American Journal of Plant Sciences
Vol.4 No.3(2013), Article ID:29148,20 pages DOI:10.4236/ajps.2013.43080
Review Article: High-Temperature Adult-Plant Resistance, Key for Sustainable Control of Stripe Rust
![]()
US Department of Agriculture, Agricultural Research Service, Wheat Genetics, Quality, Physiology, and Disease Research Unit and Department of Plant Pathology, Washington State University, Pullman, USA.
Email: xianming@wsu.edu
Received January 4th, 2013; revised February 5th, 2013; accepted February 20th, 2013
Keywords: Tricicum aestivum; Hordeum vulgare; Puccinia striiformis; Durable Resistance; Non-Race Specific Resistance
ABSTRACT
High-temperature adult-plant (HTAP) resistance expresses when plants grow old and the weather becomes warm. This non-race specific and durable type of resistance has been used successfully in control of wheat stripe rust in the US since early 1960s. This article describes practical procedures for identification and characterization of HTAP resistance and reviews recent studies on discovery of genes conferring HTAP resistance. Recent studies providing insights to the molecular basis for the durability of HTAP resistance will be presented. Strategies for improving levels of HTAP resistance and improving control of stripe rust through combining HTAP resistance with effective all-stage resistance will be discussed.
1. Introduction
Stripe rust, caused by Puccinia striiformis Westend. f. sp. tritici Erikss., is one of the most devastating diseases of wheat worldwide [1-3]. Stripe rust epidemic occurs more frequent in areas with mild climatic conditions during the wheat growing season. Because the pathogen requires relatively low temperatures for germination, infection, growth and survival, climatic conditions, especially temperatures, play an important role to the disease [1,2,4,5].
The effects of temperature on the pathogen and disease cycle are illustrated in Figure 1. Urediniospores are able to germinate between just above 0˚C and 20˚C, but the optimum temperatures for germination are from 7˚C to 12˚C. Sporulation can take place from just above 0˚C to 28˚C, but most rapidly between 12˚C to 20˚C, at which disease is also develops most rapidly. At both low and high-temperatures that are not in the optimal range but do not reach to the lethal temperatures, hyphae of the fungal pathogen may be in a dormancy state for survival. Under such conditions, the stripe rust pathogen does not die and is able to revive growth when temperatures change towards favorable for development of the disease [5,6].
Temperatures also affect the growth of plants. When temperatures are relatively high, wheat plants grow and mature relatively fast. Generally, the range of temperatures at which wheat plants can grow is wider than that for the stripe rust fungus, which allows wheat to grow under conditions unfavorable for stripe rust, especially in the late growing season. Because temperature affects both host plants and the stripe rust fungus, it can affect the interactions between the two organisms. In the long co-evolutionary course, the wheat host has developed certain traits that allow it to take the advantage of the relatively high temperatures in the late growth stage to win the battle against the pathogen infection. Such traits of resistance express mostly when plants grow old and the weather becomes warm. This plant resistance is similar to the tolerance of humans to the flu and cold viruses. Humans become more tolerance to these diseases when they grow up and also when the weather become warm.
Growing resistant cultivars is the best approach to control stripe rust. Various types of resistance to stripe rust have been observed and or reported. Line and associates [7-10] separated types of resistance into eight groups based on range of infection types on seedlings and adult-plants, race-specificity and pattern of rust development when tested at low and high temperatures in the greenhouse and in field plots. In addition to separation of resistance types based on phenotypic reactions, growth stages and temperature sensitivities, genetics has been used to characterize types of resistance. Table 1 summarizes the types of resistance based on various ways of separation. Many of these different terms characterize the same or similar types of resistance. Although
Table 1. Contrasting types of resistance based on various ways for separation.
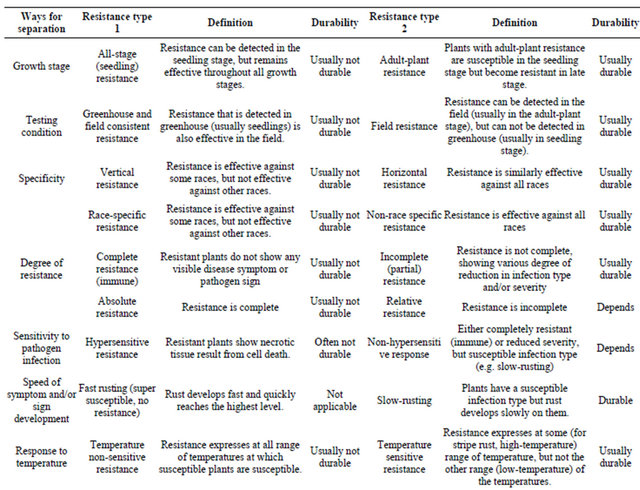
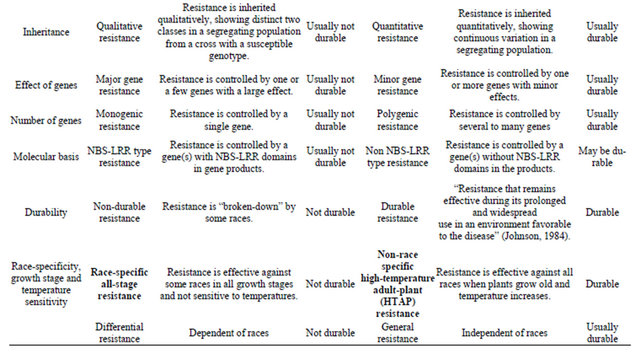
similar, two types may have differences. For example, high-temperature, adult-plant (HTAP) resistance, which will be mainly discussed in this review article, is temperature sensitive, but not all temperature-sensitive resistance is HTAP resistance because some resistances express in seedling stage at high temperature. Likewise, not all adult-plant resistances are of HTAP type, although most if not all, adult-plant resistance may be temperature sensitive.
As many cultivars with resistance controlled by a single gene have become susceptible to new virulent races developed in a pathogen population, which resulted in numerous devastating epidemics throughout the world, scientists has been seeking and utilizing durable types of resistance. Dr. Johnson’s famous definition of durable resistance as “resistance that remains effective during its prolonged and widespread use in an environment favorable to the disease” [11] is widely accepted and used as the only criterion for determining durability resistance. This definition is useful to prove durability and can be used to keep false durable type of resistance from consideration. The definition does not relate any type of resistance determined by other factors, which has been viewed by many people as a great wisdom because it can be always correct. However, the definition does not provide any value for predicting durability of resistance based on certain characteristics. Can we predict durability of a new source of resistance based on certain characteristics as listed in Table 1 without waiting for many years until the resistance is proven to be durable? With this question in mind, this short review article is to address the utilization of durable resistance to achieve sustainable control of stripe rust, using historical and current progresses in resistance research and disease control mainly in the US.
2. Durability of HTAP Resistance
Stripe rust has occurred in the US for more than 100 years [12]. From 1915 when the disease was first discovered to 1960 when the first devastating epidemic occurred in the US Pacific Northwest (PNW), there were no significant reports from North America on types of resistance, breeding and selecting for resistance, or genetics of resistance to stripe rust [6]. In the late 1950’s, about 90% of acreage was planted with “Omar”, which was resistant to common bunt caused by Tilletia tritici and T. laevis, a major disease in the PNW in that period [13], but susceptible to stripe rust, leading to the big stripe rust epidemics in 1960 and the following four years under the rust-favorable weather conditions. The devastating epidemics revived stripe rust research in the US, especially sparked breeding for stripe rust resistant cultivars.
Dr. Orville A. Vogel was the first to use partial resistance, which was later characterized as HTAP resistance, in breeding wheat cultivars for resistance to stripe rust. As Omar, which was developed by Dr. Vogel and released in 1955 [14] as a resistant cultivar to control common bunt, became predominant in early 1960s, cultivar “Brevor”, which was also developed by Dr. Vogel and released in 1949 [15], was grown in much smaller acreage. Because of the severe stripe rust in 1960, many wheat growers switched to Brevor, which had less rust and was not as severely damaged as Omar and other susceptible cultivars [6]. In 1960, some older cultivars showed similar resistance to that of Brevor. Dr. Vogel had been crossing Brevor and those cultivars with “Norin 10” to develop semidwarf cultivars, and thus had inadvertently crossed cultivars with moderate adult-plant resistance and selected progeny with moderate resistance to stripe rust. Under the severe epidemic in 1960, he purposely released cultivar “Gaines” developed from a complex cross involving Brevor and Norin 10 [16]. In 1965, he released “Nugaines” [17], a sister line of Gaines with a better level of partial resistance to stripe rust. In 1970, he and associates released “Luke”, which also has Brevor and Norin 10 in its pedigree, and have much better level of resistance to stripe rust [18,19]. As shown in Table 2, the HTAP resistance in these cultivars has remained unchanged (see data in [8]) since the cultivars were released almost 40 to 60 years ago, although the levels of HTAP resistance in Gaines and Nugaines are now considered inadequate. Nugaines and Gaines were major cultivars in the PNW from 1960s until 1981 [6] as cultivars with higher yielding and better HTAP resistance became predominant. Nugaines and Luke also showed HTAP resistance in China [20,21].
Since late 1970s, more and more wheat cultivars with high levels of HTAP resistance have been developed and
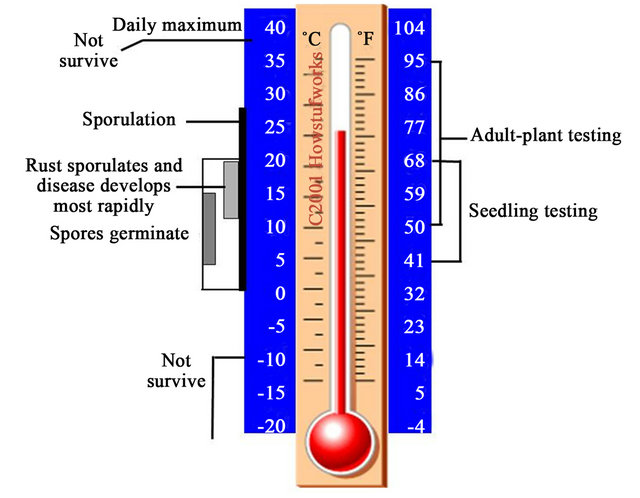
Figure 1. Ranges and optima of temperatures at which Puccinia striiformis germinates, sporulates and survives. The temperature ranges used for testing plants under lowand high-temperatures are shown at the right.
Table 2. Infection types (IT) and severities (%) of stripe rust on winter wheat cultivars developed by Vogel in experimental fields in Pullman and Mt. Vernon, Washington, USA in 2008.
