Food and Nutrition Sciences
Vol. 3 No. 6 (2012) , Article ID: 20061 , 9 pages DOI:10.4236/fns.2012.36115
A Reliable Methodology for Quantitative Extraction of Fruit and Vegetable Physiological Amino Acids and Their Subsequent Analysis with Commonly Available HPLC Systems
![]()
Wes Watkins Agricultural Research Laboratory, USDA-ARS, Lane, USA.
Email: waynefish@rocketmail.com
Received April 18th, 2012; revised May 18th, 2012; accepted May 25th, 2012
Keywords: Citrullus lanatus; Cucumis melo; Cucurbita pepo; Cucumis sativus; Cucurbita foetidissima; Brassica oleracea; Allium cepa; Prunus persica; Capiscum annum; Solanum tuberosum; Solanum lycopersicum; Malus domestica; HPLC; Free Amino Acids
ABSTRACT
Many of the extraction and amino acid analysis methodologies currently employed do not provide complete analysis of all the physiological amino acids and biogenic amines. Extraction procedures frequently employ dilute acid which partially converts gln and asn to glu and asp. A commonly used pre-column derivatizing agent, o-phthalaldehyde, does not react with the imino acids, pro and hydroxypro. The purpose of this investigation was to integrate extraction and analysis procedures into a reliable method for measuring the complete physiological amino acid profiles of fruit and vegetables using HPLC instrumentation commonly available to most laboratories. Water extraction of ground, frozen-thawed tissues effected complete recovery of the physiological amino acids as demonstrated by spiking experiments and tissue combination experiments. HPLC of dabsyl derivatives of the free amino acids allowed their quantification in a selection of fruit and vegetables. Physiological amino acid levels were determined for peach, apple, potato, onion, tomato, bell pepper, broccoli, and seven types of cucurbits. The coefficient of variation for estimation of an amino acid level generally fell in the range of 5% to 7%. Because of marked variability in physiological amino acid content as a result of growing conditions, cultural practices, and inherent cultivar differences, comparisons of results with literature values were not possible.
1. Introduction
Our interest in the physiological levels of amino acids in fruits and vegetables as a function of growing conditions and stage of fruit development led us to explore various extraction and analysis methodologies that effected quantitative extraction without modification and that did not require expensive, dedicated instrumentation for analyses. Since some fruit and vegetables possess significant quantities of non-protein amino acids, it was also imperative that an amino acid analysis method be employed which provided adequate resolution of amino acids and biogenic amines, including metabolic precursors and products, to allow identification and quantification of each. A review of the literature on amino acid analysis instrumentation and methods yields a broad array of methodologies, most of which do not meet the afore-mentioned fundamental criteria.
Widely used during the 1960’s through the 1980’s, the Moore and Stein [1,2] system of ion exchange separation of amino acids followed by post-column derivatization with ninhydrin provided a reliable method for quantification of amino acids from biological fluids as well as protein hydrolysates. With the advent of volatile derivatives of amino acids for GC [3], HPLC with preand postcolumn amino acid derivatization [4-7], and more recently, GC and HPLC with mass spectral monitoring [3, 8], ion exchange-based amino acid analysis no longer commands the prominence that it once did. Although these more contemporary methods afford a wider availability of methodology for protein hydrolysate amino acid analysis, resolution of some physiological amino acids and biogenic amines is frequently not on par with the older ion exchange systems. Secondly, a number of the amino acid analysis methodologies are hampered by incomplete reaction of specific amino acids such as pro [9] or multiple and/or overlapping derivative peaks [3]. Also, many laboratories requiring physiological amino acid composition data for specific fruit or vegetables do not have access to the latest analytical technologies because of instrument cost, so a methodology is needed that can be adapted to instrumentation commonly available to most laboratories.
Imperative to quantitative analysis of the physiological amino acids from fruit and vegetable tissues is the utilization of an extraction procedure that quantitatively releases and solubilizes all the free amino acids in their in vivo form. Many extraction methods employ dilute (~0.1 N) acid or dilute acid in combination with concentrated alcohols. Use of dilute acid routinely results in partial hydrolysis of the amide bonds of gln and asn [10]. This results in underestimates of the amide forms of these two amino acids and over-estimates of their dicarboxylic acid forms. The solubility of many of the physiological amino acids diminishes with increasing alcohol concentration, and this can potentially reduce yields of some amino acids. Finally, extraction conditions that maximize amino acid release and minimize amino acid modification would likely co-extract proteins with the physiological amino acids, and many of the amino acid analysis procedures are sensitive to the presence of protein in the sample.
One possible analytical methodology to address the challenges enumerated above is pre-column derivatization of amino acids with dabsyl chloride followed by reversed phase HPLC separation of the derivatives [11- 13]. Use of HPLC separation and quantification of dabsyl derivatives of amino acids offers several advantages over other methods currently available. First, dabsyl derivatives of amino acids possess greater stability than many other derivatives [9,14]. Second, reversed phase HPLC provides sufficient resolution of the physiologic amino acids as well as most biogenic amines for their quantifycation [12,13]. Third, the solvent system of Sethuraman et al. [13] allows analysis of physiological amino acids in the presence of proteins in the sample. Fourth, the dabsyl derivatives can be detected by their visible absorbance to provide sensitivity in the picomole to nanomole range with uv/vis detectors for HPLC that are available to most laboratories [9,12,14].
