American Journal of Plant Sciences
Vol.4 No.12(2013), Article ID:41291,6 pages DOI:10.4236/ajps.2013.412301
Development of Sanitation Protocol for Leaf Explants of B. huillensis for in Vitro Culture
![]()
1School of Life Science and Bio Engineering, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania; 2Mikocheni Agriculture Research Institute, Dar es Salaam, Tanzania.
Email: *ndakidemipa@gmail.com
Copyright © 2013 Cosmas Funguomali Ndakidemi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 26th, 2013; revised October 31st, 2013; accepted November 12th, 2013
Keywords: Asteraceae; Silver Oak; Sodium Hypochlorite in Vitro Propagation; Cefotaxime
ABSTRACT
Brachylaena huillensis (Silver Oak) is a multipurpose timber tree species in the family of Asteraceae. There has been a very high demand for B. huillensis wood and its products leading to overexploitation. B. huillensis regenerates through seedlings. However, it produces seeds with poor germination. Seeds are also difficult to be collected because of small size. Many are eaten by insects and currently there is a lack of seed bank. The facts that have hindered and rendered the natural regeneration of the tree species were uncertain. The present investigation was carried out to develop sanitation protocol of B. huillensis using leaves as explants. Juvenile leaves from the tips of B. huillensis naturally growing seedlings were collected from Bombo West Forest Reserve in Tanzania. The leaves were washed and immersed in NaOCL containing various concentrations levels and two drops of tween 20. There was significant difference between the concentrations levels employed. However, the best results were obtained when leaf explants were immersed in 1.5% v/v NaOCL for ten minutes and later in ethanol for ten seconds and cultured on woody plant media medium containing antifungal (cefotaxime). Genuinely, the protocol is vital and so opens up the way for other subsequent stages for in vitro propagation of B. huillensis.
1. Introduction
Brachylaena huillensis or Silver Oak is a multipurpose timber tree species in the family of Asteraceae [1,2]. There has been a very high demand for B. huillensis wood and its products leading to overexploitation. It is a threatened tree species [3,4]. The species is suitable for timber and carving artefacts [5], charcoal, essential oil, [5-7], sleepers, flooring blocks, furniture, and turnery [8]. Moreover, due to its durability, the species is used as fence posts, building poles, transmission poles, ornamental and medicine for schistosomiasis and leaves for diabetes [6]. The Silver Oak is illegally exploited for timber, charcoal, transmission poles, carving, building poles, fencing posts, ornamental, medicine, perfumery and toilet preparations, sleepers, flooring blocks, furniture, and turnery [7-9].
B. huillensis regenerates only through seedling and produces seeds with poor germination, seeds are also difficult to be collected because of small size. Many are eaten by insects and currently there is a lack of seed bank [5]; the facts that have hindered and rendered the natural regeneration of the tree species were uncertain. A number of endangered and threatened species have been successfully regenerated using in vitro culture methods using shoot tips, leaves, and leaf bases [10]. Nonetheless, so far in vitro propagation in B. huillensis is not yet in place. In vitro culture offers an alternative tool for rapid multiplication of disease free propagules in a short period, which will further enable both conservation and uninterrupted supply of wood and their products for this tree species.
In vitro propagation technique for plants involves various steps i.e. selection of explants, its sterilization and establishment and shoot proliferation and production of in vitro plant lets. The first condition for the success of a culture is asepsis. The maintenance of aseptic (free from all microorganisms) or sterile conditions is essential for successful tissue culture procedures. To maintain an aseptic environment, all culture vessels, media and instruments used in handling tissues, as well as explant itself must be sterilized. The importance is to keep the air, surface and floor free of dust. All operations should be carried out in lamina airflow sterile cabinet [11]. Sterilization is the process of making explants contamination free before establishment of cultures. Various sterilization agents are used to decontaminate the tissues. These sterilants are also toxic to the plant tissues. Hence proper concentration of sterilants, duration of exposing the explant to the various sterilants, and the sequences of using these sterilants have to be standardized to minimize explants injury and achieve better survival. The disinfectants widely used are sodium hypochlorite calcium hypochlorite and ethanol [12]. Among these, hypochlorite is known to be a very effective killer of bacteria. Even micromolar concentrations are enough to reduce bacterial populations significantly. Essentially, since in vitro propagation of B. huillensis is not yet developed, definitely its sterilization protocol is lacking. So, the present study was undertaken to develop efficient and reproducible sanitation protocol for B. huillensis explants for in vitro propagation.
