cteristics were assessed by Spearman’s rank correlation coefficients. In all cases the significance level was p = 0.05. 3. Results and Discussion 3.1. Peat Composition and Their Changes Peat humification process and development of peat hu- mic acids were studied in the peat profiles from two he- terogeneous ombrotrophic bogs in Latvia. The results of the paleobotanical investigations (bota- nical composition, pollen analysis) indicate both diffe- rences and similarities in the development and peat pro- perties of the studied bogs. Dzelve Bog has been formed due to paludification of sandy ground as result of ground- water level increase and wet conditions during the small depression after the Ice Age. A raised bog cotton grass peat layer covers the sandy bottom, overlaid by pine- cotton grass peat. The upper part of peat section is repre- sented by a 3.2 m thick Sphagnum fuscum peat layer with a decomposition level 9% to 17% (Figure 2). The bota- nical composition of most of the bog is relatively het- erogeneous: Sphagnum fuscum (60% - 75%), Eriopho- rum vaginatum (10% - 15%), Sphagnum rubellum (10% - 15%) and dwarf shrubs (10% - 15%). The botanical composition of Eipurs Bog is comple- tely different, although it is of a similar origin (Figure 3). The lowest part of Eipurs Bog is formed by fen wood- grass peat, Hypnum and sedge-Hypnum peat (Figure 3), and these layers are covered by transition type wood peat. The upper part is represented by a 3.45 m thick layer of raised bog peat of different types and decomposition degrees. For example, well decomposed (40% - 48%) pine-cotton grass peat occurs at the depth interval of 1.18 - 1.39 m (Figure 3). Although these bogs are located comparatively close to each other (distance 12 km), their local conditions for peat formation have been different. Basic peat properties were analyzed, using peat ele- mental (C, H, N, O, S) composition. The elemental com- position of the studied peat cores are summarized in Figure 4, Table 1. The ash contents in the studied bogs range between 0.30% ± 0.05% and 6.10% ± 0.05%, with an average content of 1.8 ± 0.05. The C concentrations range from 40 to 55%, H—from 5.4% to 6.7%, N—from 0.5% to 1.5%, S—from 0.2% to 1.7% and O—from 38% to 49%. The elemental composition of peat in Eipurs Bog is comparatively variable and reflects changes in the peat decomposition degree and peat types. C concentration in peat is increasing starting from the depth of 1 m up to the level of 53% and then again decreasing. H concentrations Copyright © 2012 SciRes. OJSS 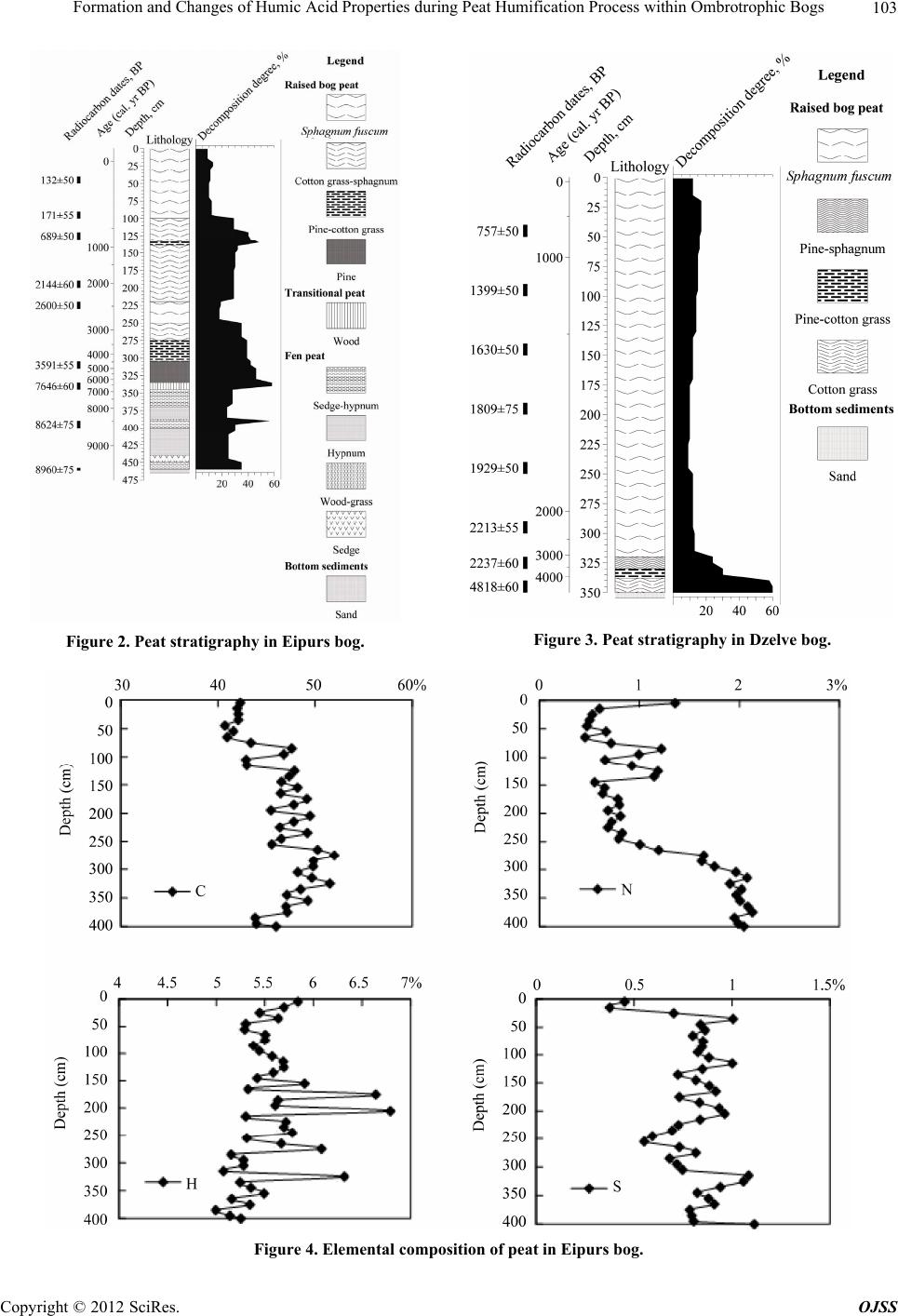 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 103 Figure 2. Peat stratigraphy in Eipurs bog. Figure 3. Peat stratigraphy in Dzelve bog. Figure 4. Elemental composition of peat in Eipurs bog. Copyright © 2012 SciRes. OJSS 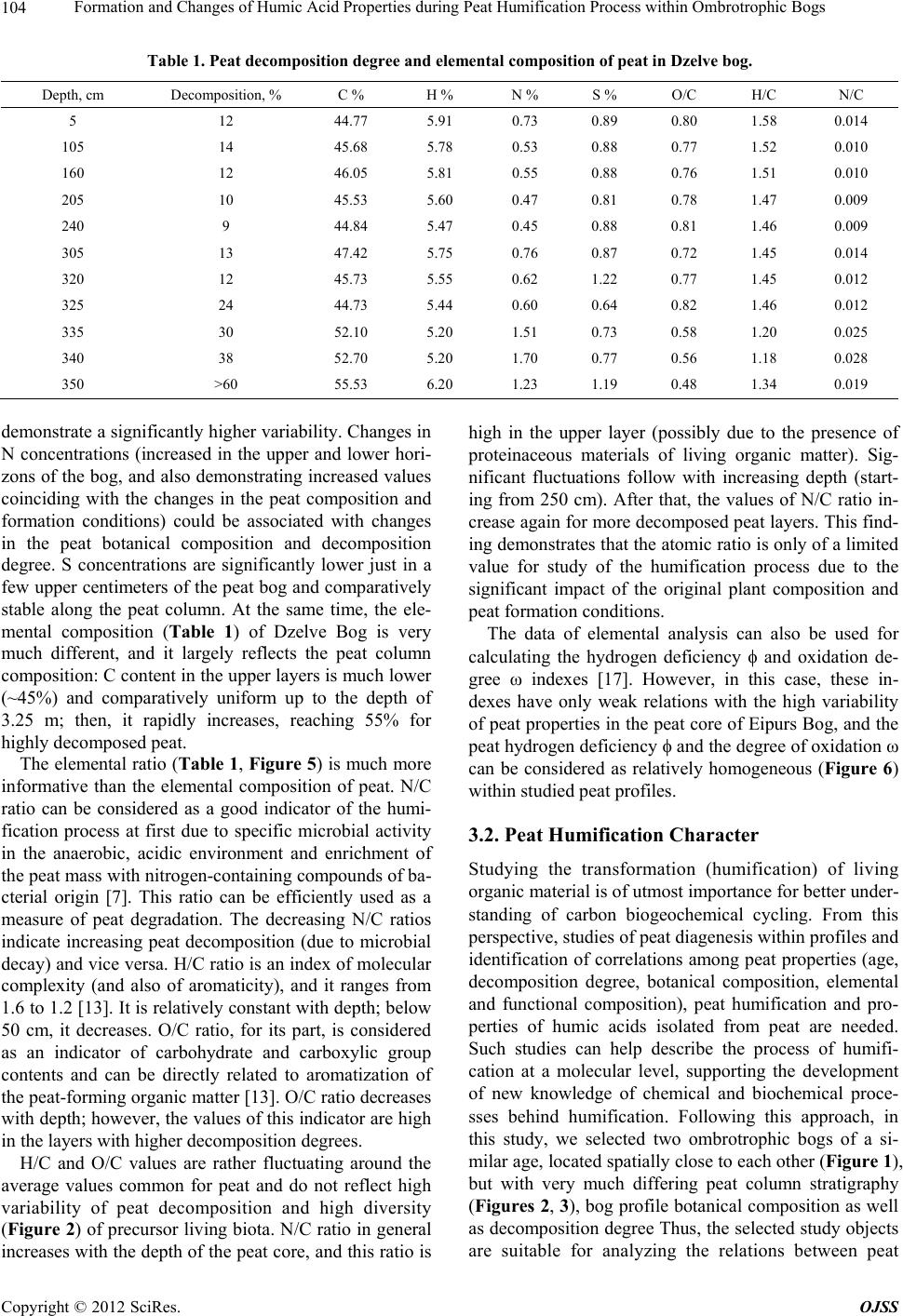 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 104 Table 1. Peat decomposition degree and elemental composition of peat in Dzelve bog. Depth, cm Decomposition, % C % H % N % S % O/C H/C N/C 5 12 44.77 5.91 0.73 0.89 0.80 1.58 0.014 105 14 45.68 5.78 0.53 0.88 0.77 1.52 0.010 160 