The purpose of this report is to document a methodology for the quantitative extraction of physiological amino acids from a variety of fruit and vegetables together with a pre-column derivatization—HPLC separation methodology for quantification of the extracted physiological amino acids. Preliminary descriptions and limited applications of this methodology have been presented earlier [15,16].
2. Materials and Methods
2.1. Fruit and Vegetable Sources
Watermelon, cultivar ACR 6177 (Citrullis lanatus), cantaloupe, cultivar Magnum 45 (Cucumis melo), pumpkin, cultivar Fall Splendor (Cucurbita pepo), cucumber, cultivar Dasher II (Cucumis sativus), and straight-necked yellow squash, cultivar Lemon Drop (Cucurbita pepo) were grown at the Wes Watkins Agricultural Research Center, Lane, OK in 2008 or 2009. Buffalo gourd fruit (Cucurbita foetidissima) were collected in July, 2010 near Terral, OK. Botswana wild watermelon (Citrullis lanatus var. citroides) fruit were obtained by growing them in a greenhouse from seed, PI 54211301 SD, obtained from the USDA ARS Genetic Resource Conservation Unit (Griffin, GA). Broccoli, (Brassica oleracea), purple onion (Allium cepa), white onion (Allium cepa), cling peach (Prunus persica), bell pepper (Capiscum annuum), Russet potato (Solanum tuberosum), Roma tomato (Solanum lycopersicum), Golden Delicious apple (Malus domestica), and Red Delicious apple (Malus domestica) were purchased at a local supermarket.
2.2. Sample Preparation
Peels and seed cavities were removed from the squash, pumpkin, cucumber, Golden and Red Delicious apples, bell pepper, cling peach, and cantaloupe. Sections of edible flesh midway between the stem and blossom ends were then removed for extraction. The skin was removed from the tomato and the potato, and cross sections of the remaining tissue used for extraction. Outer dry layers of onions were removed, and cross sections of the remaining tissue used for extraction. Broccoli florets with their immediately attending stalks were used for extraction. Watermelon tissue came from the center of the heart. Watermelon rind, used for the combinatorial and spiking experiments, was taken from the center top of the fruit (opposite the ground spot). The peel was removed from the rind, and no red or pink flesh was left on the inner rind. Botswana wild watermelon tissue came from the center of the fruit inside the ring of seeds. The exterior chlorophyll-containing skin was scraped from the rind of the Buffalo gourd, and 2 cm cross sections, including rind and seeds, were taken from the center of the fruit. Tissue dry weights were determined by drying at 105˚C - 108˚C in a forced air drying oven (Precision Scientific Corp., Chicago, IL) until the weight was constant (~24 - 36 hr).
2.3. Extraction Procedure
Tissues were frozen at −20˚C (or −80˚C for long term storage) for 24 hr, weighed, slightly thawed, diluted with 2 times the tissue weight of water, and thoroughly homogenized for 3 min with a PolytronTM PT 10 - 35 grinder (Kinematica AB, Lucerne, Switzerland) set at the highest speed. The sample tube was kept in an ice bath while grinding. After grinding, samples were centrifuged at 10,000 × g for 10 min at 15˚C to pellet insoluble debris. A 200 μL aliquot of the supernatant was removed for amino acid derivatization and analysis.
2.4. Amino Acid Separation and Quantitation
Dabsyl chloride (4-(4-Dimethylaminophenylazo) benzenesulfonyl chloride) was purchased from Pierce (Pierce Biotechnology, Rockford, IL, USA), and the amino acids used for calibration were purchased from Sigma (St. Louis, MO, USA) as a commercial 18 amino acid calibration mixture. Each amino acid was also purchased individually (Sigma, St. Louis, MO) and used to verify identity of HPLC peaks of the amino acids’ dabsyl derivatives. The physiological amino acids, L-citrulline, L-glutamine, L-asparagine, L-tryptophan, L-ornithine, and L-aminobutyric acid (purchased from Sigma), were quantitatively prepared individually and as a mixture. The method of Chang et al. [9], as modified by Krause et al. [12] and by Sethuraman et al. [13], for the derivatization and determination of physiologic amino acids and biogenic amines by reversed phase HPLC was modified to quantify fruit or vegetable physiological amino acids. All reagents and their concentrations were the same as described by Sethuraman et al. [13]. The only departure from their procedure was that dabsyl chloride was dissolved in acetonitrile rather than acetone to prepare the dabsyl reagent. Because we had the luxury of ample quantities of tissue, reactions were carried out using ten times the volume of samples and reagents as were those of Sethuraman et al. [13] (i.e., hundreds of μL instead of tens of μL). Twenty-five μL of dabsyl derivatized sample or standards were injected onto the column. Separation/ quantification was performed on a Varian ProStar ternary solvent HPLC system equipped with an autosampler and diode array detector (Varian, Walnut Creek, CA, USA). A 250 mm × 4.6 mm i.d. 5 μm LunaTM C18 reversed phase column was employed (Phenomenex, Torrance, CA, USA) at 50˚C and at a flow rate of 1.0 ml/min. Because the ratio of flow rate to column volume for our system was within 5% that of the system of Sethuraman et al. [13], an elution program was employed that was identical to theirs with no apparent impairment of resolution. The dabsyl derivatives eluting from the column were monitored by absorbance at 468 nm, the maximal absorbance for a majority of the dabsyl amino acid derivatives. The suspected identity of each unknown peak in a derivatized extract was confirmed by co-chromatography of the extract with the derivatized authentic individual amino acid and by comparison of the absorption spectrum of the dabsyl derivative of the unknown with that of the pure, known amino acid. This latter approach demonstrated an unknown compound whose dabsyl derivative co-eluted with met so that met could not be quantified in extracts from some fruit and vegetables (see footnotes to Tables 1 and 2). Calibration curves for each amino acid ranged between 0.1 and 1.6 nmoles of the amino acid injected onto the column, and this was the range to which unknowns were diluted for analysis. Under the method parameters described, the lower level of amino acid in fresh tissue that could be detected and quantified was approximately 50 nmoles/g fresh weight of tissue. The precision of the amino acid analysis was estimated by comparing the measured values for cit, gln, arg, and orn in a watermelon rind sample at different steps in the derivatization and quantification steps. Replicates from the same derivatized sample exhibited a ±0.5% coefficient of variation (n = 5) about the mean. Replicates from the same prepared tissue sample, but derivatized separately, exhibited a ±1.5% same-day coefficient of variation about the mean (n = 5) and a ±3.1% day-to-day coefficient of variation about the mean (n = 5).