2. Materials and Methods
2.1. Plant Material
Plant materials were obtained from the healthy naturally growing seedlings in Bombo West Forest Reserve (BWFR). The BWFR is located in Korogwe district, Tanga region. The reserve lies between latitude 4˚52′ and longitude 4˚47′ S and 38˚39′ and 38˚43′ E. It is situated 60 kilometres from Korogwe town on Lwengera valley about 380 m to 680 m above sea level [13]. Alternatively it is 83 kilometres from Tanga town through Tanga— Mashewa road via Maramba. The reserve is owned by the central government; it was gazetted in 1959 with a Government Notice (GN) 1 of 1959 and has an area of 3523.5 ha [14].
2.2. Explants Source
The second pair of leaves from the tips B. huillensis seedlings was the source of explants material that was collected from the healthy naturally growing seedlings in Bombo West Forest Reserve in early May 2013. The tip leaves were chopped from the naturally growing seedlings and then preserved in a cool box with ice blocks and transported to Mikocheni Agriculture Research Institute (MARI) laboratory in Dar es Salaam where this research was carried out. The leaves spent twenty four hours on transit before culture initiation.
2.3. Surface Sterilization of the Explants
In the laboratory, the leaves were placed in a bottle containing distilled water. The water contained two detergents, namely liquid soap and tween-20, which enhance the effectiveness of the disinfectant by breaking the surface tension between water and the plant tissues. For effectiveness, the explants in the solution were agitated continuously for 5 minutes, later the leaves were rinsed four times with distilled water. The bottles containing the already washed leaves were transferred to the transfer room (lamina flow) and immersed in sodium hypochlorite (NaOCl-3.85) at four concentration levels (0.8%, 1.2%, 1.5%, & 1.9% v/v) with two drops of tween-20 for 10 minutes. Subsequently the leaves were rinsed four times with sterile distilled water and later dipped in 70% ethanol for 10 seconds. Thereafter, the leaves were rinsed four times using sterile distilled water before culturing for callus induction. The sterilized explants were trimmed suitably to remove sterilizing agent affected parts. Leaf discs of about 1 square centimeter with and without midrib were sliced from the sterile leaves and cultured.
2.4. Inoculation
The media was composed of basal woody plant medium (WPM) [15] with full strength supplemented with antifungal namely cefotaxime 0.03 mg/liter in the medium after cooling to body temperature. The pH of the medium was adjusted to 5.6 before autoclaving at 121 degrees centigrade. The surface sterilized explants (leaf discs) were inoculated on the WPM medium abaxial side in contact with the medium (right side up) and labeled properly. Each petri dish (sterile) with 20 ml of the WPM medium contained five explants. Four replications with 5 explants in each were maintained for each treatment and 20 explants in each treatment were evaluated. The culture without sodium hypochlorite treatment served as a control. The cultures containing the explants (leaf discs) in a petridish were kept in a growth room at a temperature of 25 ± 2 degrees centigrade, 60% - 70% relative humidity and white fluorescent light with a 16-h photoperiod. Contamination was evaluated for 15 days after the first incubation. The number of uncontaminated explants was counted.
2.5. Statistical Analysis
Five explants (leaf discs) were placed into each petri dish. Twenty explants were established in each treatment. Each treatment was replicated 4 times. Records of clean and live leaf discs were observed for 15 days. The numbers of clean and live leaf discs were determined. The obtained data were subjected to STATISTICA program and analyzed using one-way analysis of variance (ANOVA). The means are reported with standard errors. The fisher least significance difference (L.S.D.) was used to compare treatment means at p = 0.05 level of significance [16].