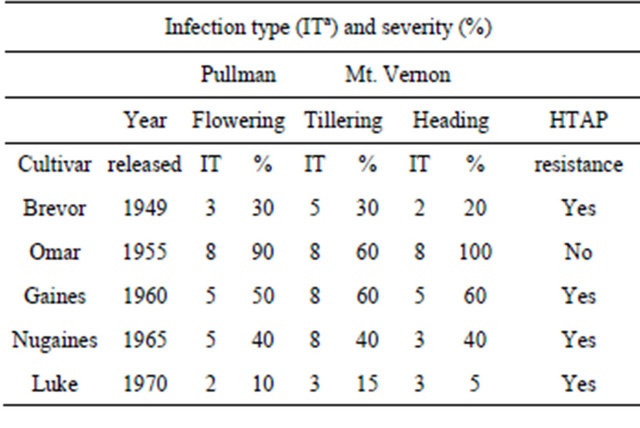
aInfection types based on the 0 - 9 scale [12] was used, in which 0 - 3 are considered resistant, 4 - 6 intermediate, and 7 - 9 susceptible.
grown in the US PNW. Stephens, which was released by the Oregon Agricultural Experiment Station and USDAARS in 1977 [22], is still one of the major cultivars grown in the PNW. The cultivar was developed from the cross “Nord Desprez”/“Pullman Selection 101”. “Pullman Selection 101” was developed by Dr. Vogel from Norin10/Brevor. Nord Desprez, released in France in 1945 [23], has always showed excellent HTAP resistance in our experiment plots. Thus, Stephens inherited HTAP resistance from Nord Desprez and/or Brevor. Cultivars “Madsen”, “Eltan”, and “Rod” have been widely grown for at least 20 years since their releases in 1988, 1990, and 1992, respectively (Figure 2). Their HTAP resistance is still effective. For the cultivars that are no longer in production, they still exhibit HTAP resistance in our field experiments year after year. Our data show that HTAP resistance from Brevor and other sources has lasted for more than 60 years. Thus, HTAP resistance has proven to be durable.
3. Determination of HTAP Resistance
The pre-condition for determination of HTAP resistance is the availability of races (pathotypes) of the stripe rust pathogen that are virulent on seedlings of the cultivar. Without a virulence race to the seedlings, whether a cultivar has HTAP resistance cannot be determined except to use reliable molecular markers. This is important for cultivars that may have combinations of effective allstage resistance and HTAP resistance. If a cultivar has only HTAP resistance, it should be susceptible to all races in the seedling stage. Nugaines is a good example of such cultivars. It is susceptible to all races of P. striiformis f. sp. tritici that has been identified so far in the US [2,12,24,25]. Plus its good plant type, we have, therefore, been using it in the greenhouse to increase urediniospores of stripe rust samples collected from various regions [25,26].
In contrast to the typical slow-rusting, in which infection type (IT) cannot be used as indication of resistance as ITs are usually high, IT can be used to indicate HTAP resistance. Plants with HTAP resistance typically show lower ITs than susceptible plants. Infection types of HTAP resistant plants often range from IT 2 to 7 in a 0 - 9 scale [12] with various necrotic stripes with or without uredia. Similar to slow-rusting, the pathogen produces much smaller amount of spores on HTAP-resistant plants than susceptible plants.
HTAP resistance can be determined in the field. However, only the areas, where stripe rust can infect and sporulate on seedlings and the temperatures are relatively low in the early crop growth stage, are suitable for detecting HTAP resistance. Under such conditions, plant reactions to stripe rust can be recorded before and after stem elongation stage. Comparison of the late data to the
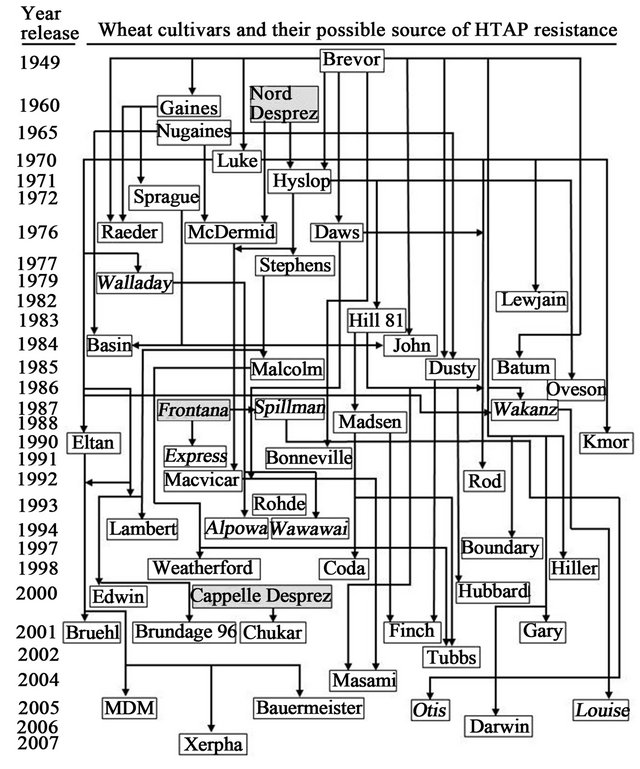
Figure 2. Possible sources of high-temperatures adult-plant (HTAP) resistance to stripe rust in wheat cultivars that were developed and grown in the US Pacific Northwest (PNW) since late 1940s. Cultivars in italic are spring and the others are winter wheat. Cultivars in the gray background were not grown in the PNW, but contributed HTAP resistance to some of the regionally developed cultivars. HTAP resistance in these cultivars has been tested for years in our field experiments and for the majority of them in tests under controlled greenhouse conditions. The sources of HTAP resistance are mostly based on their pedigrees and just a few of them were proved by genetic or molecular marker studies.
early data may indicate HTAP resistance. For example, Nugaines had IT 8 when stripe rust was recorded at the stem elongation stage, but had IT 3 on flag leaves when recorded at the heading stage at Mt. Vernon, WA, USA, where the mild winter and summer allows stripe rust occur almost all year long.
However, field data can be sometimes misleading. Let us assume that two races, one avirulent and one virulent, occur simultaneously in a field and the former produces IT 2 (necrotic stripes without uredia) and the later produces IT 9 (uredia without necrosis) on a cultivar. Plants of the cultivar will be likely to have ITs from 3 to 7, depending upon the relative frequencies of the two races and heavy or light infection. Such reactions appear like HTAP resistance, but in fact was the results of mixture infections by both virulent and avirulent races.
More clear data of HTAP resistance should be obtained from a 4-way testing of individual races under controlled greenhouse conditions, as shown in Figure 3. The 4-way testing includes seedling and adult-plant tests at the low and high temperature profiles. The seedling testing is to inoculate plants at the two-leaf stage and the adult-plant testing is to inoculate plants at the flagleaf fully expanded to soft dough stage. Line and associates [19,27-29] standardized temperatures conditions for testing for HTAP resistance. We use a low diurnal cycle of temperatures gradually changing from 4˚C to 20˚C and a high diurnal cycle from 10˚C to 35˚C for grown plants after inoculation. These temperature ranges were selected based on temperatures in the early and late growth seasons for cereal crops in the US PNW and reflect the big variation between the night and day time temperatures. As shown by Chen and Line [28], susceptible cultivar ‘Michigan Amber’ had IT 8 in all 4-way tests under these temperature conditions, while “Stephens” had IT 8 on seedlings under the high and low temperature profiles and on adult-plants under the low temperature profile, but had ITs 0 - 3 on adult plants under the high-temperature profile when tested with PST-25. Similar results were obtained for spring wheat cultivar “Alpowa” and breeding line WA3039HF when tested with race PST- 127 (Figure 3).
If it is difficult to set such wide temperature ranges, the ranges can be modified by increasing the low limit and reducing the high limit. For example, Carter et al. [30] used 28˚C for the day time and 15˚C for the night time to detect HTAP resistance. Similarly, diurnal temperature cycle gradually changing from 8˚C to 16˚C can be used for the low-temperature testing. For the high temperature testing, the upper limit of the temperatures should not be too high and too long as a long exposure to temperatures beyond 35˚C may kill rust hyphae in leaf tissue, but should allow plants to express resistance. Qayoum and Line [19] showed that plants expressing HTAP resistance at the high-temperature profile can become susceptible or less resistance if temperature changes to lower. The level of HTAP resistance tends to be higher when plants are grown at higher post-inoculated temperatures. High temperatures should allow susceptible plants to have a fully susceptible reaction. Therefore, it is essential to include a susceptible cultivar as a control for indicating a right range of temperatures for screening germplasm with HTAP resistance.
The low and high temperature ranges discussed above are for post inoculation. The temperature used for incubating inoculated plants under a high humidity for dew formation should be the same or similar for both low and high-temperature tests. We use 10˚C, which is within the optimum temperature range (7˚C to 12˚C), for inoculation.
The 4-way testing can be difficult for large-scale screening of germplasm for HTAP resistance. Practically, the problem can be solved by just conducting the seedling low-temperature and the adult-plant high-temperature tests. The 2-way testing would include high-temperature resistance, which also expresses in the seedling stage, but may eliminate plants with adult-plant resistance expressing only at low temperatures. The recently cloned Yr36 was classified as HTAP resistance based the 2-way testing [31], but was late identified more accurately as temperature-sensitive resistance using the 4-way testing [32]. Under field conditions, temperature-sensitive resistance mainly express in the adult-plant stage because temperatures are usually low in the early crop season, which does not allow high-temperature resistance to express.
4. Genetics of HTAP Resistance
Among plant diseases, wheat stripe rust was the first to be studied for genetics of resistance. The early genetic
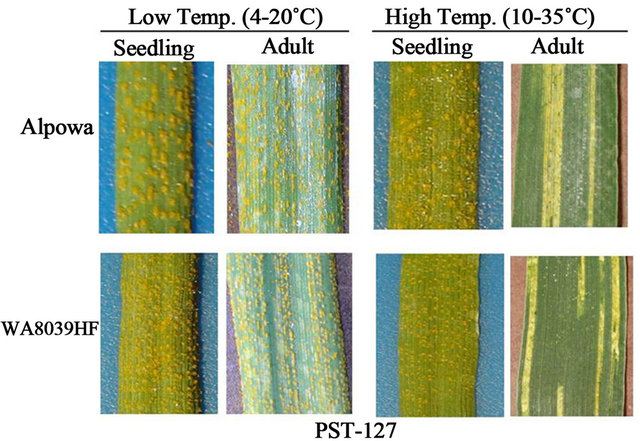
Figure 3. 4-way tests (seedling-low temperature, adult-low temperature, seedling-high temperature, and adult-high temperature) to detect high-temperature adult-plant (HTAP) resistance to stripe rust. Plants showing a resistant reaction only or mostly in the adult-plant stage at high-temperatures are identified to have HTAP resistance.
studies of wheat resistance to stripe rust were all on allstage resistance (also called “seedling resistance”) even the reaction evaluations were done on adult-plants in fields in many of the studies [33-38]. Allan and associates [39,40] in the US PNW were the first to notice that adult-plant resistance in some crosses observed in the field was controlled by different genes from or additional to those conferring resistance observed in the seedling stage. Because some loci conditioning adult-plant resistance in the field did not influence seedling resistance in the greenhouse, Allan et al. [40] pointed out the need for evaluation of reaction to stripe rust under both environments and stages of growth. Resistance in some of the wheat genotypes, such as Brevor and “C.I. 13431” (with Norin 10/Brevor in the pedigree) in these studies can be characterized as HTAP resistance, and the resistance detected in the field could be under influence of both temperature and growth stage.
Straib [41] studied temperature effects on resistance and discovered that resistance of the progeny was more often influenced by temperature (also see [38]). Sharp and associates [42-45] were the first to study inheritance of stripe rust resistance that are temperature sensitive. They identified three minor genes in wheat genotype ‘PI 178383’ and found them under influence of temperature. F3 lines from the cross of PI 178383 with susceptible ‘Lemhi’ with one, two, and three of the genes had IT 2 (necrosis with moderate sporulation), IT 0 (large necrosis without sporulation), and IT 00 (small necrosis without sporulation) at a 15˚C/24˚C (night/day) temperature cycle, but had IT 3 (susceptible reaction), IT 2, and IT 0 on a 0 - 4 scale, respectively at a 2˚C/18˚C (night/day) temperature cycle [42].