2.5. Statistical Evaluation of the Method’s Reliability
The objective of this study was to test the overall reliability of the methodology when it was applied to various fruits and vegetables; it was not to compare compositions among individual samples of a given type of fruit or vegetable. Therefore, a single fruit or vegetable was assayed multiple times in order to test only the methodology. Multiple samples (three or more) were removed from each fruit or vegetable and carried through extraction and analysis. Depending on the free amino acid composition of each species, analyses were performed at two or more dilutions of the extract. The experimental uncertainty in amino acid determination was expressed as the coefficient of variation about the mean in order to more clearly compare the experimental variation about both large and small means. Statistical analyses were performed with the aid of Statistica software version 6 (StatSoft, Tulsa, OK).
3. Results
3.1. Development and Evaluation of the Extraction Procedure
The extraction procedure utilized to quantitatively extract the physiological amino acids had to meet several criteria.
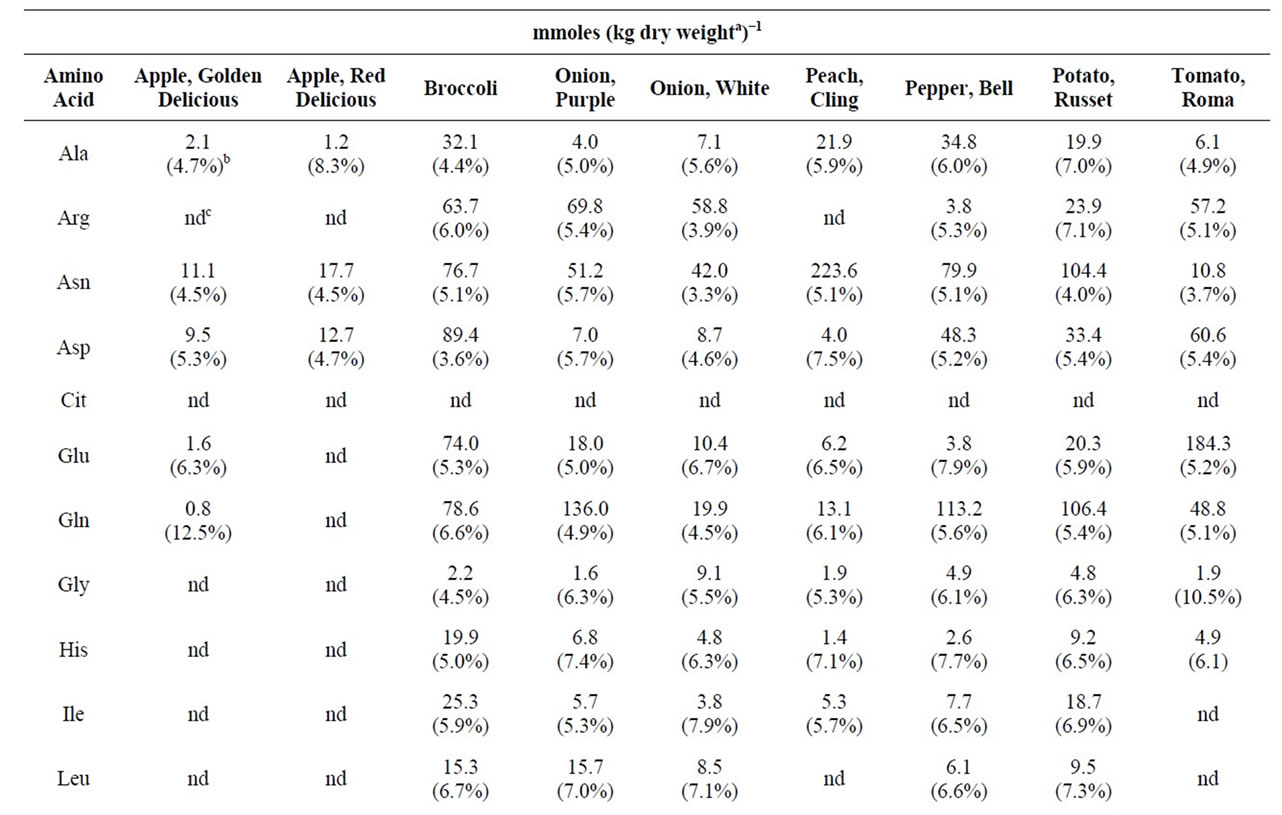
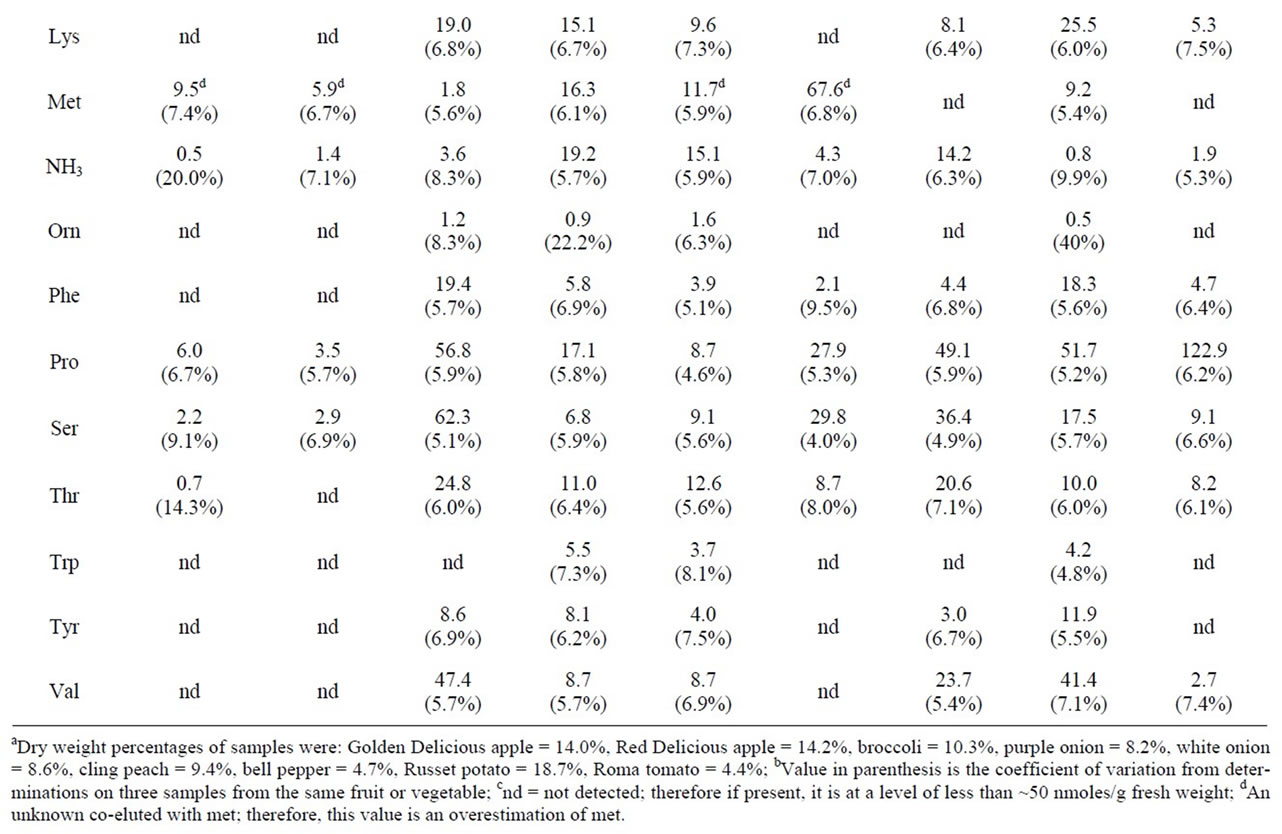
Table 1. The physiological amino acid content of selected fruits and vegetables.
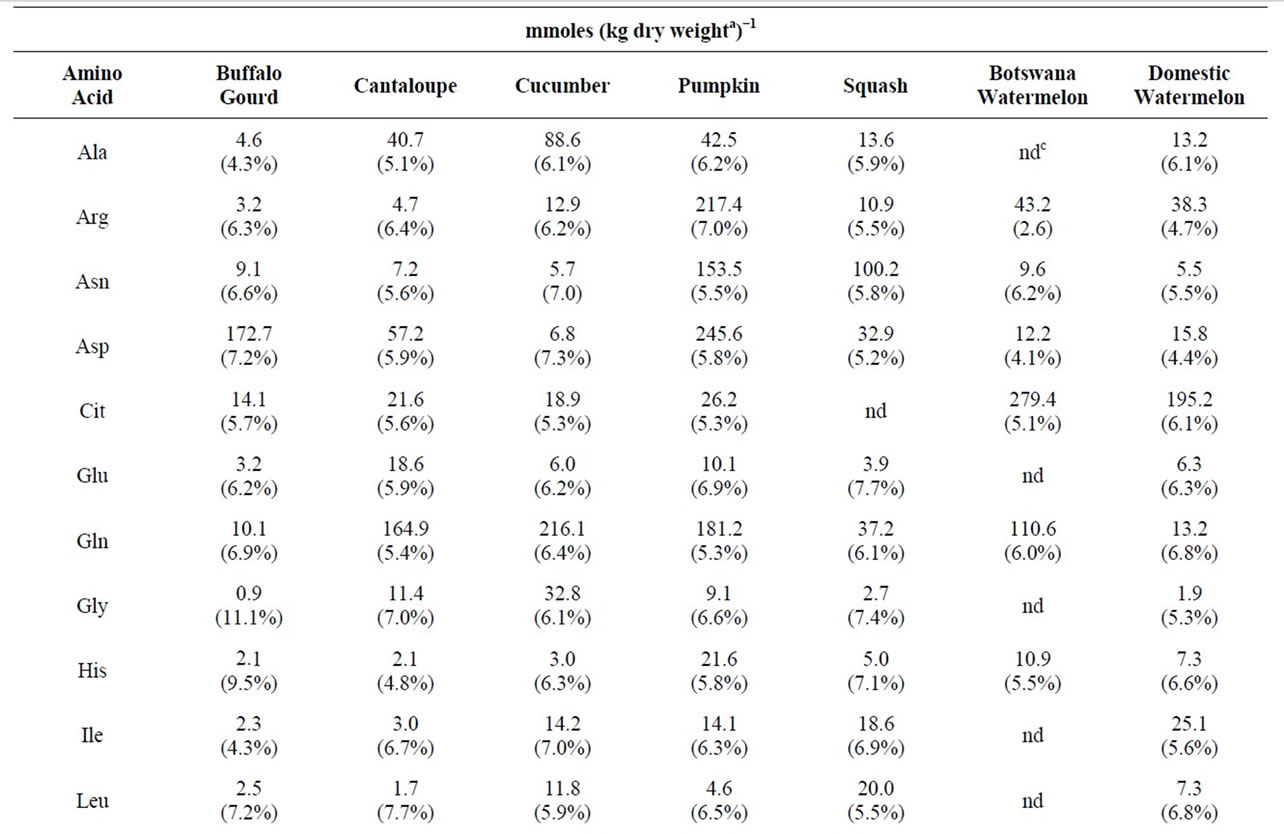
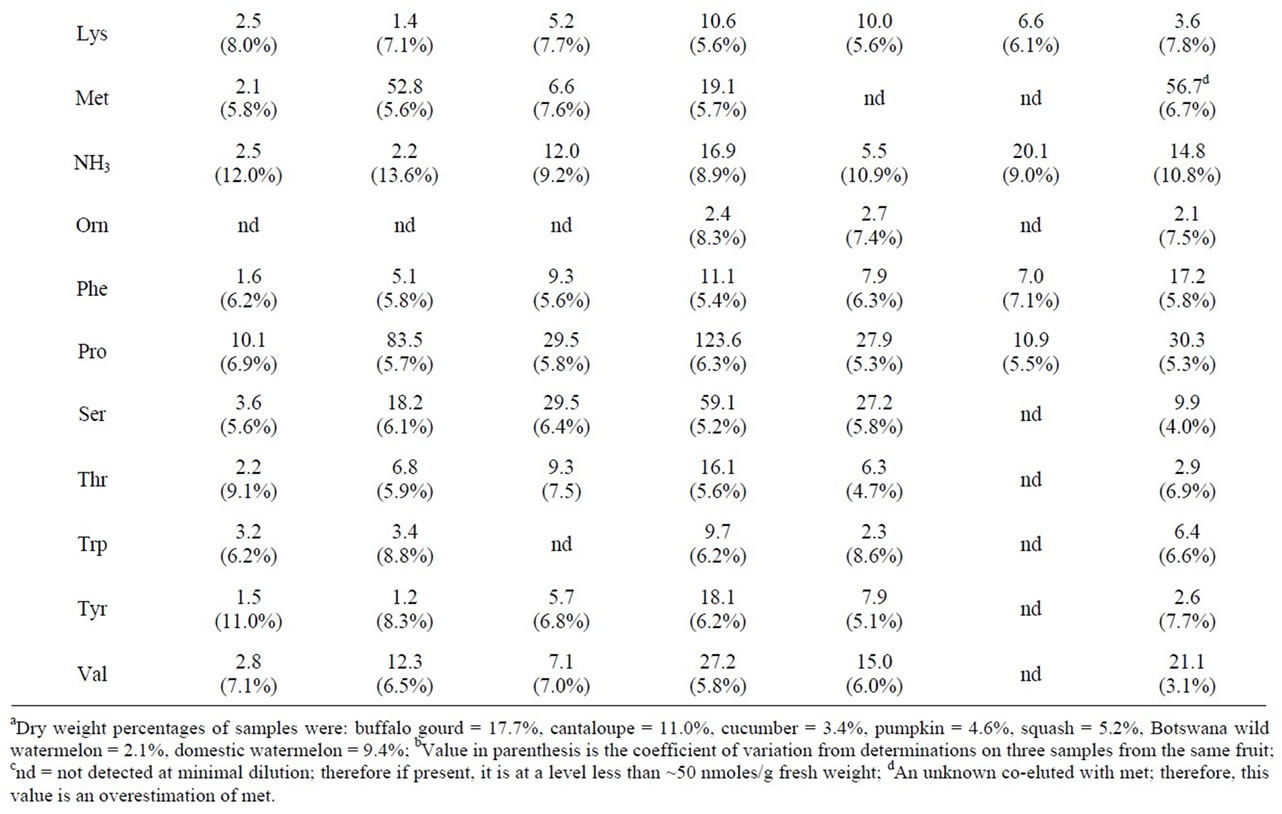
Table 2. Physiological amino acid levels in fruit of selected cucurbits.