3. Results
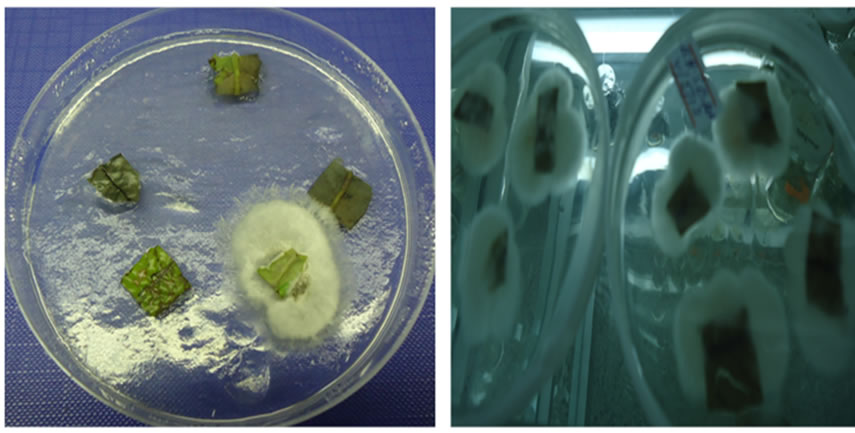
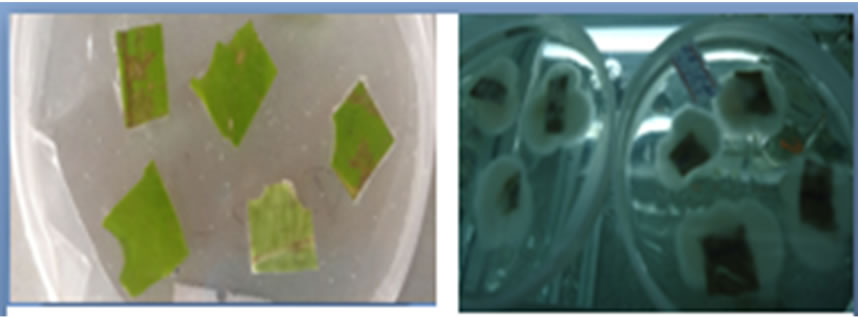
The leaves discs of B. huillensis could be sterilized by immersing them in sodium hypochlorite at various concentrations for ten minutes and in ethanol for ten seconds. Four different concentration levels of NaOCl (0.8%, 1.2%, 1.5%, & 1.9% v/v) were used in this sterilization experiment. Continuous monitoring was carried out for fifteen days while the explants were in the culture media. Obtaining clean cultures from the leaf discs of B. huillensis was problematic. However, addition of antifungal (cefotaxime) 0.03 mg/liter in the medium noticeably improved the percentage of clean cultures (Figure 1(b)). Fungal contamination was observed as hyphal growth from the explants, and bacterial contamination was identified by observing colonies, seen as watery or slimy buildups on the agar surface. Still a substantial number of leaf cultures were contaminated when only ethanol and NaOCl were used (Figure 2(b)). The results of the present study showed that most explants in the control group were contaminated after a few days, giving a contamination rate for the control group of 100% by day 5 (Figure 1(b)). The best sterilization was achieved by immersing the explants in 1.5% v/v NaOCl for 10 minutes followed by ten seconds dipping in 70% ethanol (Table 1). Also the results showed that there is a significant (p = 0.05) difference between the concentration levels of NaOCl at (0.8%, 1.5%, & 1.9% v/v) treatment. Though, there was no significant difference between the concentration levels of NaOCl (0.8%, 1.5%, & 1.9% v/v) and 1.2% v/v). Generally, the results showed that lower concentration had poor response; the same was the case for high concentration of NaOCl (Table 1).
Values represent means ± standard error. Means followed by the same later(s) within column are not significantly different at p = 0.05 according to Fisher’s Least Significance difference.
 (a) (b)
(a) (b)
Figure 1. (a) & (b) (a) Cultured leaf explants of B. huillensis (cefotaxime); (b) Contaminated leaf explants of B. huillensis in the control group. Photos by: Cosmas Ndakidemi, 2013.