12 46.05 5.81 0.55 0.88 0.76 1.51 0.010 205 10 45.53 5.60 0.47 0.81 0.78 1.47 0.009 240 9 44.84 5.47 0.45 0.88 0.81 1.46 0.009 305 13 47.42 5.75 0.76 0.87 0.72 1.45 0.014 320 12 45.73 5.55 0.62 1.22 0.77 1.45 0.012 325 24 44.73 5.44 0.60 0.64 0.82 1.46 0.012 335 30 52.10 5.20 1.51 0.73 0.58 1.20 0.025 340 38 52.70 5.20 1.70 0.77 0.56 1.18 0.028 350 >60 55.53 6.20 1.23 1.19 0.48 1.34 0.019 demonstrate a significantly higher variability. Changes in N concentrations (increased in the upper and lower hori- zons of the bog, and also demonstrating increased values coinciding with the changes in the peat composition and formation conditions) could be associated with changes in the peat botanical composition and decomposition degree. S concentrations are significantly lower just in a few upper centimeters of the peat bog and comparatively stable along the peat column. At the same time, the ele- mental composition (Table 1) of Dzelve Bog is very much different, and it largely reflects the peat column composition: C content in the upper layers is much lower (~45%) and comparatively uniform up to the depth of 3.25 m; then, it rapidly increases, reaching 55% for highly decomposed peat. The elemental ratio (Table 1, Figure 5) is much more informative than the elemental composition of peat. N/C ratio can be considered as a good indicator of the humi- fication process at first due to specific microbial activity in the anaerobic, acidic environment and enrichment of the peat mass with nitrogen-containing compounds of ba- cterial origin [7]. This ratio can be efficiently used as a measure of peat degradation. The decreasing N/C ratios indicate increasing peat decomposition (due to microbial decay) and vice versa. H/C ratio is an index of molecular complexity (and also of aromaticity), and it ranges from 1.6 to 1.2 [13]. It is relatively constant with depth; below 50 cm, it decreases. O/C ratio, for its part, is considered as an indicator of carbohydrate and carboxylic group contents and can be directly related to aromatization of the peat-forming organic matter [13]. O/C ratio decreases with depth; however, the values of this indicator are high in the layers with higher decomposition degrees. H/C and O/C values are rather fluctuating around the average values common for peat and do not reflect high variability of peat decomposition and high diversity (Figure 2) of precursor living biota. N/C ratio in general increases with the depth of the peat core, and this ratio is high in the upper layer (possibly due to the presence of proteinaceous materials of living organic matter). Sig- nificant fluctuations follow with increasing depth (start- ing from 250 cm). After that, the values of N/C ratio in- crease again for more decomposed peat layers. This find- ing demonstrates that the atomic ratio is only of a limited value for study of the humification process due to the significant impact of the original plant composition and peat formation conditions. The data of elemental analysis can also be used for calculating the hydrogen deficiency and oxidation de- gree ω indexes [17]. However, in this case, these in- dexes have only weak relations with the high variability of peat properties in the peat core of Eipurs Bog, and the peat hydrogen deficiency and the degree of oxidation ω can be considered as relatively homogeneous (Figure 6) within studied peat profiles. 