First, all free amino acids had to be released from tissue cells. Second, amino acids had to be highly soluble in the extracting solvent. Third, the extracting solvent could not cause modification of any amino acids. Fourth, the extraction conditions had to minimize non-specific or specific binding of free amino acids to tissue or macromolecules as well as prevent enzymatic modifications of amino acids to occur. Dilute acid extractions, though as effective as deionized water in extracting maximal levels of amino acids, reduced levels of gln and asn while increasing the respective levels of glu and asp (data not shown). Water soluble organic solvents, e.g. 50% 2-propanol or 70% methanol, yielded reduced levels of physiological amino acids. Presumably, this occurred because of reduced solubility of many of the amino acids in high concentrations of alcohols or because of adverse effects of alcohols on plant tissues that precluded total cellular release of free amino acids. Chaotropic agents such as 2 M guanidinium thiocyanate or 0.2% sodium dodecyl sulfate in water produced lower yields of amino acids than did deionized water alone. The reasons leading to these reduced yields were not investigated. Comparison of a number of solvent systems and tissue treatments demonstrated that highest yields of physiological amino acids were achieved by first freezing the tissue, then grinding the tissue in cold deionized water, and finally separating tissue debris from the aqueous extract by centrifugation in the cold. This methodology, though apparently effective at free amino acid release, had the potential to be susceptible to non-specific amino acid binding or enzymatic degradation. Two types of recovery experiments were performed to test the efficacy of the extraction procedure: a spiking experiment and a tissue combinatorial experiment. The spiking experiment was to test for the loss of amino acids by binding or enzyme action. The tissue combinatorial experiment was employed as a measure for consistent extraction of amino acids from tissues.
Frozen watermelon rind samples were spiked with a known level of cit (1 μmole citrulline in ~1 g fresh weight of watermelon rind), carried through the extraction procedure, and analyzed. For six separate experiments, the recovery of spiked cit was 99.8% ± 6.3%.
For the tissue combinatorial experiment, duplicate samples were taken from four different watermelon rinds that had yielded widely different cit levels in earlier determinations. One of the duplicate pieces from each of the four rinds was weighed, processed, and assayed individually. The other duplicate piece was weighed individually and combined with pieces from each of the other three watermelons. The combined pieces were then mixed, processed, and assayed. Using the gln, cit, arg, and asp values determined for each individual piece, together with its respective weight in the combination sample, these amino acid contents in the composite sample could be predicted. The ratio of measured to predicted amino acid levels in the combined sample were 89.3% ± 4.4% for gln, 93.3% ± 3.1% for cit, 91.7% ± 3.7% for arg, and 101.1% ± 4.0% for asp from three replications of the experiment.
3.2. Application of the HPLC Separation and Quantification Method
Application of the published HPLC separation/quantification methodology of Sethuraman et al. [13] to extracts of fruit and vegetable tissues demonstrated that the method adequately separated amino acids to allow their individual quantification. Though gln and cit exhibited similar retention times, they separated sufficiently to allow reliable quantification of each. Acceptable resolution by a chromatography column lasted for 125 to 150 runs. Column resolution began to deteriorate at that point so that resolution between some dabsyl-amino acids became unacceptable for quantification, and the column was replaced.
3.3. Physiological Amino Acid Content of Selected Fruit and Vegetables
Table 1 compares the physiological amino acid levels in a number of fruits and vegetables. To allow direct comparisons, the amino acid contents were expressed on a dry weight basis. For the analyses, the coefficients of variation about the means varied between 5% and 7%. Redand Golden Delicious apples yielded similar physiological amino acid contents. Though most amino acid levels appeared to be similar in purple and white onions, gln was seven times higher in purple onion than in white onion (136 mmoles/kg dry weight versus 19.9 mmoles/kg dry weight). Cling peach was unusually high in asn. The amino acid, pro, was found to be present in high levels in broccoli (56.8 mmoles/kg dry weight), bell pepper (49.1 mmoles/kg dry weight), russet potato (51.7 mmoles/kg dry weight), Roma tomato (122.9 mmoles/kg dry weight), cantaloupe (83.5 mmoles/kg dry weight), and pumpkin (123.6 mmoles/kg dry weight). An unknown compound whose dabsyl derivative co-eluted with dabsyl-met was present in extracts from Golden Delicious apple, Red Delicious apple, purple onion, cling peach, and domestic watermelon. Apparent met values are given for those samples in Tables 1 and 2, but they represent overestimates of the true met values and are so indicated. Absorption spectra of the dabsyl derivatives that eluted at the retention time of dabsyl-met did not exhibit the same wavelength of maximal absorbance as that of dabsyl-met. Authentic dabsyl-met exhibited an absorption maximum at 460 nm; the wavelengths for the unknown dabsyl derivatives’ maximal absorbances were 466 - 470 nm. All other fruit and vegetable samples yielded dabsyl derivatives with chromatographic and spectral properties identical to those of authentic dabsyl-met.
3.4. Physiological Amino Acid Levels in Selected Cucurbit Fruit
Table 2 presents a comparison of the physiological amino levels in a variety of cucurbit fruit. With the exception of the cultivar of squash that was tested, cit appeared in all the cucurbits examined. Domestic watermelon and a Botswana wild-type watermelon were high in this amino acid. Pumpkin and cantaloupe were high in pro. Pumpkin contained a proportionally high concentration of total free amino acids as compared to the other cucurbits and the other fruit and vegetables that were tested. Pumpkin possessed a total of 1340 mmoles of free amino acids/kg dry weight, whereas most other fruit and vegetables possessed 500 or less mmoles/kg dry weight. High concentrations of arg, asn, asp, gln, and pro significantly contributed to this large total in pumpkin.