 (a)
(a)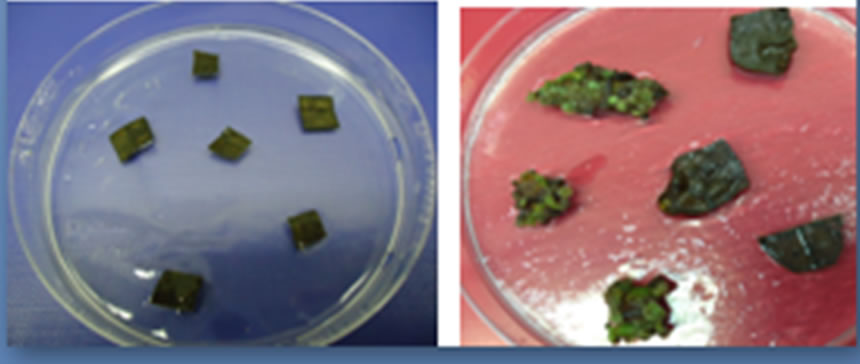 (b)
(b)
Figure 2. Leaf explants of B. huillensis treated with sodium hypochlorite at various concentrations. (a) Initiated leaf explants B. huillensis of on woody plant media; (b) Contaminated dead leaf explants B. huillensis of treated with sodium hypochlorite (0.8%); (c) Live and clean leaf explants of B. huillensis uncontained treated with 1.5% (optimal) sodium hypochlorite; (d) Live and clean leaf explants of B. huillensis callusing on a woody plant media following successful sterilization. Photos by: Cosmas Ndakidemi, 2013.
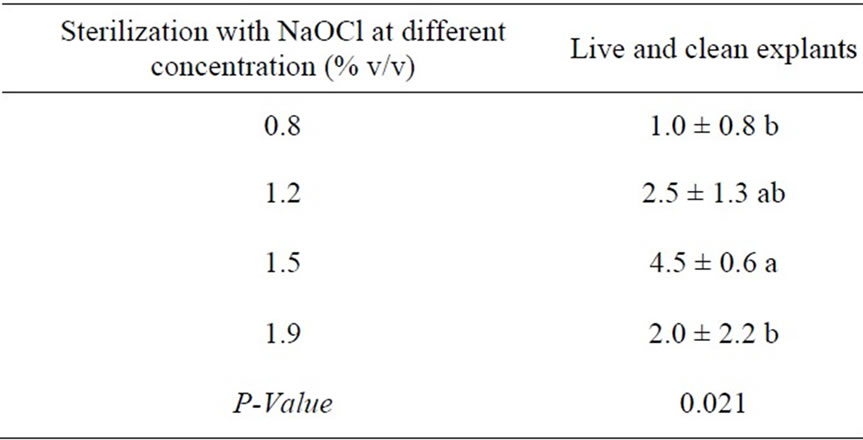
Table 1. Effects of ethanol and NaOCl in the sterilization of B. huillensis leaf discs.
4. Discussion
Plant tissue culture is a system of growing plant cells, tissue or organs that have been separated from the mother plant (called explants) in artificial medium under aseptic condition [17]. In this study, the protocol for sterilization of leaf explants of B. huillensis has been developed using NaOCL, ethanol and surfactant tween 20 and addition of antifungal i.e. cefotaxime. The results of this study revealed that all explants in the control group were contaminated after five days of inoculation (Figure 1(b)). Apparently, it is an obvious result for explants collected from naturally grown plants. [13,18,19] found that the use of field grown plants as direct sources of explants for the production of “clean” in vitro plantlets, presents a major challenge in in vitro cultures. Also, Mathias, [20] established that the incidence of bacterial and fungal contamination was higher in explants taken from their natural environments. This is because the surface of plants carries a wide range of microbial contaminants. Micropropagation of field-grown woody plants can be more difficult because of contamination and exudates from cut woody stems [21-24]. Also the contaminants can be present in the explants (endophytic) or can be reintroduced from poor aseptic handling, unhygienic conditions in the laboratory or from laboratory instruments [19]. However, explants contamination depends on several plant and environmental related factors such as species, age, explant source and prevailing weather condition [13]. [19] found that leaves collected from greenhouse-grown plants had low levels of contamination. Furthermore, young leaves may be less contaminated compared with mature ones. Thus, it is suggested that prudent selection of explants from the healthy parent plants coupled with an effective surface sterilization method should be the goal in avoiding culture contamination. So, to avoid this source of infection, the explants must be thoroughly surface-sterilized using various sterilant agents before inoculating them onto nutrient medium [25]. The problem is exacerbated when explants are sourced directly from field grown plants [18].