3.2. Peat Humification Character Studying the transformation (humification) of living organic material is of utmost importance for better under- standing of carbon biogeochemical cycling. From this perspective, studies of peat diagenesis within profiles and identification of correlations among peat properties (age, decomposition degree, botanical composition, elemental and functional composition), peat humification and pro- perties of humic acids isolated from peat are needed. Such studies can help describe the process of humifi- cation at a molecular level, supporting the development of new knowledge of chemical and biochemical proce- sses behind humification. Following this approach, in this study, we selected two ombrotrophic bogs of a si- milar age, located spatially close to each other (Figure 1), but with very much differing peat column stratigraphy (Figures 2, 3), bog profile botanical composition as well as decomposition degree Thus, the selected study objects are suitable for analyzing the relations between peat Copyright © 2012 SciRes. OJSS 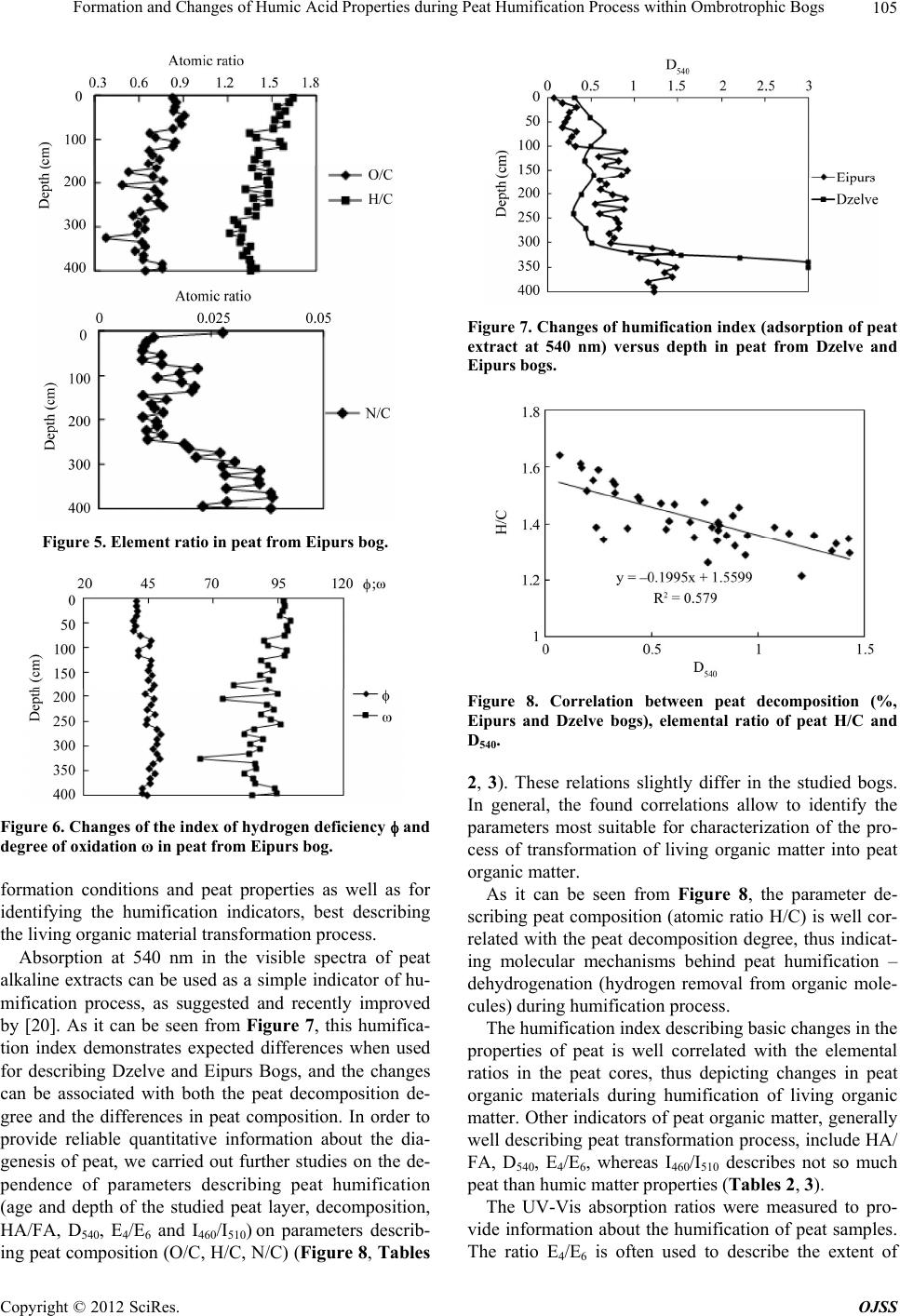 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 105 Figure 5. Element ratio in peat from Eipurs bog. Figure 6. Changes of the index of hydrogen deficiency and degree of oxidation ω in peat from Eipurs bog. formation conditions and peat properties as well as for identifying the humification indicators, best describing the living organic material transformation process. Absorption at 540 nm in the visible spectra of peat alkaline extracts can be used as a simple indicator of hu- mification process, as suggested and recently improved by [20]. As it can be seen from Figure 7, this humifica- tion index demonstrates expected differences when used for describing Dzelve and Eipurs Bogs, and the changes can be associated with both the peat decomposition de- gree and the differences in peat composition. In order to provide reliable quantitative information about the dia- genesis of peat, we carried out further studies on the de- pendence of parameters describing peat humification (age and depth of the studied peat layer, decomposition, HA/FA, D540, E4/E6 and I460/I510) on parameters describ- ing peat composition (O/C, H/C, N/C) (Figure 8, Tables Figure 7. Changes of humification index (adsorption of peat extract at 540 nm) versus depth in peat from Dzelve and Eipurs bogs. Figure 8. Correlation between peat decomposition (%, Eipurs and Dzelve bogs), elemental ratio of peat H/C and D540. 