4. Discussion
Freeze-thaw of tissues followed by grinding and extraction in cold deionized water efficiently extracted free amino acids from a variety of fruit and vegetables. This methodology allowed complete extraction of the physiological amino acids in the tested fruit and vegetable tissues as supported by spiking and combinatorial experiments. This approach also allowed quantitative recovery of gln and asn that under low pH extraction conditions can be hydrolyzed to glu and asp [10]. Furthermore, this approach eliminates any concerns about potential solubility issues of free amino acids in polar organic solvents such as methanol, ethanol, or propanol. Microwave-assisted aqueous extraction also has been shown to be an efficient and rapid means for extraction of free amino acids from foods [17].
As was first demonstrated for measuring physiological amino acids in cerebrospinal fluid, the addition of a nonionic surfactant and a chaotropic agent enabled the precolumn derivatization of fruit and vegetable free amino acids with dabsyl chloride without the need for deproteinization of the extract [13]. Furthermore, the presence of certain buffer salts and chaotropic agents in the derivatization medium enhanced separation and detection sensitivity of the dabsyl amino acid derivatives [13]. The pre-column derivatization/reversed phase column separation employed in this study was used at a less sensitive level, nmoles versus pmoles, than must be applied to animal physiological fluids. For the types of fruit and vegetable samples examined in this study, this less sensitive method is better adapted for the quantities generally encountered.
It was not possible to support validation of the method described herein by comparing its estimated amino acid levels to values reported in the literature. This occurred for two reasons. First, it has been repeatedly demonstrated that marked differences occur among different varieties grown in the same year [18], for the same variety produced in different growing seasons [19], or for the same variety subjected to different cultural practices [20]. Second, no published analyses could be found for a number of the fruit and vegetables that were assayed in this study. In general, amino acids reported to be proportionately high in each fruit or vegetable were determined to be high by our assay methodology. The dabsyl chloride derivativization methodology offers an advantage over methods employing o-phthalaldehyde as the amino derivatizing agent since the dabsyl-associated methodology allows quantification of pro whereas o-phthalaldehyde does not. Pro levels proved to be a substantial proportion of the free amino acids in a number of the fruit and vegetables examined. Since pro is thought to act as an osmotic protectant in plants, the methodology described herein would be applicable to plant studies dealing with drought and salt stress.
A pitfall associated with all chromatographic methods was encountered in this study, and that was the co-elution of an unknown with a known constituent. An unknown dabsyl derivative co-eluted with dabsyl-met from extracts of five of the fruit and vegetables. Accordingly, accurate met values could not be estimated for those five samples. This situation was recognized because of differences in the wavelength of maximum absorbance, and it emphasizes the necessity of using other criteria in addition to chromatographic elution position to verify column peak identity.
One of the stimuli for development of this methodology was to examine levels of cit and its associated metabolic amino acids in various cucurbits with changes in growing conditions, cultural practices, and fruit development. The results of this work demonstrate that such investigations will be possible, not only for the arg-cit metabolic family, but other physiological amino acid families as well. Preliminary cursory examinations of growing conditions and fruit development on amino acids of the arg-cit family relying on the methodology described above support the claim for its applicability for such investigations [15,16]. Availability of the described methodology further provides the potential to define research problems heretofore unrecognized. For example, it was demonstrated that all but one of the cucurbits examined in this study produced cit in their mature fruit. It is presently not clear why in two cucurbits of the same genus and species, pumpkin and straight-necked yellow squash, both Cucurbita pepo, one produced cit and one did not.
5. Conclusion
The methodology reported herein provides quantitative extraction and subsequent quantification of the physiological amino acids. Though generally applicable, it was developed for analysis of fresh fruit and vegetables. Its application not only allows quantification of the free amino acids, it also facilitates evaluation of the physiological interplay among amino acids of a given metabolic family such as demonstrated for those of the arg family.
6. Acknowledgements
The author thanks Rick Houser and Shelia Magby for providing valuable technical support and Sara Duke for guidance on statistical applications.
7. Disclaimer
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. All programs and services of the US Department of Agriculture are offered on a nondiscriminatory basis without regard to race, color, national origin, religion, sex, age, marital status, or handicap. The article cited was prepared by a USDA employee as part of his official duties. Copyright protection under US copyright law is not available for such works. Accordingly, there is no copyright to transfer. The fact that the private publication in which the article appears is itself copyrighted does not affect the material of the US Government, which can be freely reproduced by the public.