In the present study, sodium hypochlorite at various concentration and ethanol was employed in all experiments. [12] showed that the disinfectants widely used are sodium hypochlorite calcium hypochlorite, ethanol, mercuric chloride, hydrogen peroxide, silver nitrate and bromine water. Among these, hypochlorite is known to be a very effective killer of bacteria; even micromolar concentrations are enough to reduce bacterial populations significantly. [26] showed that Sodium hypochlorite produced the highest reduction in bacterial and fungal contamination at time intervals between 20 - 45 minutes. [27] concluded that the use of locally produced bleach containing 3.85% hypochlorite for 30 min is as effective. Consequently, we would recommend its usage because of its simplicity and economy. [28] established that the common sterilizing agents like sodium or calcium hypochlorite (5% - 10%) and ethyl alcohol (50% - 95%) are used to exclude the surface contaminants by washing in the appropriate solution for 10 - 25 min. Sodium hypochlorite, usually purchased as laundry bleach is the most frequent choice for surface sterilization. It is readily available and can be diluted to proper concentrations. A balance between concentration and time must be determined empirically for each type of explant because of phytotoxicity [27].
Ethanol is a powerful sterilizing agent but also extremely phototoxic. Therefore, the explant is typically exposed to it for only a few seconds or minutes. To enhance effectiveness in sterilization procedure, a surfactant like Tween 20 is frequently added to the sterilizing solution (and in some laboratories a mild vacuum is applied during the procedure). In general, the sterilizing solutions containing the explants are continuously stirred during the sterilization period [29].
In the present study, NaOCL and ethanol were successfully used for sterilization protocol for B. huillensis leaf explants. These sterilants are preferred due to their availability and economic merits. Similarly, these sterilants have been used in many studies for sterilization of a wide of plant explants for in vitro cultures successfully. Some studies of which NaOCL and ethanol have been used successfully include [12,26,30-32] among others.
In the present study, the best results were obtained when leaf explants were immersed in 1.5% for ten minutes and later in 70% ethanol for ten seconds. The results do not agree with many studies especially those carried out in herbaceous plants. Basically, requirements on the concentration and time of exposure differ from one plant to another and for different parts of plants depending on their morphological characters like softness /hardness of the tissue [29]. Mostly, many plants (woody plants inclusive) appear to have microbial contamination within the vascular system and other tissues [33]. Essentially, surface sterilization of woody plant explants for in vitro culture is comparatively a difficult step in culture initiation due to its nature and the environment in which they are grown. Another feasible reason may be due slow growth in woody plants, the longer life span of trees may add to the problem of contamination in vitro by the symbiotic association of microorganisms. [15] found that the growth rates of shoots and other tissues such as callus of woody plants in microculture are usually slower than observed with herbaceous plant. In a study by [28] it was observed that phase change or maturation in woody plants not only results in changes in growth behavior, but results in increased difficulty in vegetative propagating selected individuals. This slow growth can complicate all stages of microculture. Thus, as observed in this study, the sterilization process obviously could be difficult in woody plants compared with herbaceous plants [34-36].