2, 3). These relations slightly differ in the studied bogs. In general, the found correlations allow to identify the parameters most suitable for characterization of the pro- cess of transformation of living organic matter into peat organic matter. As it can be seen from Figure 8, the parameter de- scribing peat composition (atomic ratio H/C) is well cor- related with the peat decomposition degree, thus indicat- ing molecular mechanisms behind peat humification – dehydrogenation (hydrogen removal from organic mole- cules) during humification process. The humification index describing basic changes in the properties of peat is well correlated with the elemental ratios in the peat cores, thus depicting changes in peat organic materials during humification of living organic matter. Other indicators of peat organic matter, generally well describing peat transformation process, include HA/ FA, D540, E4/E6, whereas I460/I510 describes not so much peat than humic matter properties (Tables 2, 3). The UV-Vis absorption ratios were measured to pro- vide information about the humification of peat samples. The ratio E4/E6 is often used to describe the extent of Copyright © 2012 SciRes. OJSS 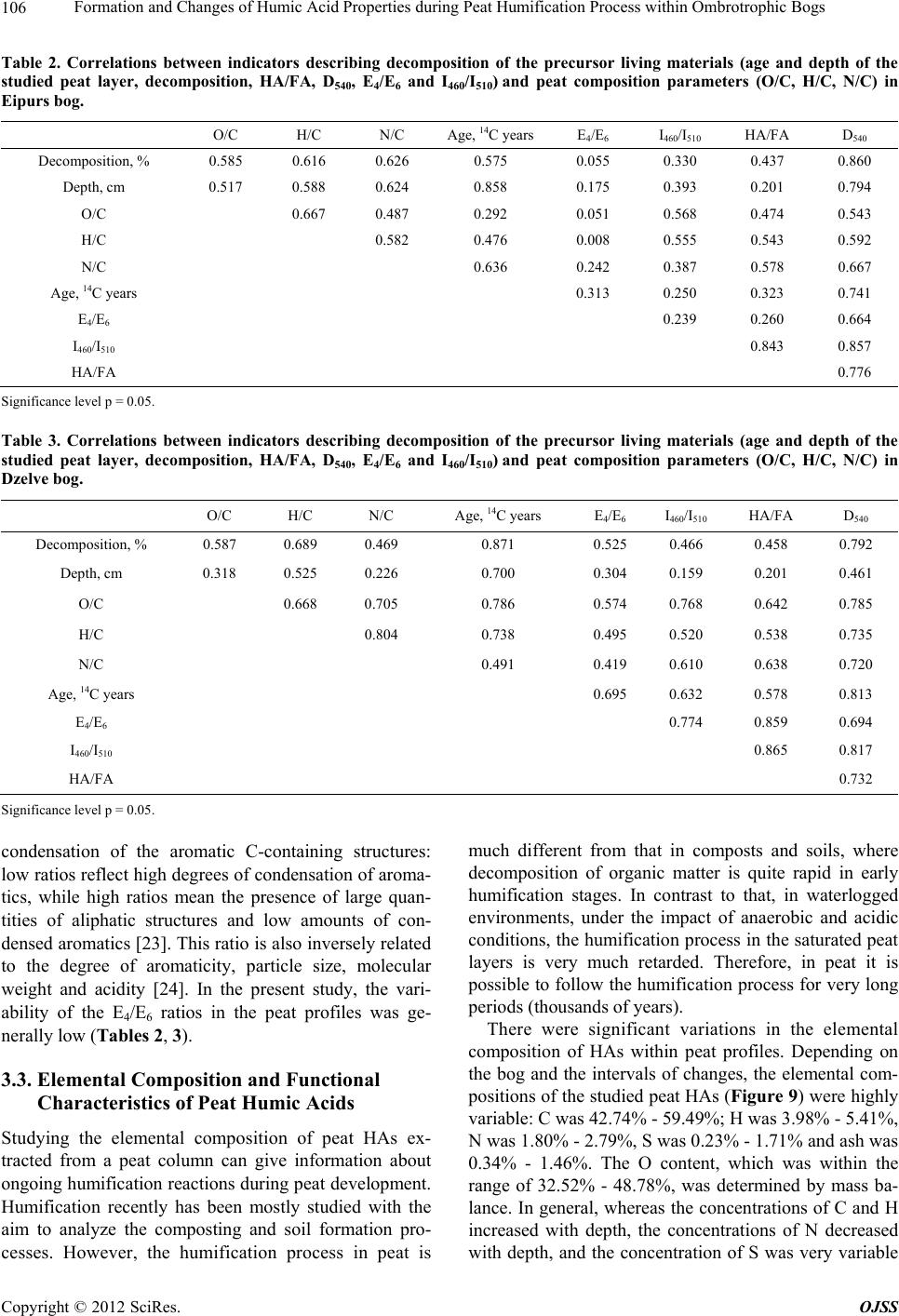 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs Copyright © 2012 SciRes. OJSS 106 Table 2. Correlations between indicators describing decomposition of the precursor living materials (age and depth of the studied peat layer, decomposition, HA/FA, D540, E4/E6 and I460/I510) and peat composition parameters (O/C, H/C, N/C) in Eipurs bog. O/C H/C N/C Age, 14C years E4/E6 I 460/I510 HA/FA D540 Decomposition, % 0.585 0.616 0.626 0.575 0.055 0.330 0.437 0.860 Depth, cm 0.517 0.588 0.624 0.858 0.175 0.393 0.201 0.794 O/C 0.667 0.487 0.292 0.051 0.568 0.474 0.543 H/C 0.582 0.476 0.008 0.555 0.543 0.592 N/C 0.636 0.242 0.387 0.578 0.667 Age, 14C years 0.313 0.250 0.323 0.741 E4/E6 0.239 0.260 0.664 I460/I510 0.843 0.857 HA/FA 0.776 Significance level p = 0.05. Table 3. Correlations between indicators describing decomposition of the precursor living materials (age and depth of the studied peat layer, decomposition, HA/FA, D540, E4/E6 and I460/I510) and peat composition parameters (O/C, H/C, N/C) in Dzelve bog. O/C H/C N/C Age, 14C years E4/E6 I 460/I510 HA/FA D540 Decomposition, % 0.587 0.689 0.469 0.871 0.525 0.466 0.458 0.792 Depth, cm 0.318 0.525 0.226 0.700 0.304 0.159 0.201 0.461 O/C 0.668 0.705 0.786 0.574 0.768 0.642 0.785 H/C 0.804 0.738 0.495 0.520 0.538 0.735 N/C 0.491 0.419 0.610 0.638 0.720 Age, 14C years 0.695 0.632 0.578 0.813 E4/E6 0.774 0.859 0.694 I460/I510 0.865 0.817 HA/FA 0.732 Significance level p = 0.05. condensation of the aromatic C-containing structures: low ratios reflect high degrees of condensation of aroma- tics, while high ratios mean the presence of large quan- tities of aliphatic structures and low amounts of con- densed aromatics [23]. This ratio is also inversely related to the degree of aromaticity, particle size, molecular weight and acidity [24]. In the present study, the vari- ability of the E4/E6 ratios in the peat profiles was ge- nerally low (Tables 2, 3). 