REFERENCES
- D. H. Spackman, W. H. Stein and S. Moore, “Automatic Recording Apparatus for Use in the Chromatography of Amino Acids,” Analytical Chemistry, Vol. 30, No. 7, 1958, pp. 1190-1206. doi:10.1021/ac60139a006
- J. V. Benson Jr. and J. A. Patterson, “Accelerated Chromatographic Analysis of Amino Acids Commonly Found in Physiological Fluids on a Spherical Resin of Specific Design,” Analytical Biochemistry, Vol. 13, No. 2, 1965, pp. 265-280. doi:10.1016/0003-2697(65)90196-X
- K. L. Woo and D. S. Lee, “Capillary Gas Chromatographic Determination of Proteins and Biological Amino Acids as N(O)-tert.-Butyldimethylsilyl Derivatives,” Journal of Chromatography B, Vol. 665, No. 1, 1995, pp. 15-25. doi:10.1016/0378-4347(94)00515-7
- P. Lindroth and K. Mopper, “High-Performance Liquid Chromatographic Determination of Subpicomole Amounts of Amino Acids by Precolumn Fluorescence Derivatization with o-Pthaldialdehyde,” Analytical Chemistry, Vol. 51, No. 11, 1979, pp.1667-1674. doi:10.1021/ac50047a019
- R. Schuster, “Determination of Free Amino Acids by High Performance Liquid Chromatography,” Analytical Chemistry, Vol. 52, No. 4, 1980, pp. 617-620. doi:10.1021/ac50054a005
- S. Weiner and A. Tishbee, “Separation of Dns-Amino Acids Using Reversed-Phase High-Performance Liquid Chromatography: A Sensitive Method for Determining N-Termini of Peptides and Proteins,” Journal of Chromatography, Vol. 213, No. 3, 1981, pp. 501-506. doi:10.1016/S0021-9673(00)80501-4
- S. Einersson, B. Josefsson and S. Lagerkvist, “Determination of Amino Acids with 9-Fluorenylmethyl Chloroformate and Reversed-Phase High-Performance Liquid Chromatography,” Journal of Chromatography, Vol. 282, 1983, pp. 609-618. doi:10.1016/S0021-9673(00)91638-8
- K. Shimbo, T. Oonuki, A. Yahashi, K. Hirayama and H. Miyano, “Precolumn Derivatization Reagents for HighSpeed Analysis of Amines and Amino Acids in Biological Fluid Using Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry,” Rapid Communications in Mass Spectrometry, Vol. 23, No. 10, 2009, pp. 1483-1492. doi:10.1002/rcm.4026
- J.-Y. Chang, R. Knecht and D. G. Braun, “Amino Acid Analysis in the Picomole Range by Precolumn Derivatiztion and High-Performance Liquid Chromatography,” Methods in Enzymology, Vol. 91, 1983, pp. 41-48. doi:10.1016/S0076-6879(83)91009-1
- S. J. Leach and H. Lindley, “The Kinetics of Hydrolysis of the Amide Group in Proteins and Peptides. Part 1. The Acid Hydrolysis of L-Asparagine and L-Asparaginylglycine,” Transactions of the Faraday Society, Vol. 49, 1953, pp. 915-920. doi:10.1039/tf9534900915
- J.-Y. Chang, R. Knecht and D. G. Braun, “Amino Acid Analysis at the Picomole Level. Application to the C-Terminal Sequence Analysis of Polypeptides,” Biochemical Journal, Vol. 199, 1981, pp. 547-555.
- I. Krause, A. Bockhardt, H. Neckermann, T. Henle and H. Klostermeyer, “Simultaneous Determination of Amino Acids and Biogenic Amines by Reversed-Phase HighPerformance Liquid Chromatography of the Dabsyl Derivatives,” Journal of Chromatography A, Vol. 715, No. 1, 1995, pp. 67-79. doi:10.1016/0021-9673(95)00578-B
- R. Sethuraman, T. L. Lee and S. Tachibana, “Simple Quantitative HPLC Method for Measuring Physiologic Amino Acids in Cerebrospinal Fluid without Pretreatment,” Clinical Chemistry, Vol. 50, No. 3, 2004, pp. 665- 669. doi:10.1373/clinchem.2003.026195
- H.-J. Schneider, “Amino Acid Analysis Using DABSCl,” Chromatographia, Vol. 28, No. 1, 1989, pp. 45-48. doi:10.1007/BF02290382
- W. W. Fish and B. D. Bruton, “Quantification of Lcitrulline and Other Physiologic Amino Acids in Watermelon and Selected Cucurbits,” Cucurbitaceae, 2010, pp. 152-154.
- A. R. Davis, C. L. Weber III, W. W. Fish, T. C. Wehner, S. King and P. Perkins-Veazie, “L-citrulline Levels in Watermelon Cultigens Tested in Two Environments,” HortScience, Vol. 46, No. 12, 2011, pp. 1572-1575.
- A. Kovács, K. Ganzier and L. Simon-Sarkadi, “Microwave-Assisted Extraction of Free Amino Acids From Foods,” Zeitschrift fuer Lebensmittel-Untersuchung und, Forschung A, Vol. 207, No. 1, 1998, pp. 26-30.
- F. Zhu, Y.-Z, Cai and H. Corke, “Compositions of Phenolic Compounds, Amino Acids and Reducing Sugars in Commercial Potato Varieties and Their Effects on Acrylamide Formation,” Journal of the Science of Food and Agriculture, Vol. 90, No. 13, 2010, pp. 2254-2262. doi:10.1002/jsfa.4079
- M. H. Gomes and E. Rosa, “Free Amino Acid Composition in Primary and Secondary Inflorescences of 11 Broccoli (Brassica oleracea var Italica) Cultivars and Its Variation Between Growing Seasons,” Journal of the Science of Food and Agriculture, Vol. 81, No. 3, 2001, pp. 295-299. doi:10.1002/1097-0010(200102)81:3<295::AID-JSFA811>3.0.CO;2-#
- J. Lee, J. W. Finley and J. M. Harnly, “Effect of Selenium Fertilizer on Free Amino Acid Composition of Broccoli (Brassica oleracea Cv. Majestic) Determined by Gas Chromatography with Flame Ionization and Mass Selective Detection,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 23, 2005, pp. 9105-9111. doi:10.1021/jf051221x