As HTAP resistance is often incomplete in degree, its inheritance often deviated from completely dominant or recessive. Milus and Line [27,46] studied inheritance of HTAP resistance in Gaines, Nugaines and Luke and found that resistance in the three cultivars was partially recessive. Chen and Line [28] found that HTAP resistances in wheat cultivars Stephens and “Druchamp” were partially recessive or partially dominant, depending upon the reciprocal crosses. They also found that the heterozygous plants were less resistant than either parent when Stephens was the female parent. HTAP resistance conditioned by Yr39 in spring wheat cultivar “Alpowa” was found to be partial recessive as more F3 lines were between the middle parent value and the susceptible parent [47]. The distribution of BC7:F3 lines from the “Avocet S” (AvS) x Compair indicated that the HTAP resistance in Compair and the AvSYr8NIL line is partially recessive [48]. Yan and Chen [49] determined that HTAP resistance in ‘Bancroft’ spring barley is a dominant trait, which is in contrast to the finding that genes for race-specific all-stage resistance in many barley genotypes are recessive [50,51]. The dominant inheritance of Bancroft HTAP resistance was determined with F1 and F2 progenies under the controlled greenhouse conditions using the IT data. However, when F5 RILs were tested in the field, the severity data showed continuous variation, the HTAP resistance was more like partially dominant [49]. Thus, HTAP resistance can be dominant or recessive, but in most cases partially dominant or partially recessive.
Transgressive segregation is often observed in genetic studies of HTAP or adult-plant resistance, especially when resistance is partial [30,40,46,47,52,53]. Such transgressive segregation is contributed by an additional gene(s) from the other parent in a cross considered as susceptible. It is also possible that the transgressive segregation can be attributed to disease scoring methods or errors. The usefulness of transgressive segregation of HTAP resistance is that it is possible to obtain progeny lines with higher level of resistance than the resistance donor even incorporate the resistance gene into a cultivar generally considered susceptible.
Scientists previously thought that horizontal resistance, which is often durable, is controlled by polygenes [54]. However, only in a few cases, adult-plant resistance in wheat against stripe rust was reported to be controlled by more than five QTL [55-57]. Most studies reported that HTAP or adult plant resistances are controlled by one to three QTL. A major QTL controlled HTAP resistance was reported in Alpowa (Yr39) [47], Compair and the AvSYr9NIL line [48], Louise [30] and PI 183527 (Yr52) [58] wheat genotypes and Bancroft barley [49]. TwoQTL controlled HTAP resistance was reported in wheat cultivars Stephens [53], Luke [21], PI 610750 (including Yr48), UC1110 [59] and Rio Blanco [56]. Three-QTL controlled HTAP resistance was reported in wheat cultivar Express [52]. In addition, the recently cloned Yr36, a single-gene or QTL conditioning HTAP resistance was reported in wheat genotypes transgressed from Triticum dicoccoides [31,32]. Similarly, the Yr18 conditioning stripe rust resistance we considered as HTAP resistance was also recently cloned as the same gene as Lr34 for durable resistance to leaf rust [60]. In addition, adultplant resistance to stripe rust controlled by a single gene or QTL was reported in wheat genotypes “Bersee” as Yr16 [61], in “Pavon F76” as Yr29 [62], in wheat genotype “Lgst. 79 - 74” as Yrns-B1 [63], in “Opata 85” as Yr30 [64], in “Cook” [65], in a T. boeoticum transgressed wheat genotype as QYrtb.pau-5A and a T. monococcum transgressed wheat line as QYrtm.pau-2A [66], and in wheat genotype “Aquileja” [21]; and by three genes or QTL was reported in “Guardian” [67]. In winter wheat “IDO444”, HTAP resistance is controlled by two major QTL and three minor QTL [56]. It should be noted that not all of the mentioned QTL contribute to HTAP or adult-plant resistance as many of the genotypes also have genes conferring all-stage resistance. Because of the effect of different race frequencies in the pathogen populations as mentioned above and frequency changes during a crop season, a race-specific resistance gene may appear to contribute to the quantitative “adult-plant resistance” in the field experiments.
So far, there is no evidence that HTAP resistance controlled by a single gene is less durable than that controlled by more genes. This is because that there is no evidence to show any HTAP resistance gene is no longer effective. Thus, the durability of HTAP resistance could be independent of the number of genes conditioning the resistance. For example, both HTAP resistances in ‘Alpowa’ and ‘Express’ are still effective, even though they have a single gene (Yr39) [47] and three genes (QYrex. wgp-6AS, QYrex.wgp-3BL, and QYrex.wgp-1BL) [52] and have been grown since their releases in 1994 and 1991, respectively. In fact, “Alpowa” has been more widely grown than “Express”. If single gene controlled HTAP resistance is equally durable to more genes controlled HTAP resistance, there is much advantage to choose individual genes conferring higher levels of resistance because of its relative easiness to be used in breeding program. With this thinking in mind, our program put more effort on identification of QTL with major or large effects [30,47,48,49,52,53,58]. However, it should be better to use diverse HTAP resistance genes, either deployed in different cultivars or pyramided in single cultivars. For the genes or QTL identified in our program and those we tested in our greenhouse and/or field experiments, we have found that QYrlo.wgp-2BS in Louise and the HTAP QTL in the AvSYr8NIL line provide a higher level of resistance than Yr39 in Alpowa. Yr39 is better than Yr36; Yr36 is better than Yr18; and Yr18 is better than Yr29. Yr39 alone provide about the similar level of resistance as the three QTLs from Express together. This information allows us to selected HTAP resistance genes either used individually or in combination to develop cultivars with adequate levels, if not complete, of resistance.
5. Molecular Mapping of Genes for HTAP Resistance and Marker-Assisted Selection
Molecular mapping has been an efficient approach for identification of not only genes for race-specific all-stage resistance to stripe rust, but also for genes or QTL for non-race specific HTAP resistance. We have used resistance gene analog polymorphism (RGAP) [68,69] as the major marker technique to identify markers linked to a resistance locus. If identified RGAP markers or allelic markers are present in wheat genotype “Chinese Spring” (CS), the set of CS nulli-tetrasomic lines and ditellosomic lines can be used to localize the markers and the genes to wheat chromosomes and chromosomal arms [47,69]. Then simple sequence repeat (SSR) markers specific to the chromosome and chromosomal arms are screened to find markers polymorphic between the parents of the mapping population and the SSR markers will confirm the chromosome and serve as additional markers to map the resistance gene to a chromosomal region. More recently, single nucleotide polymorphism (SNP) markers have been used in mapping genes for stripe rust resistance [70]. The integrated approach allowed us having mapped numerous genes or QTL for HTAP resistance, as well as genes for all-stage resistance [47,58, 69-76].
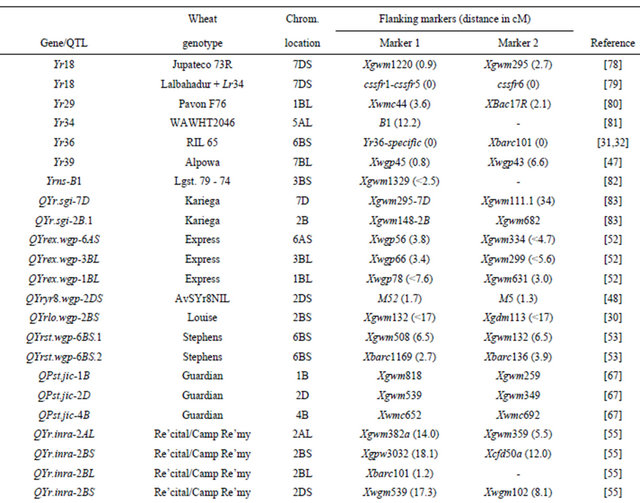
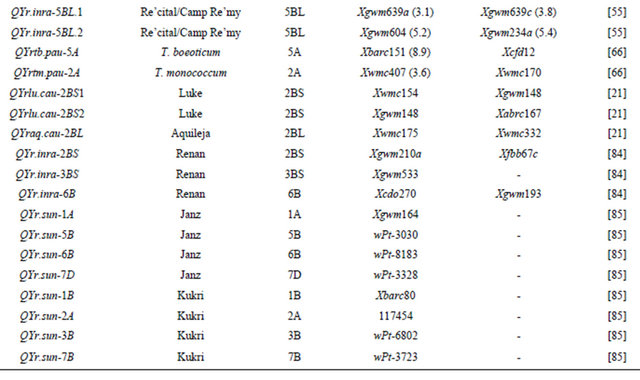
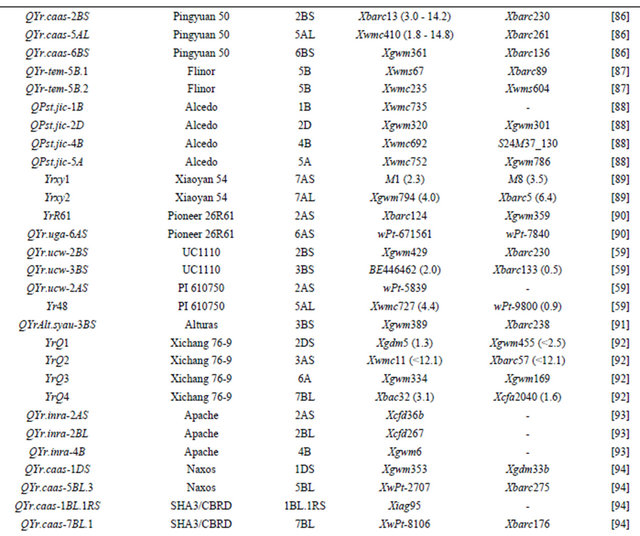
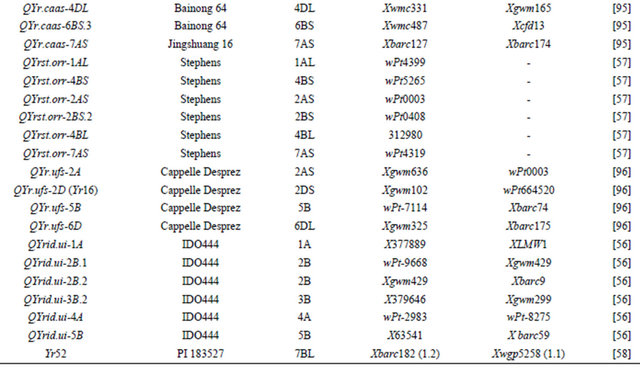
Table 3 lists chromosomal locations and linked markers for genes or QTL conferring HTAP or HTAP-alike resistance (resistance can be detected in adult plants under high temperatures, such as temperature sensitive and slow-rusting) to stripe rust in wheat. A total of 88 genes or QTL conferring various levels of resistance have been mapped to all 21 chromosomes except 3D and 5D and 24 chromosomal arms. Of the 88 genes, 57 are mapped to chromosomal arms and 31 only to chromosomes. The B genome carries 51 genes, the A genome carries 25 and the D genome carries 12 genes reported from various studies. The genes mapped to the same chromosomal arm have possibility to be the same gene. For example, QYrlo.wgp-2BS in spring wheat Louise and one of the QTL in winter wheat Luke may be the same gene as both cultivars have Brevor in their pedigree (Figure 2) and share the SSR marker Xgwm148 in their linkage groups [21,30]. The possibility that QYrex.wgp-1BL in Express and Yr29 in “Pavon F76” are the same gene could not be ruled out [52]. Similarly, the two or more genes on other chromosomal arms can be either the same or different. In contrast, the two QTL in “Stephens” and Yr36 on 6BS are at different loci as determined by a diallel cross and different map distances [53]. Nevertheless, most of the genes or QTL listed in Table 3 are different from each other based on their different chromosomal locations. Durability of most of the resistances conferred by these genes has been demonstrated. These genes are great genetic resources for developing cultivars with durable resistance to stripe rust. The markers identified to be linked to these genes (Table 3) could be useful in marker assisted selection. However, the markers should be tested for polymorphism and reliability in particular parental lines before they can be used in the breeding populations. In general, the markers more closely linked to the resistance loci will be more reliable for marker-assisted selection. The markers for Yr18 and Yr36 are perfect as they were developed from the cloned resistance genes [32,60].