3.3. Elemental Composition and Functional Characteristics of Peat Humic Acids Studying the elemental composition of peat HAs ex- tracted from a peat column can give information about ongoing humification reactions during peat development. Humification recently has been mostly studied with the aim to analyze the composting and soil formation pro- cesses. However, the humification process in peat is much different from that in composts and soils, where decomposition of organic matter is quite rapid in early humification stages. In contrast to that, in waterlogged environments, under the impact of anaerobic and acidic conditions, the humification process in the saturated peat layers is very much retarded. Therefore, in peat it is possible to follow the humification process for very long periods (thousands of years). There were significant variations in the elemental composition of HAs within peat profiles. Depending on the bog and the intervals of changes, the elemental com- positions of the studied peat HAs (Figure 9) were highly variable: C was 42.74% - 59.49%; H was 3.98% - 5.41%, N was 1.80% - 2.79%, S was 0.23% - 1.71% and ash was 0.34% - 1.46%. The O content, which was within the range of 32.52% - 48.78%, was determined by mass ba- lance. In general, whereas the concentrations of C and H increased with depth, the concentrations of N decreased with depth, and the concentration of S was very variable 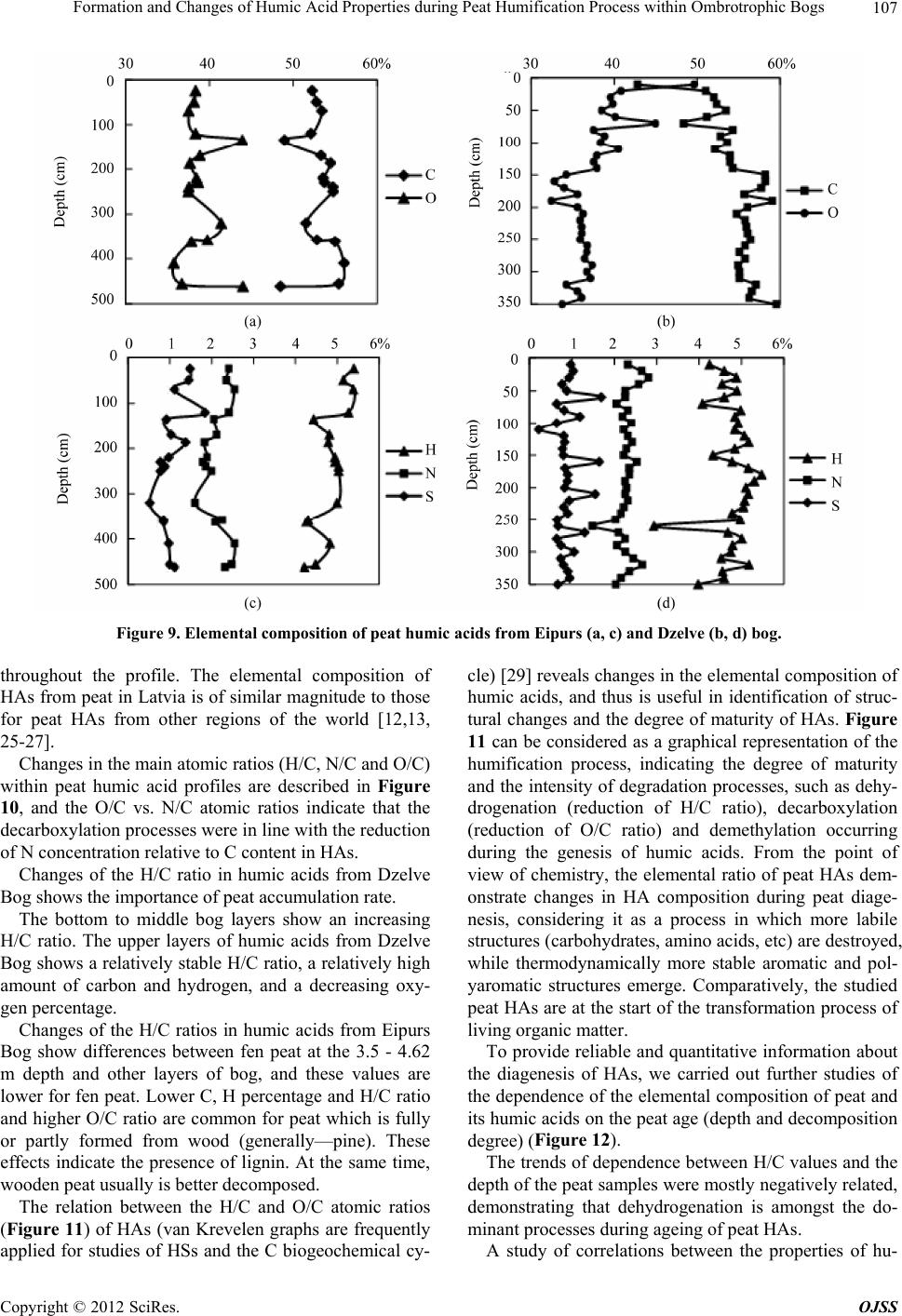 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 107 Figure 9. Elemental composition of peat humic acids from Eipurs (a, c) and Dzelve (b, d) bog. throughout the profile. The elemental composition of HAs from peat in Latvia is of similar magnitude to those for peat HAs from other regions of the world [12,13, 25-27]. Changes in the main atomic ratios (H/C, N/C and O/C) within peat humic acid profiles are described in Figure 10, and the O/C vs. N/C atomic ratios indicate that the decarboxylation processes were in line with the reduction of N concentration relative to C content in HAs. Changes of the H/C ratio in humic acids from Dzelve Bog shows the importance of peat accumulation rate. The bottom to middle bog layers show an increasing H/C ratio. The upper layers of humic acids from Dzelve Bog shows a relatively stable H/C ratio, a relatively high amount of carbon and hydrogen, and a decreasing oxy- gen percentage. Changes of the H/C ratios in humic acids from Eipurs Bog show differences between fen peat at the 3.5 - 4.62 m depth and other layers of bog, and these values are lower for fen peat. Lower C, H percentage and H/C ratio and higher O/C ratio are common for peat which is fully or partly formed from wood (generally—pine). These effects indicate the presence of lignin. At the same time, wooden peat usually is better decomposed. The relation between the H/C and O/C atomic ratios (Figure 11) of HAs (van Krevelen graphs are frequently applied for studies of HSs and the C biogeochemical cy- cle) [29] reveals changes in the elemental composition of humic acids, and thus is useful in identification of struc- tural changes and the degree of maturity of HAs. Figure 11 can be considered as a graphical representation of the humification process, indicating the degree of maturity and the intensity of degradation processes, such as dehy- drogenation (reduction of H/C ratio), decarboxylation (reduction of O/C ratio) and demethylation occurring during the genesis of humic acids. From the point of view of chemistry, the elemental ratio of peat HAs dem- onstrate