The results of the present study showed that treatment with low concentration of NaOCl had poor response; likewise an increase in concentration of NaOCL had adverse effect on the cultured explants (Table 1). Possible reason for ineffective results in low concentration may be due to the fact that the plant under study is woody in nature and also the explants were collected from their natural environment. Regarding to poor response in high concentration of NaOCL, the probable reasons could be due to the fact that the sterilants are also toxic to the plant tissues. [29] observed that proper concentration of sterilants, duration of exposing the explants to various sterilants, the sequences of using these sterilants has adverse effect so sterilants need to be standardized to minimize explants injury and achieve better survival. The results showed that there is a significant difference between the employed concentration levels of NaOCL except for 1.2%. In this case, the plausible cause may be due to the fact that different explants need different level concentration for sterilization. The finding is in line with a study by [29] which found that requirements on the concentration and time of exposure differ from one plant to another and for different parts of plants depending on their morphological characters like softness/ hardness of the tissue.
In an attempt to obtain clean in vitro cultures, sources of contamination other than surface contaminants need to be considered. Systemic contaminants, for example, are not killed by surface sterilization [19]. Because of that, a systemic fungicide such as Benomyl can be helpful to control the incidence of internal fungal infection in explants. In the present study, antifungal cefotaxime was added in the medium. Essentially, the addition of antifungal (cefotaxime) 0.03 mg/liter in the medium noticeably improved the percentage of clean cultures. The finding of this study concurs with many studies of this nature, among others the studies include those conducted by; [37-39]. Also, [40] found that pre-sterilization using 0.2% Benomyl for 15 minutes improved the number of “clean and alive” individuals of all types of explants, especially when followed by surface sterilization.
5. Conclusion and Recommendations
The present study has developed sterilization protocol for B. huillensis leaf explants in in vitro culture. Since the plant under study was woody in nature, it involved addition of fungicide. Significant difference between the concentrations levels of NaOCL was noticed. However, the study revealed that immersing leaf explants of B. huillensis in NaOCL in 1.5% v/v for ten minutes and later in 70% ethanol for ten seconds with addition of fungicide, cefotaxime produced the best results. Fundamentally, this protocol offers a way forward for in vitro propagation of this tree species. It also opens up ways for further stages of in vitro culture in B. huillensis and proposes further research on important woody plant species in the family of Asteraceae.
6. Acknowledgement
Our earnest thanks go to the Nelson Mandela African Institution of Science and Technology, the Commission for Science and Technology (COSTECH) for financial support of this study, and Mikocheni Agriculture Research Institute (MARI) Dar es Salaam, for allowing us to use their laboratory facilities. We are also indebted to the Tanzania Forest Service (TFS) under the Ministry of Natural Resources and Tourism for permission to collect explant materials (samples) in BWFR.
REFERENCES
- WCMC, “Provision of Data on Rare and Threatened Tropical Timber Species,” Chapman and Hall Ltd., London, 1991, 58 Pages.
- S. K. Chonge, “Study of Economic Aspects of the Wood Carving Industry in Kenya: Implications for Policy Development to Make the Industry More Sustainable,” Thesis for Award of Msc Degree at University of Natal, Natal, 2002, 55 pages. Unpublished.
- C. K. Ruffo and S. M. Maliondo, “Forest Plant Genetic Resources in Tanzania,” Proceedings of the 1st National Workshop, Arusha, 16-20 January 1990.
- IUCN, “IUCN Red List of Threatened Species,” 2008. http://www.iucnredlist.org
- L. P. Mbuya, H. P. Msanga C. K. Ruffo A. Birniel and B. Tengnas, “Useful Trees and Shrubs for Tanzania: Identification, Propagation and Management for Agricultural and Pastoral Communities,” Regional Soil Conservation Unit, Nairobi, 1994, 542 Pages.
- B. Cunningham, “Kenya’s Carvings, the Ecological Footprint of the Wooden Rhino Africa,” Wildlife and Environment, Vol. 6, No. 2, 1998, pp. 43-50.
- J. M. Bryce and A. W. Chihongo, “The Commercial Timbers of Tanzania,” KAD Publishers, Dar es Salaam, 1999, 293 Pages.
- N. T. Marshall and M, Jenkins, “Hard Times for Hardwood: Indigenous Timber and the Timber Trade in Kenya Traffic International,” London, 1994, 53 Pages.