Molecular markers can be more valuable for assistance to develop cultivars with HTAP resistance than with all-stage resistance because screening for HTAP resistance needs to be done in adult-plant stages. In the US
Table 3. Genes or quantitative trait loci conditioning HTAP resistance, their chromosomal locations, and flanking molecular markers in wheat.
Continued
four genotype laboratories have been established at Raleigh, North Carolina; Manhattan, Kansas; Fargo, North Dakota; and Pullman, Washington to support cereal breeding programs using molecular markers for various important traits including resistance to stripe rust. Among genes for the Wheat CAP (coordinated agricultural projects) using marker-assisted selection, Yr18/Lr34, Yr29/ Lr46 and Yr36 for adult-plant (HTAP) resistance to stripe/leaf rust have been commonly used [77]. The linkage of Yr36 to Gpc-B1, a high protein gene, makes these genes very useful for both stripe rust resistance and better quality. The markers tightly linked to these genes have been intensively used to develop improved wheat germplasms and cultivars [97,98]. Several cultivars have been released, of which “Farnum” (PI 638535), a hard red winter wheat, released by Washington State University and the USDA-ARS in 2008 is a good example. The cultivar was developed by selecting the stripe rust resistance and high protein with the flanking markers Xgwm508 and Xgwm644 [99]. The releases of “Farnum” and “Bauermeister” (PI 634717), which was released in 2005 with HTAP resistance from soft white winter wheats [100] and has become a major hard red winter wheat cultivar in the state of Washington, have changed the previous situation that hard red winter wheat cultivars as a group were highly susceptible to stripe rust in the PNW. Breeding programs have started using markers for the HTAP resistance QTL identified in Alpowa, Express, Stephens and Louise. Similarly, barley breeding programs have been using markers for the HTAP QTL in Bancroft [49] and those developed in Oregon State University for quantitative resistance to stripe rust on barley chromosomes 3H and 5H [101,102], presumably HTAP resistance.
Molecular markers are even more useful for pyramiding HTAP QTL to achieve high levels of resistance. HTAP resistance in many cultivars protects the crop from major yield losses in comparison with susceptible cultivars. However, these cultivars may suffer considerable yield losses compared to completely resistant cultivars under severe epidemic conditions. For example, spring wheat cultivars Alpowa and Express can have up to 15% yield losses. As shown in Table 4, Alpowa had a 22.64% relative area under the disease progress curve (rAUDPC) value and 12.33% yield loss, Express had a 27.17% rAUDPC value and 13.02% yield loss in 2004 when stripe rust was severe, which were much lower than the 100% rAUDPC and 47.80% yield loss of the susceptible check ‘Lemhi’. Similarly, the yield losses of Alpowa and Express were 10.52% and 7.50% in 2008, a relatively low stripe rust season, which also were much low than the 29.75% in the non-fungicide treated Lemhi plots. In both 2004 and 2008, races detected from the region were virulent on seedlings of Express [25,103] and seedlings of Alpowa are susceptible to all races except the least virulent races PST-21 and PST-1 [47]. Thus, resistance of Alpowa and Express exhibited in the fields were contributed only by their HTAP resistance. Even though the level of HTAP resistance in both Alpowa and Express is adequate when stripe rust is relatively low such as in 2008 when commercial fields did not have stripe rust without fungicide application based on our disease surveys, they can have substantial yield losses as shown in Table 4.
To develop wheat genotypes with improved level of HTAP resistance, we developed F8 recombinant inbred lines (RILs) from the Alpowa x Express cross and selected lines with two to four QTL, Yr39 from Alpowa and QYrex.wgp-6AS and QYrex.wgp-3BL from Express, using flanking markers for these genes. As shown in Table 4, the four RILs almost had no any obvious infection in both non-fungicide sprayed and fungicide sprayed plots. The yield differences between sprayed and nonsprayed were not significant. All of the four RILs have the three above genes except for AlpExp44 that has Yr39 and QYrex.wgp-6AS, the strongest QTL in Express [52]. One of the RILs, AlpExp53, had grain yield not significantly different from that of either Alpowa or Express. Under more severe epidemics, the RILs with a complete level of HTAP resistance should produce more grains than Alpowa and Express. This study demonstrates that a complete level of HTAP resistance can be achieved by combining different genes for partial levels of HTAP resistance.
6. Breeding for Cultivars with HTAP Resistance
HTAP resistance, not just resistance, to stripe rust has been one of the top priorities for wheat breeding programs in the US PNW, and has become accepted in other regions. As discussed above, the non-race specific nature makes this type of resistance preferred over race-specific all-stage resistance as virulence and virulence combinations change rapidly in the rust population and new race migration. If resources and technologies allow only one choice from HTAP or all-stage resistance, my suggestion is to select HTAP resistance first and fix it into adapted cultivars because this process can be accomplished without sophisticated technology such as marker-assisted selection and any breeding program in stripe rust epidemic regions can conduct the selection. It is much easier to select HTAP resistance in regions where stripe rust often infects seedlings. In such regions, lines showing susceptibility should be selected at the seedling stage and these selected lines can be further screened for resistant lines in the adult-plant stages. This process will almost guarantee the select lines with HTAP resistance.
Many HTAP resistant and also slow-rusting cultivars
Table 4. Relative area under the disease progress curve (rAUDPC) values of stripe rust severity on fungicide applied (FA) and non-fungicide applied (NFA) plots of HTAP resistant spring wheat cultivars Alpowa, Express, selected F8 lines from the Alpowa/Express cross with increased level of HTAP resistance, and susceptible cultivar Lemhi near Pullman, Washington, USA in 2004 and 2008.
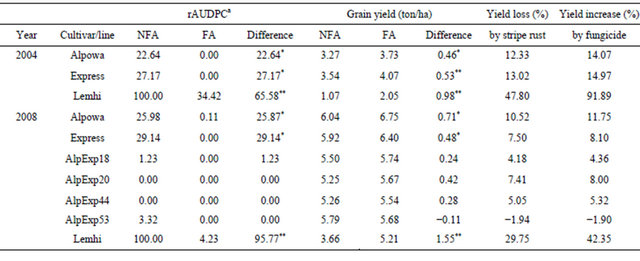 aArea under the disease progress curve (AUDPC) data were calculated from severities (percentage of diseased leaf areas, including mostly necrotic stripes with limited uredia for Alpowa, Exprese and their F8 recombinant inbred lines) using the formula AUDPC = ∑[rust severity (i) + rust severity (i + 1)]/2*days; and sporulated leaf areas for the “Lemhi” susceptible check. The relative AUDPC values were calculated by treating the mean AUDPC value of non-fungicide-treated “Lemhi” as 100 for each year. The experiments were a randomized split-block design with four replications. The plot sizes ranged from 5.75 - 7.03 m2 in 2004 and 7.20 - 8.32 m2 in 2008; and each plot was individually measured for calculating yield. The 2004 plots were planted on 22nd April and harvested on 15th August; and the 2008 plots were planted on 28th April and harvested on 3rd September when grain was naturally dry. Fungicide Tilt was used at 4 fl oz/A mixed with crop oil concentrate (COC) at 1% v/v. In 2004, the plots were spayed on 18 June at early boot stage when “Lemhi” had 5% - 10% rust severities and severity notes were recorded for each plot on 21 June (boot stage), 3rd July (early flowering), and 15th July (milk) when stripe rust developed to 20%, 90%, and 100% severity on non-treated “Lemhi”. In 2008, the plots were sprayed on 1st July (late boot to early heading) when Lemhi had 1% - 5% rust severity and severity notes were recorded for each plot 3rd July (early heading), 10th July (early flowering), 16th July (late flowering), 21st July (milk), and 30th July (soft dough). Compared to 2004, stripe rust developed relative late and the epidemic was relatively low in 2008. The data shown in this table were subtracted from experiments consisting of 16 cultivars/lines in each year. *= significant at 0.05 > P > 0.01 and **= significant at P < 0.01 in t tests.
aArea under the disease progress curve (AUDPC) data were calculated from severities (percentage of diseased leaf areas, including mostly necrotic stripes with limited uredia for Alpowa, Exprese and their F8 recombinant inbred lines) using the formula AUDPC = ∑[rust severity (i) + rust severity (i + 1)]/2*days; and sporulated leaf areas for the “Lemhi” susceptible check. The relative AUDPC values were calculated by treating the mean AUDPC value of non-fungicide-treated “Lemhi” as 100 for each year. The experiments were a randomized split-block design with four replications. The plot sizes ranged from 5.75 - 7.03 m2 in 2004 and 7.20 - 8.32 m2 in 2008; and each plot was individually measured for calculating yield. The 2004 plots were planted on 22nd April and harvested on 15th August; and the 2008 plots were planted on 28th April and harvested on 3rd September when grain was naturally dry. Fungicide Tilt was used at 4 fl oz/A mixed with crop oil concentrate (COC) at 1% v/v. In 2004, the plots were spayed on 18 June at early boot stage when “Lemhi” had 5% - 10% rust severities and severity notes were recorded for each plot on 21 June (boot stage), 3rd July (early flowering), and 15th July (milk) when stripe rust developed to 20%, 90%, and 100% severity on non-treated “Lemhi”. In 2008, the plots were sprayed on 1st July (late boot to early heading) when Lemhi had 1% - 5% rust severity and severity notes were recorded for each plot 3rd July (early heading), 10th July (early flowering), 16th July (late flowering), 21st July (milk), and 30th July (soft dough). Compared to 2004, stripe rust developed relative late and the epidemic was relatively low in 2008. The data shown in this table were subtracted from experiments consisting of 16 cultivars/lines in each year. *= significant at 0.05 > P > 0.01 and **= significant at P < 0.01 in t tests.
and resources may not have an adequate level of resistance as discussed above. Also, a certain level of resistance is adequate in one region may not be good enough in another region because of the sensitivity of HTAP resistance to environment and various growing seasons in different regions. Pyramiding genes or QTL from different sources, as demonstrated using Alpowa and Express, can lead to higher levels of durable resistance. This goal can be achieved just by phenotypic selection, although marker-assisted selection can speed up the selection process if useful markers are available.
The second approach to achieve high level of durable resistance is to combine genes for HTAP resistance with genes for effective all-stage resistance. This is an ideal approach as cultivars with both types of resistance will be completely protected from disease damage when the all-stage resistance is still effective and will remain resistant, although at a reduced level, after the all-stage resistance becomes ineffective to new races. Markerassisted selection will be more useful for this approach. Markers for numerous HTAP resistance genes (Table 3) and all-stage resistance [2,74,75] are available with various degrees of usefulness. In the US, many wheat breeding programs combining Yr5, Yr15, Yr26 and other genes for effective all-stage resistance with HTAP resistance genes such as Yr18, Yr29, Yr36, Yr39, and other QTL in Stephens, Eltan, Madsen, Jagger and Louise using and without using molecular markers. If marker technologies are not available, the combination approach can still be used. However, introgression which type of resistance first is critical. Because effective all-stage resistance masks HTAP resistance, HTAP resistance cannot be detected if effective all-stage resistance gene is already in the lines. Thus, it is better to incorporate HTAP resistance genes first into susceptible adapted lines and then incorporate an all-stage resistance gene or genes into the selected HTAP lines through backcrossing. As shown by Lin and Chen [52] using molecular markers, “Expresso” which was developed from Express by West Plant Breeders keeps the three HTAP resistance QTL from Express. The new cultivar that has Yr15, which is effective against all races identified so far in the US [25,103], has been completely resistant in all field and greenhouse tests.