changes in HA composition during peat diage- nesis, considering it as a process in which more labile structures (carbohydrates, amino acids, etc) are destroyed, while thermodynamically more stable aromatic and pol- yaromatic structures emerge. Comparatively, the studied peat HAs are at the start of the transformation process of living organic matter. To provide reliable and quantitative information about the diagenesis of HAs, we carried out further studies of the dependence of the elemental composition of peat and its humic acids on the peat age (depth and decomposition degree) (Figure 12). The trends of dependence between H/C values and the depth of the peat samples were mostly negatively related, demonstrating that dehydrogenation is amongst the do- minant processes during ageing of peat HAs. A study of correlations between the properties of hu- Copyright © 2012 SciRes. OJSS 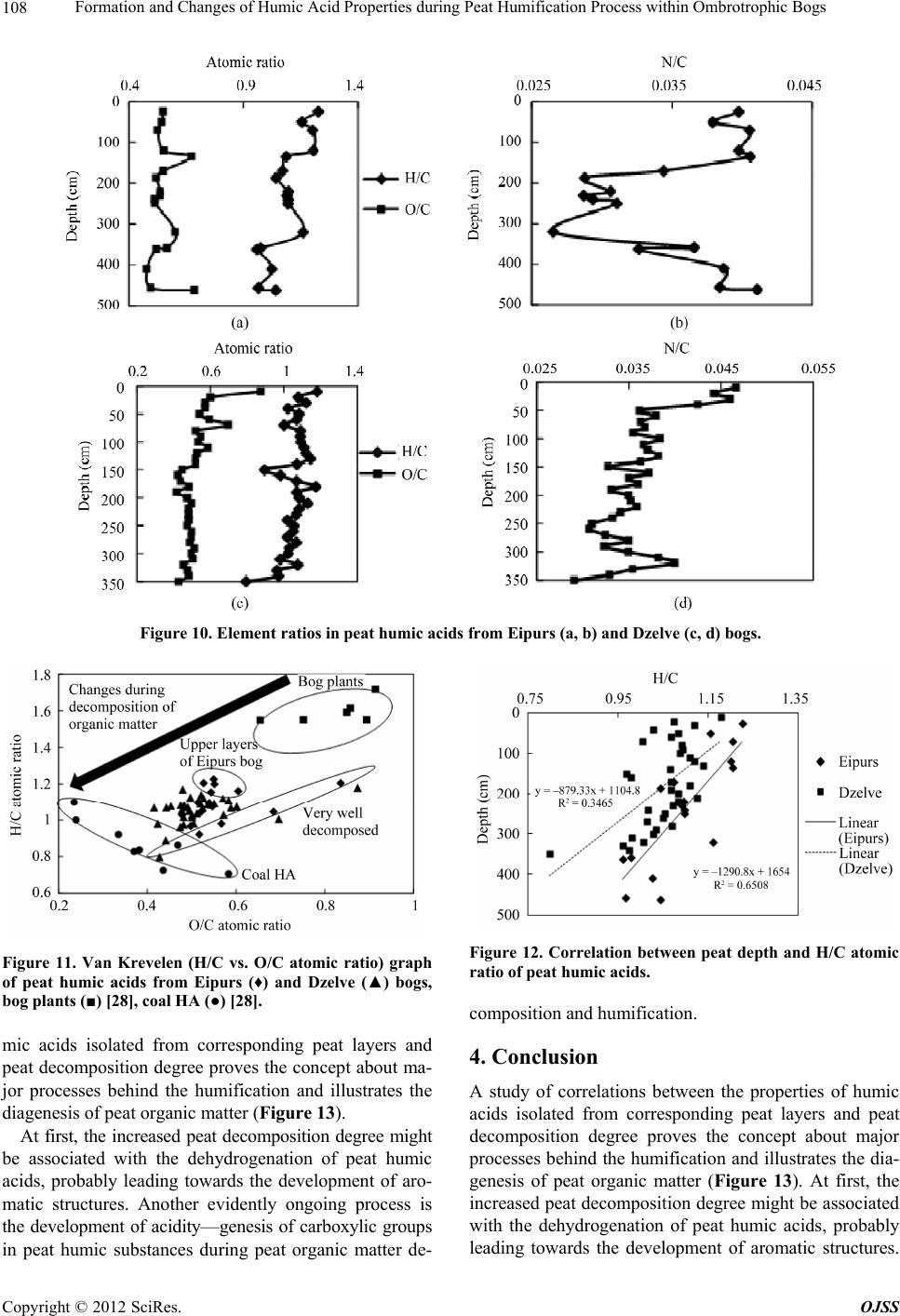 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 108 Figure 10. Element ratios in peat humic acids from Eipurs (a, b) and Dzelve (c, d) bogs. Figure 11. Van Krevelen (H/C vs. O/C atomic ratio) graph of peat humic acids from Eipurs (♦) and Dzelve (▲) bogs, bog plants (■) [28], coal HA (●) [28]. mic acids isolated from corresponding peat layers and peat decomposition degree proves the concept about ma- jor processes behind the humification and illustrates the diagenesis of peat organic matter (Figure 13). At first, the increased peat decomposition degree might be associated with the dehydrogenation of peat humic acids, probably leading towards the development of aro- matic structures. Another evidently ongoing process is the development of acidity—genesis of carboxylic groups in peat humic substances during peat organic matter de- Figure 12. Correlation between peat depth and H/C atomic ratio of peat humic acids. composition and humification. 4. Conclusion A study of correlations between the properties of humic acids isolated from corresponding peat layers and peat decomposition degree proves the concept about major processes behind the humification and illustrates the dia- genesis of peat organic matter (Figure 13). At first, the increased peat decomposition degree might be associated with the dehydrogenation of peat humic acids, probably leading towards the development of aromatic structures. Copyright © 2012 SciRes. OJSS 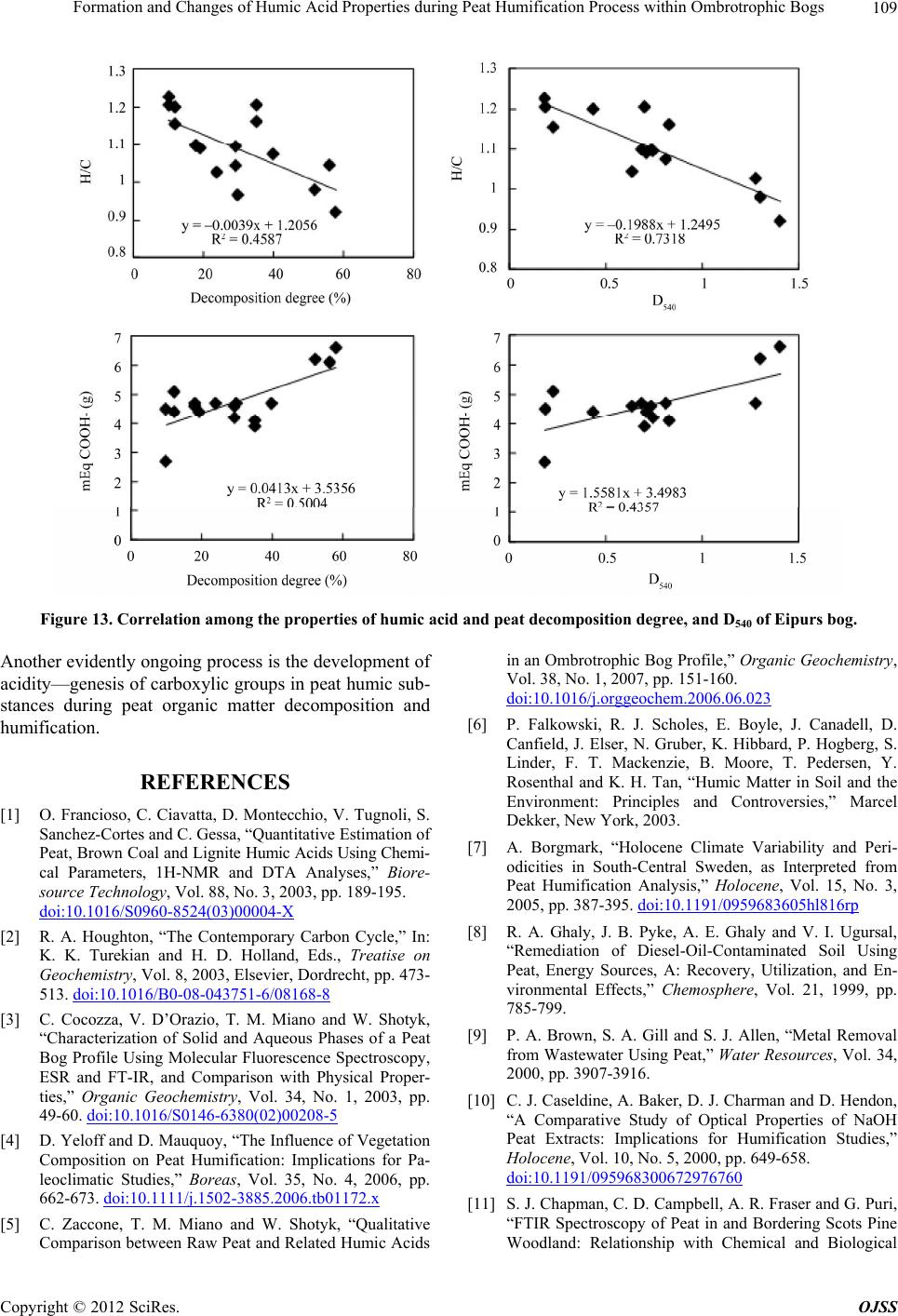 Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 109 Figure 13. Correlation among the properties of humic acid and peat decomposition degree, and D540 of Eipurs bog. Another evidently ongoing process is the development of acidity—genesis of carboxylic groups in peat humic sub- stances during peat organic matter decomposition and humification. REFERENCES [1] O. Francioso, C. Ciavatta, D. Montecchio, V. Tugnoli, S. Sanchez-Cortes and C. Gessa, “Quantitative Estimation of Peat, Brown Coal and Lignite Humic Acids Using Chemi- cal Parameters, 1H-NMR and DTA Analyses,” Biore- source Technology, Vol. 88, No. 3, 2003, pp. 189-195. doi:10.1016/S0960-8524(03)00004-X [2] R. A. Houghton, “The Contemporary Carbon Cycle,” In: K. K. Turekian and H. D. Holland, Eds., Treatise on Geochemistry, Vol. 8, 2003, Elsevier, Dordrecht, pp. 473- 513. doi:10.1016/B0-08-043751-6/08168-8 [3] C. Cocozza, V. D’Orazio, T. M. Miano and W. Shotyk, “Characterization of Solid and Aqueous Phases of a Peat Bog Profile Using Molecular Fluorescence Spectroscopy, ESR and FT-IR, and Comparison with Physical Proper- ties,” Organic Geochemistry, Vol. 34, No. 1, 2003, pp. 49-60. doi:10.1016/S0146-6380(02)00208-5 [4] D. Yeloff and D. Mauquoy, “The Influence of Vegetation Composition on Peat Humification: Implications for Pa- leoclimatic Studies,” Boreas, Vol. 35, No. 4, 2006, pp. 662-673. doi:10.1111/j.1502-3885.2006.tb01172.x [5] C. Zaccone, T. M. Miano and W. Shotyk, “Qualitative Comparison between Raw Peat and Related Humic Acids in an Ombrotrophic Bog Profile,” Organic Geochemistry, Vol. 38, No. 1, 2007, pp. 151-160. doi:10.1016/j.orggeochem.2006.06.023 [6] P. Falkowski, R. J. Scholes, E. Boyle, J. Canadell, D. Canfield, J. Elser, N. Gruber, K. Hibbard, P. Hogberg, S. Linder, F. T. Mackenzie, B. Moore, T. Pedersen, Y. Rosenthal and K. H. Tan, “Humic Matter in Soil and the Environment: Principles and Controversies,” Marcel Dekker, New York, 2003. [7] A. Borgmark, “Holocene Climate Variability and Peri- odicities in South-Central Sweden, as Interpreted from Peat Humification Analysis,” Holocene, Vol. 15, No. 3, 2005, pp. 387-395. doi:10.1191/0959683605hl816rp [8] R. A. Ghaly, J. B. Pyke, A. E. Ghaly and V. I. Ugursal, “Remediation of Diesel-Oil-Contaminated Soil Using Peat, Energy Sources, A: Recovery, Utilization, and En- vironmental Effects,” Chemosphere, Vol. 21, 1999, pp. 785-799. [9] P. A. Brown, S. A. Gill and S. J. Allen, “Metal Removal from Wastewater Using Peat,” Water Resources, Vol. 34, 2000, pp. 3907-3916. [10] C. J. Caseldine, A. Baker, D. J. Charman and D. Hendon, “A Comparative Study of Optical Properties of NaOH Peat Extracts: Implications for Humification Studies,” Holocene, Vol. 10, No. 5, 2000, pp. 649-658. doi:10.1191/095968300672976760 [11] S. J. Chapman, C. D. Campbell, A. R. Fraser and G. Puri, “FTIR Spectroscopy of Peat in and Bordering Scots Pine Woodland: Relationship with Chemical and Biological Copyright © 2012 SciRes. OJSS  Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs 110 Properties,” Soil Biology and Biochemistry, Vol. 33, No. 9, 2001, pp. 1193-1200. doi:10.1016/S0038-0717(01)00023-2 [12] C. Zaccone, C. Cocozza, A. K. Cheburkin, W. Shotyk and T. M. Miano, “Enrichment and Depletion of Major and Trace Elements, and Radionuclides in Ombrotrophic Raw Peat and Corresponding Humic Acids,” Geoderma, Vol. 141, No. 3-4, 2007, pp. 235-246. doi:10.1016/j.geoderma.2007.06.007 [13] H. Anderson and A. Hepburn, “Variation of Humic Sub- stances within Peat Profile,” In: C. H. Fuchsman, Ed., Peat and Water, Academic Press, New York, 1986, pp. 177-194. [14] C. Zaccone, C. Cocozza, A. K. Cheburkin, W. Shotyk and T. M. Miano, “Distribution of As, Cr, Ni, Rb, Ti and Zr between Peat and Its Humic Fraction along an Undis- turbed Ombrotrophic Bog Profile (NW Switzerland),” Applied Geochemistry, Vol. 23, No. 1, 2008, pp. 