- H. N. Daud, J. Shashita and R. Mohamed, “An Improved Surface Sterilization Technique for Introducing Leaf, Nodal and Seed Explants of Aquilaria malaccensis from Field Sources into Tissue Culture,” Asia-Pacific Journal of Molecular Biology and Biotechnology, Vol. 20, No. 2, 2012, pp. 55-58
- S. Seeni and P. G. Latha, “In Vitro Multiplication and Ecorehabilitation of the Endangered Blue Vanda,” Plant Cell, Tissue and Organ Culture, Vol. 61, 2000, pp. 1-8. http://dx.doi.org/10.1023/A:1006444614657
- H. S. Chawla, “Plant Biotechnology: Laboratory Manual for Plant Biotechnology,” Oxford & IBH Publishing Co. Pvt Ltd., New Delhi, 2003.
- A. Badoni and J. S. Chauhan, “In Vitro Sterilization Protocol for Micropropagation of Solanum tuberosum cv ‘Kufri Himalini’,” Academia Arena, Vol. 2, No. 4, 2010, pp. 24-27.
- G. R. T. Rout, S. Samantaray and P. Das, “In Vitro Manipulation and Propagation of Medicinal Plants,” Biotechnology Advances, Vol. 18, No. 2, 2000, pp. 91-120. http://dx.doi.org/10.1016/S0734-9750(99)00026-9
- J. C. Lovett and T. Pocs, “Assessment of the Condition of the Catchment Forest Reserves: A Botanical Appraisal,” Government Printers, Dar es Salaam, 1993, 300 Pages.
- B. H. McCown, “Recalcitrance of Woody and Herbaceous Perennial Plants: Dealing with Genetic Predetermination,” In Vitro Cellular and Developmental Biology Plant, Vol. 36, No. 3, 2000, pp. 149-154. http://dx.doi.org/10.1007/s11627-000-0030-6
- R. G. D. Steel and J. H. Torrie, “Principles and Procedures of Statistics,” 2nd Edition, McGraw Hill Book Co. Inc., New York, 1980, pp. 232-249.
- I. B. Omamor, A. O. Asemota, C. R. Eke and E. I. Eliashi, “Fungal Contamination of the Oil Palm Tissue Culture in Nigerian Institute for Oil Palm Research (NIFOR),” African Journal of Agriculture Research, Vol. 2, No. 10, 2007, pp. 534-537.
- O. I. Odutayo, N. A. Amusa, O. O. Okutade and Y. R. Ogunsanwo, “Sources of Microbial Contamination in Tissue Culture Laboratories in Southwestern Nigeria African,” Journal of Agricultural Research, Vol. 2, No. 3, 2007, pp. 67-72.
- S. K. Webster, J.A. Seymour, S.A. Mitchell and M. H. Ahmad, “A Novel Surface Sterilization Method for Reducing Microbial Contamination of Field Grown Medicinal Explants Intended for In Vitro Culture,” Biotechnology Centre, Kingston, 2003.
- P. J. Mathias, P. G. Alderson and R. R. B. Leakey, “Bacterial Contamination in Tropical Hardwood Cultures,” Acta Horticulture, Vol. 212, 1987, pp. 43-48
- N. Brassard, C. Richer, D. Tousignant and J. A. Rioux, “Multiplication végé Tative de L’acer Saccharum: Contribution,” Micropropagation, Vol. 33, 2003, pp. 682-690.
- H. R. Kerns and M. M. Meyer, “Tissue Culture Propagation of Acer X Freemanii Using Thidiazuron to Stimulate Shoot Tip Proliferation,” Hortisciences, Vol. 21, No. 5, 1986, pp. 1209-1210.
- M. G. Padilla and C. L. Encina, “Micropropagation of Adult Cherimoya (Annona cherimola Mill ) cv Fino de Jete,” In Vitro Cellular & Developmental Biology—Plant, Vol. 40, No. 2, 2004, pp. 210-214. http://dx.doi.org/10.1079/IVP2003521
- J. E. Preece, “Practical Regulation of Woody Plant Growth and Development Using Biotechnology,” Acta Horticulturae, Vol. 300, 1991, pp. 23-33.