The purpose of this review is to promote more widely use of HTAP resistance for sustainable control of stripe rust as it has been demonstrated to be durable, effective and also workable for breeding programs. However, all-stage resistance has played an important role in reducing frequency of stripe rust epidemics in the last century throughout the world, and particular genes for allstage resistance should be last longer if used by proper strategies such as pyramiding, cultivar mixtures and multiline cultivars. These approaches have been using in the PNW and some of them are also used in other regions of the world for control of stripe rust and other diseases. One of the most successful examples is the multiline cultivar “Rely” purposely developed for stabilizing control of stripe rust on club wheat in the PNW [104]. The overall resistance in this cultivar of 10 individual components with more than 10 stripe rust resistance genes has remained effective in commercial fields and our field nurseries since it was released in 1991. However, HTAP resistance has been most effective in stripe rust control in the PNW.
7. Epidemiological Mechanisms of HTAP Resistance
Except for wheat cultivars or lines purposely developed for a complete level of resistance as shown above, so far there is no report that complete resistance in a wheat germplasm is naturally conditioned only by genes conferring HTAP, slow-rusting or other incomplete types of resistance. Because HTAP resistance is often partial as exampled by Alpowa and Express, the disease on wheat plants, even in a much lower level than susceptible plants, tends to keep breeders from selecting these lines for releasing as new cultivars even in the current era, in which partial but durable resistance is much more accepted than over 40 years ago when Dr. Vogel was releasing Gaines and Nugaines. We can still hear people talking about preference of completely clean (completely resistant) plants. People have good reasons for worrying about disease damage as many HTAP resistant or slow-rusting cultivars can suffer as discussed above. However, we should think about such issue from an epidemiological viewpoint, not just a particular crop growth season when the disease is severe and substantial acreage of susceptible cultivars providing huge amount of spores which may cause damage to partial HTAP resistant cultivars.
There is no doubt that plants with HTAP resistance provide much fewer spores than susceptible plants, and therefore, reduce the inoculum pressure. Such reduction is more dramatic after several infection cycles as illustrated in Figure 4. As shown in Table 4, Alpowa has about 23% - 26% of severity, but produce much less than 10% of spores on adult plants under high temperatures (Figures 3 and 5) and in fields as relative to the 100% of susceptible Lemhi. From the model of inoculum decline in Figure 4, the percentage of spore production by a cultivar, which produces as much as 10% spores as a susceptible cultivar can produce, will decrease to close to 0% after 4 to 5 infection cycles compared to the 100% of a susceptible cultivar. In field conditions, most of initial and subsequent infections in fields grown with HTAP resistant cultivars, such as Alpowa, are caused by spores from outside of the field.
From 2002 to 2005, stripe rust infection producing necrotic stripes was very common in Alpowa fields, and fungicide application is commonly used to reduce yield losses. However, from 2006 to 2008, infections in Alpowa fields were observed, but too low to warrant fungicide application. Actually, almost no fungicide application was used in Alpowa fields in the three years. Although the difference can be attributed to the relatively unfavorable conditions in the later three years compared to 2002-2005 (the weather conditions were extremely favorable for stripe rust epidemic in 2005 [24,25]), it can be, at least partially, attributed to the reduction of stripe rust inoculum by reducing susceptible wheat acreage and early application of fungicide in the area of inoculum sources. The rust inoculum in the spring of 2002 was mainly from fields grown with spring wheat cultivars, such as “Zak” and “Jubilee” and other susceptible cultivars, that became susceptible to the group of races first identified in 2000 in California and the south-central states [2] and spread to the PNW in 2002 [105]. Such susceptible spring wheat cultivars were gradually withdrew from production in the following years.

Figure 4. Theoretical demonstration of inoculum decline (reducing spores) by growing cultivars with various levels of HTAP resistance measured by sporulation potential as percent of sporulation on susceptible cultivars (as 100%).

Figure 5. Leaves of spring wheat cultivar Alpowa in fields showing infection type 2 - 3 at the 0 - 9 scale, necrotic stripes without (IT 2) and with limited number of uredia (IT 3).
If susceptible cultivars are not grown in a region so large that inoculum from outside of the region alone does not contribute much to an epidemic. We can imagine such effect of inoculum decline in reducing epidemic potential from one growth season to another growth season in a region like the PNW, where such good cycles of stripe rust control is basically achieved by growing cultivars with HTAP resistance, except few cases that susceptible cultivars caused problems in recent years. If such level of resistance is achieved throughout the country, the continent and the world, stripe rust should be under control and such control should be sustainable.
8. Molecular Mechanisms of HTAP Resistance
Studies on molecular mechanisms of stripe rust resistance were attempted as early as in 1960s [106]. However, a better understanding of molecular basis for stripe rust resistance was not possible until the recent cloning of resistance genes and transcriptomics profiling studies. This is also true for resistance to many other diseases.
So far, three genes for resistance to wheat stripe rust have been cloned. Yr10 is a NBS-LRR (nucleotide binding site—leucine rich repeats) type gene [107]. The gene is effective in many countries. However, races virulent to Yr10 have been identified in the US since 1972 [12] as Yr10 was widely used in wheat breeding programs in 1960s to 1980s. Recently identified races, such as PST- 114, with virulence to Yr10 have been predominant in the PNW in the past several years [25,103].
In contrast, both Yr18 and Yr36, which are considered as HTAP resistance, do not have an LRR in these gene’s protein products. Yr18, the same gene as Lr34 for resistance to leaf rust and Pm38 for resistance to powdery mildew, encodes a putative ATP-binding cassette (ABC) transporter [60]. Yr18 has a DNA sequence of 11,805 bp, 24 exons in its full-length cDNA sequence, and a predicted 1401 amino acid protein that belongs to the pleotropic drug resistance subfamily of ABC transporters. The gene protein product has two cytosolic nucleotide binding domains (NBD) and two hydrophobic transmembrane domains. The pleotropic gene also shows leaf tip necrosis. The expression level of the gene was very low in 14-day-old seedlings grown at 20˚C, but clearly higher in flag leaves of adult plants before (53-day-old plants) and after the development (63-day-old plants) of leaf tip necrosis. The authors postulated that the Yr18/ Lr34/Pm38 resistance is the result of senescence-like process or the gene product may play a more direct role in resistance by exporting metabolites that affect fungal growth.
The product of Yr36 includes a predicted kinase and a steroidogenic acute regulatory protein-related lipid transfer (START) domain. Both the kinase and START domains are necessary for the resistance function. Temperature up-regulates expression of the resistance alleles, but down-regulates the susceptible alleles [32]. Stripe rust inoculation up-regulated expression of the resistance allele at high temperatures, but consistently down-regulated the susceptible allele. The authors postulated that the START domain has the ability to bind lipids from stripe rust fungus at high temperature and change its conformation, which may cause the kinase domain to initiate a signaling cascade leading to the observed programmed cell death. The combination of the kinase and START domains is unique because they are not found together in other organisms.
Yr10, Yr18, Yr36 and other identified resistance genes are all regulatory genes that control expression of other genes leading to resistance reactions. Therefore, comparisons of how many genes and what functions of genes regulated by HTAP verse all-stage resistance genes may lead to the understanding of their resistance mechanisms, which may provide answers to the question why HTAP resistance is durable and all-stage resistance not durable. Hulbert et al. [108] used the Affymetrix GeneChip to study gene expression patterns in near-isogenic lines for Lr34/Yr18. They identified 57 transcripts that were upregulated in mock-inoculated leaf tips of resistant plants. These genes with functions generally associated with ABA inducibility, osmotic stress, cold stress and/or seed maturation. Five transcripts were also up-regulated in resistant leaf bases. These patterns of gene expression of resistant isolines with Lr34/Yr18 showed a characteristic abiotic stress expression signature. In leaf rust inoculated plants, a set of 59 pathogenesis-related (PR) proteins, such as chitinases, glucanases and thaumatin-like proteins, was up-regulated in both resistant and susceptible flag leaves. However, resistant plants showed higher levels of express than susceptible plants. The same set of PR proteins was not up-regulated in resistant flag leaves compared to that in susceptible flag leaves. The rust infection down-regulated two genes, one homologous to cysteine proteinases and one predicted to code for ACC oxidase.
We conducted a series of studies to determine transcripts regulated by genes for all-stage and those for HTAP resistance and compare their different expression transcripts to determine the mechanisms of different types of resistance [109-113]. Through comparison of transcripts regulated by Yr5 for race-specific all-stage resistance verses Yr39 for HTAP resistance, we found the following differences between the two types of resistance conferred by these two genes: 1) Expression of genes regulated by Yr39 is not as fast as and is not as dramatic as that of those regulated by Yr5. In Yr5 plants, expression of the genes was significantly increased 24 hours after stripe rust inoculation, while expression of the genes in Yr39 plants was not increased until 48 hours. Such relatively slow and small increase of the gene expression may explain the partial level of HTAP resistance; 2) Yr39 regulates more genes than Yr5. A total of 99 genes specifically expressed in plants expressing HTAP resistance after stripe rust inoculation, while only 61 specifically expressed in plants expressing the Yr5 resistance (Figure 6); 3) Genes regulated by Yr39 have more diverse functions than those by Yr5 [110]. More hyposensitive response protein and PR protein genes were identified for Yr5, while more genes involved in phenylpropanoid and miscellaneous (pleotropic drug resistance/ABC transporter, putative disease resistance protein, and latex protein allergen) defense, transport and signal transduction. More interestingly, nine R (resistance) gene-like transcripts, including a homologue of the Yr10 all-stage stripe rust resistance protein, a Cf2/Cf5 disease resistance protein homologue and three protein kinase transcripts with similarity to the barley stem rust resistance protein Rpg1. The involvement of these R genes in the Yr39 HTAP resistance may be related to the necrotic stripe phenotype, resembling a hypersensitive response but large in dead tissue size, of Yr39 plants after infection (Figure 5). The results may indicate that Yr39 regulates these R genes that may induce expression of many other genes involved in resistance in a cascade manner. Because so many R genes involved in the Yr39 controlled HTAP resistance, they collectively provide resistance to all different races. Such hypothesis should be tested in future studies.