25-33. doi:10.1016/j.apgeochem.2007.09.002 [15] I. I. Lishtvan and N. T. Korol, “Basic Properties of Peat and Methods for Their Determination,” Nauka I Teknika, Minsk, 1975 (in Russian). [16] K. H. Tan, “Soil Sampling, Preparation, and Analysis,” Second Edition, Taylor & Francis Group, New York, 2005. [17] S. S. Fong and M. Mohamed, “Chemical Characterization of Humic Substances Occurring in the Peats of Sarawak, Malaysia,” Organic Geochemistry, Vol. 38, No. 6, 2007, pp. 967-976. doi:10.1016/j.orggeochem.2006.12.010 [18] Y. Chen, N. Senesi and M. Schnitzer, “Information Pro- vided on Humic Substances by E4/E6 Ratios,” Soil Sci- ence Society of America Journal, Vol. 41, No. 2, 1977, pp. 352-358. doi:10.2136/sssaj1977.03615995004100020037x [19] J. J. Blackford and F. M. Chambers, “Determining the Degree of Peat Decomposition for Peat-Based Paleocli- matic Studies,” International Peat Journal, Vol. 5, 1993, pp. 7-24. [20] A. Borgmark, “The Colour of Climate: Changes in Peat Decomposition as a Proxy for Climate Change—A Study of Raised Bogs in South-central Sweden,” PhD Thesis, Stockholm University, Stockholm, 2005. [21] A. G. Zavarzina, V. V. Demin, T. I. Nifanteva, V. M. Shkinev, T. V. Danilova and B. Y. Spivakov, “Extraction of Humic Acids and Their Fractions in Poly(ethylene gly- col)-Based Aqueous Biphasic Systems,” Analytica Chimica Acta, Vol. 452, No. 1, 2002, pp. 95-103. doi:10.1016/S0003-2670(01)01428-3 [22] D. M. B. P. Milori, L. M. Neto, C. Bayer, J. Mielniczuk and V. S. Bagnato, “Humification Degree of Soil Humic Acids Determined by Fluorescence Spectroscopy,” Soil Science, Vol. 167, No. 1, 2002, pp. 739-749. doi:10.1097/00010694-200211000-00004 [23] Y. Chin, G. R. Aiken and K. M. Danielsen, “Binding of Pyrene to Aquatic and Commercial Humic Substances: The Role of Molecular Weight and Aromaticity,” Envi- ronmental Science and Technology, Vol. 31, No. 6, 1997, pp. 1630-1635. doi:10.1021/es960404k [24] C. S. Uyguner, C. Hellriegel, W. Otto and C. K. Larive, “Characterization of Humic Substances: Implications for Trihalomethane Formation,” Analytical and Bioanalytical Chemistry, Vol. 378, No. 6, 2004, pp. 1579-1586. doi:10.1007/s00216-003-2451-7 [25] T. Qiamg, S. Xiaoquan and N. Zheming, “Comparative Characteristic Studies on Soil and Commercial Humic Acids,” Fresenius Journal of Analytical Chemistry, Vol. 347, No. 8-9, 1993, pp. 330-336. doi:10.1007/BF00323816 [26] T. Yamaguchi, H. Hayashi, Y. Yazawa, M. Uomori, F. Yazaki and N. N. Bambalov, “Comparison of Basic Characteristics of Humic Acids Extracted from Peats and Other Sources,” International Peat Journal, Vol. 8, 1998, pp. 87-94. [27] E. Garnier-Sillam, S. Hariyento and Y. Bourezgui, “Hu- mic Substances in Peats,” Analysis, Vol. 27, No. 5, 1999, pp. 405-408. doi:10.1051/analusis:1999270405 [28] J. Šīre, M. Kļaviņš, O. Purmalis and V. Melecis, “Ex- perimental Study of Peat Humification Indicators,” Pro- ceedings of Latvian Academy of Sciences, B, Vol. 62, No. 1-2, 2008, pp. 18-27. [29] D. W. Van Krevelen, “Graphical-Statistical Method for the Study of Structure and Reaction Processes of Coal,” Fuel, Vol. 29, 1950, pp. 269-228. Copyright © 2012 SciRes. OJSS
|