- M. Kane, “Bacterial and Fungal Indexing of Tissue Cultures,” 2003
- O. B. Oyebanji, O. Nweke, O. Odebunmi, N. B. Galadima, M. S. Idris, U. N. Nnodi, A. S. Afolabi and G. H. Ogbadu, “Simple, Effective and Economical ExplantSurface Sterilization Protocol for Cowpea, Rice and sorghum seeds,” African Journal of Biotechnology, Vol. 8, No. 20, 2009, pp. 5395-5399.
- N. Srivastava, B. Kamal V. Sharma, Y. K. Negi, A. K. Dobriyal, S. Gupta and V. S. Jadon, “Standardization of Sterilization Protocol for Micropropagation of Aconitum Heterophyllum—An Endangered Medicinal Herb,” Academia Arena, Vol. 2, No. 6, 2010, 37-42.
- M. S. Greenwood, “Rejuvenation in Forest Trees,” Journal of Plant Growth Regulation, Vol. 6, 1987, pp. 1-12. http://dx.doi.org/10.1007/BF00043947
- H. Colgecen, U. Koca and G. Toker, “Influence of Different Sterilization Methods on Callus Initiation and Production of Pigmented Callus in Arnebia Densifl ora Ledeb,” Turkish Journal of Biology, Vol. 35, No. 4, 2011, pp. 513-520.
- A. N. Ahmed, “In Vitro Production of Somatic Embryos from Nucellus of Mango (Mangifera indica L.),” Life Science Journal, Vol. 10, No. 2, 2013, pp. 1164-1174.
- A. S. Oluwaseun and A. B. Erhinmeyoma of Callus and Somatic Embryogenesis from Cotyledon Explants of Parkia biglobosa (Jacq ) Benth,” African Journal of Biotechnology, Vol. 4, No. 1, 2005, pp. 68-71.
- T. C. Siang, S. T. Soong, A. Yien and T. Su, “Plant Regeneration Studies of Jatropha curcas Using Induced Embryogenic Callus from Cotyledon Explants,” African Journal of Biotechnology, Vol. 11, No. 31, 2012, pp 8022-8031
- R. H. Smith, “Plant Tissue Culture Techniques and Experiments,” 2nd Edition, Academic Press, San Diego, 2000, 231 Pages.
- S. S. Bhojwani and M. K. Rozdan, “Plant Tissue Cultue: Theory and Practice: Development in Crop Science,” Elsevier, Amsterdam, 1983.
- M. N. Amin and V. S. Jaiswal, “In Vitro Response of Apical Bud Explants from Immature Trees of Jackfruit (Artocarpus heterophyllus),” Plant Cell, Tissue and Organ Culture, Vol. 33, 1993, pp. 59-65. http://dx.doi.org/10.1007/BF01997599
- V. S. Jaiswal and M. N. Amin, “In Vitro Shoot Proliferation and Plantlet Formation from Somatic Tissues of Guava (Psidium guajava L.),” 6th International Congress of Plant Tissue and Cell Culture, University of Minnesota, Minneapolis (Abstract), 1986, p. 279.
- B. M. Reed and P. Tanprasert, “Detection and Control of Bacterial Contaminants of Plant Tissue Cultures. A Review of Recent Literature,” Plant Tissue Culture and Biotechnology, Vol. 1, No. 3, 1995, pp. 137-142.
- S. M. Woo and H. Y. Wetzstein, “Morphological and Histological Evaluations of in Vitro Regeneration in Elliottia racemosa Leaf Explants Induced on Media with Thidiazuron,” Journal of the American Society for Horticultural Science, 2008 (in Press).
- R. Mohamed, P. L. Jong and M. S. Zali, “Fungal Diversity in Wounded Stems of Aquilaria Mala,” Fungal Diversity, Vol. 43, No. 1, 2010, pp. 67-74. http://dx.doi.org/10.1007/s13225-010-0039-z
- K. Welsh, K. C. Sink and H. Davidson, “Progress on in Vitro Propagation of Red Maple,” Combined Proceedings—International Plant Propagators’ Society, Vol. 29, 1979, pp. 382-386.
NOTES
*Corresponding author.