To determine if the regulated genes identified for Yr5 and Yr39 are common for different genes controlled racespecific all-stage resistance and non-race specific HTAP
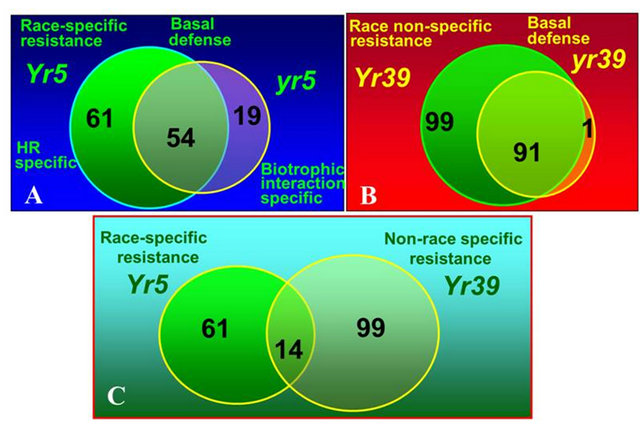
Figure 6. Numbers of unique transcripts significantly regulated for different interactions in wheat near-isogenic lines with the Yr5 for race-specific all-stage resistance and Yr39 for non-race specific high-temperature adult-plant (HTAP) resistance. (A) Comparison of genes up-regulated in Yr5 plants and yr5 plants; (B) Comparison of genes up-regulated in Yr39 plants and yr39 plants; (C) Comparison of genes up-regulated in Yr5 plants and Yr39 plants.
resistance, respectively, we constructed a custom oligonucleotide GeneChip containing 343 unique genes identified for the Yr5, Yr39 and Yr18/Lr34 resistances from the previous studies [108,110,111]. Eight race-specific resistance genes, Yr1, Yr5, Yr7, Yr8, Yr9, Yr10, Yr15 and Yr17 in “Avocet Susceptible” near-isogenic lines were used to study genes commonly and uniquely involving in all-stage resistance. We identified 28 transcripts as significantly involved in the race-specific resistance phenotype across all single gene lines. Unique defense-related transcripts significant in each genotype were also identified, which highlighted some transcriptional events specific to certain genotypes. These transcripts revealed key gene expression events and defense pathways involved in race-specific resistance. The results confirm the activity of known R-gene mediated pathways in the race-specific resistance response, including an oxidative burst that likely contributes to a hypersensitive response, as well as PR protein expression and activity of the phenylpropanoid pathway. To identify common and unique genes regulated by different HTAP resistance genes, transcripts of Yr18, Yr29, Yr36 and Yr39 single gene lines were used in microarray hybridizations with the custom oligonucleotide GeneChip. In contrast to 28 genes shared by different race-specific all-stage resistance, only one gene was found in common to the four HTAP resistant genotypes. This gene is predicted as a nonclathrin coat protein with a putative function in transport. Nonclathrin coat protein is able to bind to the cytoplasmic dilysine motifs of membrane proteins of the early secretory pathway [114]. Expression of this gene in other HTAP resistant wheat genotypes and its function need to be further studied.
Using real-time polymerase chain reaction (RT-PCR), Moldenhauer et al. [115] detected greater transcript accumulation for PR-1, PR2 (glucanase), PR-5 (Thaumatin) and PR-9 (peroxidase) in stripe rust-infected flag leaves of the necrosis-exhibiting line than in leaves of the non-necrotic line developed from adult-plant resistant “Kariega”. In contrast, no expression of PR proteins was seen in any of the mock-inoculated control plants. The findings are generally in agreement with our results discussed above. However, they could not determine transcription changes for many other genes, because their study was limited to a few PR protein genes.
Using the cDNA-AFLP technique, Mallard et al. [116] studied gene expression in adult-plant resistant cultivar “Camp Remy”. They detected transcripts differentially expressed in response to stripe rust infection. Sequencing analysis revealed genes involved in resistance/defense responses, transcription and signal transduction, and primary metabolism. Using RT-PCR, they confirmed the stage-specific expression of the genes at one or two specific stages in response to stripe rust infection and demonstrated that the cultivar modifies the expression in some resistance/defense-related genes during its transition from the seedling to adult-plant growth stages.
Using a suppressive subtractive hybridization approach, Huang et al. [117] identified 89 genes involved in defense and signal transduction during the adult-plant resistance response in wheat cultivar “Xingzi 9104” inoculated stripe rust urediniospores. The large number of genes was comparable to those identified for Yr18 and Yr39 HTAP resistance genes [106,108] as discussed above. The authors selected 12 of the genes for timecourse expression analyses using quantitative RT-PCR and found that 9 of those genes were induced by stripe rust infection, including those predicted to encode peroxidase, BAX inhibitor, Concanavalin A-like lectin/glucanase, subtilisin-chymotrypsin inhibitor, chinase, glucan endo-1,3-beta-D-glucosidase and acidic PR5 precursor. Some PR protein genes kept high expression levels longer in the adult plants than in seedlings after infection.
Based on evidence of molecular studies with wheat stripe rust and other diseases, we may generalize that race-specific all-stage resistance is controlled by typical R genes of the NBS-LRR type and these genes regulate a relatively small number of genes involved in relatively narrow based defense mechanisms. In contrast, non-race specific HTAP resistance is controlled by non-NBS-LRR genes, especially without LRR domains as such domains are thought to be involved in specific recognition of pathogen genes. However, several R genes can be involved in HTAP resistance, but through regulation by one or two master genes. HTAP resistance is more diverse in its molecular basis, each HTAP resistance gene controls more genes with diverse functions in defense, and different HTAP resistance genes share fewer genes for regulation than all-stage resistance genes. The broadly based molecular mechanisms may explain the durability of HTAP resistance.
9. Conclusions and Perspectives
Durability and effectiveness of HTAP resistance against stripe rust has been demonstrated by growing wheat cultivars with this type of resistance for 60 years in the US PNW and some other regions. This type of resistance can be recognized phenotypically by necrotic stripes and relatively low severities, and its detection should be done in adult-plants under relatively high temperatures in comparison with susceptible plants tested under the same conditions. Isolates of stripe rust virulent to seedlings of the plants are necessary for phenotypical detection of HTAP resistance. In the recent years, a large number of genes or QTL with various levels of HTAP resistance have been identified and molecular markers have been developed for these genes. The genes and markers are useful in facilitating more effective breeding for cultivars with the durable type of resistance. HTAP resistance contributes to sustainable control of stripe rust by reduceing inoculum from season to season in a large scale region, a phenomenon that can be referred as “inoculum decline”. Through such inoculum decline, partially resistant HTAP resistant cultivars can be protected from yield losses. High levels of HTAP resistance can be achieved by pyramiding different genes for HTAP resistance. HTAP resistance genes also can be combined with effective all-stage resistance genes to develop cultivars with high-level and durable resistance. Cloning of Yr18 and Yr39 has revealed that these genes are different from race-specific R genes of the NBS-LRR type. The NonNBS-LRR structure could be a general rule for HTAP resistance gene products. Such generalization needs to be further tested by cloning more HTAP resistance genes, as well as R genes for race-specific resistance to stripe rust. Several studies are currently undergoing. Transcriptomics studies also have shield lights on molecular mechanisms of HTAP resistance. Compared to race-specific all-stage resistance, the relatively large number of genes with diverse molecular functions involved in HTAP resistance can explain, at least in part, why HTAP resistance is non-race specific and durable. Durability of resistance may be predicted by its responses to races, environment conditions particularly temperature, plant growth stage; inheritance; type of the genes controlling the resistance; and genes involved in the resistance under regulations of the control genes. Sustainable control of stripe rust can be achieved by developing cultivars with high level and durable resistance through primarily use of HTAP resistance and complemented with effective all-stage resistance.
REFERENCES
- R. W. Stubbs, “Stripe Rust,” In: A. P. Roelfs and W. R. Bushnell, Eds., Cereal Rusts. Volume II. Disease, Distribution, Epidemiology, and Control, Academic Press, New York, 1985, pp. 61-101.
- X. M. Chen, “Epidemiology and Control of Stripe Rust [Puccinia striiformis f. sp. tritici] on Wheat,” Canadian Journal of Plant Pathology, Vol. 27, No. 3, 2005, pp. 314-337. doi:10.1080/07060660509507230
- C. R. Wellings, “Global Status of Stripe Rust: A Review of Historical and Current Threats,” Euphytica, Vol. 179, No. 1, 2011, pp. 129-141. doi:10.1007/s10681-011-0360-y
- F. Rapilly, “Yellow Rust Epidemiology,” Annual Review Phytopathology, Vol. 17, 1979, pp. 59-73. doi:10.1146/annurev.py.17.090179.000423
- D. Sharma-Poudyal and X. M. Chen, “Models for Predicting Potential Yield Loss of Wheat Caused by Stripe Rust in the US Pacific Northwest,” Phytopathology, Vol. 101, No. 5, 2011, pp. 544-554. doi:10.1094/PHYTO-08-10-0215
- R. F. Line, “Stripe Rust of Wheat and Barley in North America: A Retrospective Historical Review,” Annual Review of Phytopathology, Vol. 40, 2002, pp. 75-118. doi:10.1146/annurev.phyto.40.020102.111645
- R. F. Line, “Recording and Processing Data on Foliar Diseases of Cereals,” Proceedings of European and Mediterranean Cereal Rusts Conference, Prague, 1972, pp. 175-178.
- R. F. Line, C. F. Konzak and R. E. Allan, “Evaluating Resistance to Puccinia striiformis,” Induced Mutation for Disease Resistance, Crop Plants, Vol. 180, 1974, pp. 125- 132.
- R. F. Line, R. E. Allan and C. F. Konzak, “Identifying and Utilizing Resistance to Puccinia striiformis in Wheat,” Proceedings of Induced Mutations for Disease Resistance, Crop Plants (1975), FAO/IAEA, Ames, 1976, pp. 151- 158.
- C. F. Konzak, R. F. Line, R. E. Allan and J. F. Schafer, “Guidelines for the Production, Evaluation, and Use of Induced Resistance to Stripe Rust in Wheat,” Proceedings of Induced Mutation to Plant Diseases, International Atomic Energy, Vienna, 1977, pp. 437-460.
- R. Johnson, “Durable Resistance, Definition of Genetic Control, and Attainment in Plant Breeding,” Phytopathology, Vol. 71, No. 6, 1981, pp. 567-568. doi:10.1094/Phyto-71-567
- R. F. Line and A. Qayoum, “Virulence, Aggressiveness, Evolution, and Distribution of Races of Puccinia striiformis (the Cause of Stripe Rust of Wheat) in North America, 1968-1987,” US Department of Agriculture, Agricultural Research Service (USDA-ARS), Technical Bulletin, Washington DC, Vol. 1788, 1992, 44 p.
- C. S. Holton, O. A. Vogel, E. L. Kendrick and F. C. Elliott, “The Reaction of Varieties and Hybrid Selections of Wheat to Physiologic Races of Tilletia caries and T. foetida,” Agronomy Journal, Vol. 48, No. 6, 1956, 276- 278. doi:10.2134/agronj1956.00021962004800060011x
- US Department of Agriculture, Agricultural Research Service, 1995. http://www.ars-grin.gov/cgi-bin/npgs/acc/display.pl?1062296
- US Department of Agriculture, Agricultural Research Service, 1949. http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=Brevor
- US Department of Agriculture, Agricultural Research Service, 1961. http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=Gaines
- US Department of Agriculture, Agricultural Research Service, 1965. http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=Nugaines
- US Department of Agriculture, Agricultural Research Service, 1970. http://www.ars-grin.gov/cgi-bin/npgs/acc/display.pl?1067898
- A. Qayoum and R. F. Line, “High-Temperature, AdultPlant Resistance to Stripe Rust of Wheat,” Phytopathology, Vol. 75, No. 10, 1985, 1121-1125. doi:10.1094/Phyto-75-1121
- J. Feng, Z. J. Zhang, G. H. Li, Y. Zhou, H. H. Wang, Q. G. Guo and J. Sun, “Components of Quantitative, Durable Resistance to Stripe Rust in Five Wheat Cultivars and Genetic Distance among the Cultivars,” Acta Phytopathologica Sinica, Vol. 37, No. 2, 2007, pp. 175-183.
- Q. Guo, Z. J. Zhang, Y. B. Xu, G. H. Li, J. Feng and Y. Zhou, “Quantitative Trait Loci for High-Temperature Adult-Plant and Slow-Rusting Resistance to Puccinia striiformis f. sp. tritici in Wheat Cultivars,” Phytopathology, Vol. 98, No. 7, 2008, pp. 803-809. doi:10.1094/PHYTO-98-7-0803
- US Department of Agriculture, Agricultural Research Service, 1977. http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=CI17596
- US Department of Agriculture, Agricultural Research Service, 1948. http://www.ars-grin.gov/cgi-bin/npgs/acc/display.pl?1144024
- X. M. Chen, “Challenges and Solutions for Stripe Rust Control in the United States,” Australian Journal of Agricultural Research, Vol. 58, No. 6, 2007, pp. 648-655. doi:10.1071/AR07045
- X. M. Chen, L. Penman, A. M. Wan and P. Cheng, “Virulence Races of Puccinia striiformis f. sp. tritici in 2006 and 2007 and Development of Wheat Stripe Rust and Distributions, Dynamics, and Evolutionary Relationships of Races from 2000 to 2007 in the United States,” Canadian Journal of Plant Pathology, Vol. 32, No. 3, 2010, pp. 315-323. doi:10.1080/07060661.2010.499271
- X. M. Chen, M. K. Moore, E. A. Milus, D. L. Long, R. F. Line, D. Marshall and L. Jackson, “Wheat Stripe Rust Epidemics and Races of Puccinia striiformis f. sp. tritici in the United States in 2000,” Plant Disease, Vol. 86, No. 1, 2002, pp. 39-46. doi:10.1094/PDIS.2002.86.1.39
- E. A. Milus and R. F. Line, “Gene Action for Inheritance of Durable, High-Temperature, Adult-Plant Resistance to Stripe Rust in Wheat,” Phytopathology, Vol. 76, 1986, pp. 435-441. doi:10.1094/Phyto-76-435
- X. M. Chen and R. F. Line, “Gene Action in Wheat Cultivars for Durable High-Temperature Adult-Plant Resistance and Interactions with Race-Specific, Seedling Resistance to Stripe Rust Caused by Puccinia striiformis,” Phytopathology, Vol. 85, 1995, pp. 567-572. doi:10.1094/Phyto-85-567
- X. M. Chen and R. F. Line, “Gene Number and Heritability of Wheat Cultivars with Durable, High-Temperature, Adult-Plant Resistance and Race-Specific Resistance to Puccinia striiformis,” Phytopathology, Vol. 85, 1995, pp. 573-578. doi:10.1094/Phyto-85-573
- A. H. Carter, X. M. Chen, K. G. Campbell and K. K. Kidwell, “Identifying QTL for High-Temperature AdultPlant Resistance to Stripe Rust (Puccinia striiformis f. sp. tritici) in the Spring Wheat (Triticum aestivum L.) Cultivar ‘Louise’,” Theoretical and Applied Genetics, Vol. 119, No. 6, 2009, pp. 1119-1128. doi:10.1007/s00122-009-1114-2
- C. Uauy, J. C. Brevis, X. M. Chen, I. A. Khan, L. F. Jackson, O. Chicaiza, A. Distelfeld, T. Fahima and J. Dubcovsky, “High-Temperature Adult Plant (HTAP) Stripe Rust Resistance Gene Yr36 from Triticum turgidum ssp. dicoccoides Is Closely Linked to the Grain Protein Content Locus Gpc-B1,” Theoretical and Applied Genetics. Vol. 112, No. 1, 2005, pp. 97-105. doi:10.1007/s00122-005-0109-x
- D. L. Fu, C. Uauy, A. Distelfeld, A. Blechl, L. Epstein, X. M. Chen, H. Sela, T. Fahima and J. Dubcovsky, “A Kinase-START Gene Confers Temperature-Dependent Resistance to Wheat Stripe Rust,” Science, Vol. 323, No. 5919, 2009, pp. 1357-1360. doi:10.1126/science.1166289
- R. H. Biffen, “Mendel’s Law of Inheritance and Wheat Breeding,” Journal of Agricultural Sciences, Vol. 1, No. 1, 1905, pp. 4-48. doi:10.1017/S0021859600000137
- R. E. Allan and O. A. Vogel, “Stripe Rust Resistance of Suwon 92 and Its Relationship to Several Morphological Characteristics in Wheat,” Plant Disease Reporter, Vol. 45, No. 7, 1961, pp. 778-779.
- F. C. H. Lupton and R. C. F. Macer, “Inheritance of Resistance to Yellow Rust (Puccinia glumarum Erikss. and Henn.) in Seven Varieties of Wheat,” Transaction of British Mycological Society, Vol. 45, No. 1, 1962, pp. 21- 45. doi:10.1016/S0007-1536(62)80032-1
- R. C. F. Macer, “The Formal and Monosomic Genetic Analysis of Stripe Rust (Puccinia striiformis) Resistance in Wheat,” In: J. MacKey, Ed., Proceedings of the Second International Wheat Genetics Symposium, Lund, Hereditas, Suppl 2, 1966, pp. 127-142.
- R. J. Metzger and B. A. Silbaugh, “Inheritance of Resistance to Stripe Rust and Its Association with Brown Glume Color in Triticum aestivum L., ‘P.I. 178383’,” Crop Science, Vol. 10, No. 5, 1970, pp. 567-568. doi:10.2135/cropsci1970.0011183X001000050035x
- G. Röbbelen and E. L. Sharp, “Mode of Inheritance, Interaction and Application of Genes Conditioning Resistance to Yellow Rust,” Verlag Paul Parey, Berlin and Hamburg, 1978, pp. 1-88.
- L. H. Purdy and R. E. Allan, “Seedling and Mature Plant Reactions of Wheat to Stripe Rust,” Plant Disease Reporter, Vol. 47, No. 9, 1963, pp. 797-799.
- R. E. Allan, L. H. Purdy and O. A. Vogel, “Inheritance of Seedling and Adult-Plant Reaction of Wheat to Stripe Rust,” Crop Science, Vol. 6, No. 3, 1966, pp. 242-245. doi:10.2135/cropsci1966.0011183X000600030007x
- W. Straib, “Understanding of Genetics of Yellow Rust on Wheat,” Phytopathologische Zeitschrift, Vol. 7, 1934, pp. 427-477.
- E. L. Sharp and E. R. Hehn, “Major and Minor Genes in Wheat Variety P.I. 178383 Conditioning Resistance to Stripe Rust,” Phytopathology, Vol. 57, No. 8, 1967, p. 1009.
- R. T. Lewellen, E. L. Sharp and E. R. Hehn, “Major and Minor Genes in Wheat for Resistance to Puccinia striiformis and Their Responses to Temperature Changes,” Canadian Journal of Botany, Vol. 45, No. 11, 1967, pp. 2155-2172. doi:10.1139/b67-234
- R. T. Lewellen and E. L. Sharp, “Inheritance of Minor Reaction Gene Combinations in Wheat to Puccinia striiformis at Two Temperature Profiles,” Canadian Journal of Botany, Vol. 46, No. 1, 1968, pp. 21-26. doi:10.1139/b68-005
- E. L. Sharp and R. B. Volin, “Additive Genes in Wheat Conditioning Resistance to Stripe Rust,” Phytopathology, Vol. 60, No. 7, 1970, pp. 1146-1147. doi:10.1094/Phyto-60-1146
- E. A. Milus and R. F. Line, “Number of Genes Controlling High-Temperature Adult-Plant Resistance to Stripe Rust in Wheat,” Phytopathology, Vol. 76, 1986, pp. 93- 96. doi:10.1094/Phyto-76-93
- F. Lin and X. M. Chen, “Genetics and Molecular Mapping of Genes for Race-Specific All-Stage Resistance and Non-Race Specific High-Temperature Adult-Plant Resistance to Stripe Rust in Spring Wheat Cultivar Alpowa,” Theoretical and Applied Genetics, Vol. 114, No. 7, 2007, pp. 1277-1287. doi:10.1007/s00122-007-0518-0
- X. M. Chen and J. Zhao, “Identification of Molecular Markers for Yr8 and a Gene for High-Temperature, AdultPlant Resistance against Stripe Rust in the AVS/6*Yr8 Wheat Line,” Phytopathology, Vol. 97, No. 7S, 2007, p. S21.
- G. P. Yan and X. M. Chen, “Identification of a Quantitative Trait Locus for High-Temperature Adult-Plant Resistance against Puccinia striiformis f. sp. hordei in ‘Bancroft’ Barley,” Phytopathology, Vol. 98, No. 1, 2008, pp. 120-127. doi:10.1094/PHYTO-98-1-0120
- X. M. Chen and R. F. Line, “Recessive Genes for Resistance to Races of Puccinia striiformis f. sp. hordei in Barley,” Phytopathology, Vol. 89, No. 3, 1999, pp. 226- 232. doi:10.1094/PHYTO.1999.89.3.226
- X. M. Chen and R. F. Line, “Identification of Genes for Resistance to Puccinia striiformis f. sp. hordei in 18 Barley Genotypes,” Euphytica, Vol. 129, No. 1, 2002, pp. 127- 145. doi:10.1023/A:1021585907493
- F. Lin and X. M. Chen, “Quantitative Trait Loci for NonRace-Specific, High-Temperature Adult-Plant Resistance to Stripe Rust in Wheat Cultivar Express,” Theoretical and Applied Genetics, Vol. 118, No. 4, 2009, pp. 631-642. doi:10.1007/s00122-008-0894-0
- D. K. Santra, X. M. Chen, M. Santra, K. G. Campbell and K. K. Kidwell, “Identification and Mapping QTL for High-Temperature Adult-Plant Resistance to Stripe Rust in Winter Wheat (Triticum aestivum L.) Cultivar ‘Stephens’,” Theoretical and Applied Genetics, Vol. 117, No. 5, 2008, pp. 793-802. doi:10.1007/s00122-008-0820-5
- J. E. Van der Plank, “Plant Diseases: Epidemics and Control,” Academic Press, New York, 1966, 349 p.
- S. Mallard, D. Gaudet, A. Aldeia, C. Abelard, A. L. Besnard, P. Sourdille and F. Dedryver, “Genetic Analysis of Durable Resistance to Yellow Rust in Bread Wheat,” Theoretical and Applied Genetics, Vol. 110, No. 8, 2005, pp. 1401-1409. doi:10.1007/s00122-005-1954-3
- J. L. Chen, C. G. Chu, E. J. Souza, M. J. Guttieri, X. M. Chen, S. Xu, D. Hole and R. Zemetra, “Genome-Wide Identification of QTLs Conferring High-Temperature Adult-Plant (HTAP) Resistance to Stripe Rust (Puccinia striiformis f. sp. tritici) in Wheat,” Molecular Breeding, Vol. 29, No. 3, 2012, pp. 791-800. doi:10.1007/s11032-011-9590-x
- M. D. Vazquez, C. J. Peterson, O. Riera-Lizarazu, X. M. Chen, A. Heesacker, K. Ammar, J. Crossa and C. C. Mundt, “Genetic Analysis of Adult Plant, Quantitative Resistance to Stripe Rust in Wheat Cultivar Stephens in Multi-Environment Trials,” Theoretical and Applied Genetics, Vol. 124, No. 1, 2012, pp. 1-11. doi:10.1007/s00122-011-1681-x
- R. S. Ren, M. N. Wang, X. M. Chen and Z. J. Zhang, “Characterization and Molecular Mapping of Yr52 for High-Temperature Adult-Plant Resistance to Stripe Rust in Spring Wheat Germplasm PI 183527,” Theoretical and Applied Genetics, Vol. 125, No. 5, 2012, pp. 847-857. doi:10.1007/s00122-012-1877-8
- I. Lowe, L. Jankuloski, S. M. Chao, X. M. Chen, D. See and J. Dubcovsky, “Mapping and Validation of QTL Which Confer Partial Resistance to Broadly Virulent Post- 2000 North American Races of Stripe Rust in Hexaploid Wheat,” Theoretical and Applied Genetics, Vol. 123, No 1, 2011, pp. 143-157. doi:10.1007/s00122-011-1573-0
- [61] S. G. Krattinger, E. S. Lagudah, W. Spielmeyer, R. P. Singh, J. Huerta-Espino, H. McFadden, E. Bossolini, L. L. Selter and B. Keller, “A Putative ABC Transporter Confers Durable Resistance to Multiple Fungal Pathogens in Wheat,” Science, Vol. 323, No. 5919, 2009, pp. 1360- 1363. doi:10.1126/science.1166453
- [62] A. J. Worland and C. N. Law, “Genetic Analysis of Chromosome 2D of Wheat. I. The Location of Genes Affecting Height, Day-Length Insensitivity, Hybrid Dwarfism and Yellow Rust Resistance,” Plant Breeding, Vol. 96, No. 4, 1986, pp. 331-345.
- [63] R. P. Singh, J. C. Nelson and M. E. Sorrells, “Mapping Yr28 and Other Genes for Resistance to Stripe Rust in Wheat,” Crop Science, Vol. 40, No. 4, 2000, pp. 1148- 1155. doi:10.2135/cropsci2000.4041148x
- [64] A. Börner, M. S. Röder, O. Unger and A. Meinel, “The Detection and Molecular Mapping of a Major Gene for Non Specific Adult Plant Disease Resistance against Stripe Rust (Puccinia striiformis) in Wheat,” Theoretical and Applied Genetics, Vol. 100, No. 7, 2000, pp. 1095- 1099. doi:10.1007/s001220051391
- [65] H. M. William, R. P. Singh, J. Huerta-Espino and G. Rosewarne, “Characterization of Genes for Durable Resistance to Leaf Rust and Yellow Rust in CIMMYT Spring Wheats,” In: H. T. Buck, J. E. Nisi and N. Salomón, Eds., Wheat Production in Stressed Environments, Developments in Plant Breeding, Springer, New York, Vol. 11, 2007, pp. 65-70. doi:10.1007/1-4020-5497-1_7
- [66] A. Navabi, J. P. Tewari, R. P. Singh, B. McCallum, A. Laroche, K. G. Briggs, “Inheritance and QTL Analysis of Durable Resistance to Stripe and Leaf Rusts in an Australian Cultivar, Triticum aestivum ‘Cook’,” Genome, Vol. 48, No. 1, 2005, pp. 97-106. doi:10.1139/g04-100
- [67] P. Chhuneja, S. Kaur, T. Garg, M. Ghai, S. Kaur, M. Prashar, N. S. Bains, R. K. Goel, B. Keller, H. S. Dhaliwal and K. Singh, “Mapping of Adult Plant Stripe Rust Resistance Genes in Diploid A Genome Wheat Species and Their Transfer to Bread Wheat,” Theoretical and Applied Genetics, Vol. 116, No. 3, 2008, pp. 313-324. doi:10.1007/s00122-007-0668-0
- [68] J. P. E. Melichar, S. Berry, C. Newell, R. MacCormack and L. A. Boyd, “QTL Identification and Microphenotype Characterization of the Developmentally Regulated Yellow Rust Resistance in the UK Wheat Cultivar Guardian,” Theoretical and Applied Genetics, Vol. 117, No. 3, 2008, pp. 391-399. doi:10.1007/s00122-008-0783-6
- [69] X. M. Chen, R. F. Line and H. Leung, “Genome Scanning for Resistance-Gene Analogs in Rice, Barley, and Wheat by High-Resolution Electrophoresis,” Theoretical and Applied Genetics, Vol. 97, No. 3, 1998, pp. 345-355. doi:10.1007/s001220050905
- [70] Z. X. Shi, X. M. Chen, R. F. Line, H. Leung and C. R. Wellings, “Development of Resistance Gene Analog Polymorphism Markers for the Yr9 Gene Resistance to Wheat Stripe Rust,” Genome, Vol. 44, No. 4, 2001, pp. 509-516.
- [71] X. M. Chen, M. N. Wang, P. Cheng, X. L. Zhou, R. S. Ren, L. Hou, Y. Lu, J. Y. Feng and D. See, “Molecular Mapping of Wheat Genes for Effective All-Stage Resistance and High-Temperature Adult-Plant Resistance to Stripe Rust,” In: W.-Q. Chen, Ed., Disease Risk and Food Security, Proceedings of the 13th International Cereal Rusts and Powdery Mildews Conference, Beijing, 28 August-1 September 2012, China Agricultural Science and Technology Press, 2012, pp. 179-180.
- [72] F. Lin and X. M. Chen, “Molecular Mapping of Genes for Race-Specific Overall Resistance to Stripe Rust in Wheat Cultivar Express,” Theoretical and Applied Genetics, Vol. 116, No. 6, 2008, pp. 797-806. doi:10.1007/s00122-008-0713-7
- [73] X. X. Sui, M. N. Wang and X. M. Chen, “Molecular Mapping of a Stripe Rust Resistance Gene in Spring Wheat Cultivar ‘Zak’,” Phytopathology, Vol. 99, No. 10, 2009, 1209-1215. doi:10.1094/PHYTO-99-10-1209
- [74] P. Cheng and X. M. Chen, “Molecular Mapping of a Gene for Stripe Rust Resistance in Spring Wheat Cultivar IDO377s,” Theoretical and Applied Genetics, Vol. 121, No. 1, 2010, pp. 195-204. doi:10.1007/s00122-010-1302-0
- [75] Q. Li, X. M. Chen, M. N. Wang and J. X. Jing, “Yr45, a New Wheat Gene for Stripe Rust Resistance Mapped on the Long Arm of Chromosome 3D,” Theoretical and Applied Genetics, Vol. 122, No. 1, 2010, pp. 189-197. doi:10.1007/s00122-010-1435-1
- [76] L. S. Xu, M. N. Wang, P. Cheng, Z. S. Kang, S. H. Hulbert and X. M. Chen, “Molecular Mapping of Yr53, a New Gene for Stripe Rust Resistance in Durum Wheat Accession PI 480148 and Its Transfer to Common Wheat,” Theoretical and Applied Genetics, Vol. 126, No. 2, 2013, pp 523-533. doi:10.1007/s00122-012-1998-0
- [77] M. N. Wang, X. M. Chen, L. S. Xu, P. Cheng and H. E. Bockelman, “Registration of 70 Common Spring Wheat Germplasm Lines Resistant to Stripe Rust,” Journal of Plant Registrations, Vol. 6, No. 1, 2012, pp. 104-110. doi:10.3198/jpr2011.05.0261crg
- [78] UCDavis, 2011. http://maswheat.ucdavis.edu/protocols/index.htm
- [79] W. Spielmeyer, R. P. Singh, H. McFadden, C. R. Wellings, J. Huerta-Espino, X. Kong, R. Appels and E. S. Lagudah, “Fine Scale Genetic and Physical Mapping Using Interstitial Deletion Mutants of Lr34/Yr18: A Disease Resistance Locus Effective against Multiple Pathogens in Wheat,” Theoretical and Applied Genetics, Vol. 116, No. 4, 2008, pp. 481-490. doi:10.1007/s00122-007-0684-0
- [80] E. S. Lagudah, S. G. Krattinger, S. Herrera-Foessel, R. P. Singh, J. Huerta-Espin, W. Spielmeyer, G. Brown-Guedira, L. L. Selter and B. Keller, “Gene-Specific Markers for the Wheat Gene Lr34/Yr18/Pm38 Which Confers Resistance to Multiple Fungal Pathogens,” Theoretical and Applied Genetics, Vol. 119, No. 5, 2009, pp. 889-898. doi:10.1007/s00122-009-1097-z
- [81] G. M. Rosewarne, R. P. Singh, J. Huerta-Espino, H. M. William, S. Bouchet, S. Cloutier, H. McFadden and E. S. Lagudah, “Leaf Tip Necrosis, Molecular Markers and β1- Proteasome Subunits Associated with the Slow Rusting Resistance Genes Lr34/Yr29,” Theoretical and Applied Genetics, Vol. 112, No. 3, 2006, pp. 500-508. doi:10.1007/s00122-005-0153-6
- [82] H. S. Bariana, N. Parry, L. I. Barclay, R. Loughman, R. J. McLean, M. Shankar, R. E. Wilson, N. J. Willey and M. Francki, “Identification and Characterization of Stripe Rust Resistance Gene Yr34 in Common Wheat,” Theoretical and Applied Genetics, Vol. 112, No. 6, 2006, pp. 1143-1148. doi:10.1007/s00122-006-0216-3
- [83] E. K. Khleskina, M. S. Röder, O. Unger, A. Meinel and A. Börner, “More Precise Map Position and Origin of a Durable Non-Specific Adult Plant Disease Resistance against Stripe Rust (Puccinia striiformis) in Wheat,” Euphytica, Vol. 153, No. 1, 2007, pp. 1-10. doi:10.1007/s10681-006-9182-8
- [84] V. P. Ramburan, Z. A. Pretorius, J. H. Louw, L. A. Boyd, P. H. Smith, W. H. P. Boshoff and R. Prins, “A Genetic Analysis of Adult Plant Resistance to Stripe Rust in the Wheat Cultivar Kariega,” Theoretical and Applied Genetics, Vol. 108, No. 7, 2004, pp. 1426-1433. doi:10.1007/s00122-003-1567-7
- [85] F. Dedryver, S. Paillard, S. Mallard, O. Robert, M. Trottet, S. Nègre, G. Verplancke and J. Jahier, “Characterization of Genetic Components Involved in Durable Resistance to Stripe Rust in the Bread Wheat ‘Renan’,” Phytopathology, Vol. 99, No. 8, 2009, pp. 968-973. doi:10.1094/PHYTO-99-8-0968
- [86] H. S. Bariana, U. K. Bansal, A. Schmidt, A. Lehmensiek, J. Kaur, H. Miah, N. Howes and C. L. McIntyre, “Molecular Mapping of Adult Plant Stripe Rust Resistance in Wheat and Identification of Pyramided QTL Genotypes,” Euphytica, Vol. 176, No. 2, 2010, pp. 251-260. doi:10.1007/s10681-010-0240-x
- [87] C. Lan, S. Liang, X. Zhou, G. Zhou, Q. Lu, X. Xia and Z. He, “Identification of Genomic Regions Controlling AdultPlant Stripe Rust Resistance in Chinese Landrace Pingyuan 50 through Bulked Segregant Analysis,” Phytopathology, Vol. 100, No. 4, 2010, pp. 313-318. doi:10.1094/PHYTO-100-4-0313
- [88] J. Feng, L. L. Zuo, Z. Y. Zhang, R. M. Lin, Y. Y. Cao and S. C. Xu, “Quantitative Trait Loci for Temperature-Sensitive Resistance to Puccinia striiformis f. sp. tritici in Wheat Cultivar Flinor,” Euphytica, Vol. 178, No. 3, 2011, 321-329. doi:10.1007/s10681-010-0291-z
- [89] L. J. Jagger, C. Newell, S. T. Berry, R. MacCormack and L. A. Boyd, “The Genetic Characterisation of Stripe Rust Resistance in the German Wheat Cultivar Alcedo,” Theoretical and Applied Genetics, Vol. 122, No. 4, 2011, pp. 723-733. doi:10.1007/s00122-010-1481-8
- [90] X. L. Zhou, W. L. Wang, L. L. Wang, D. Y. Hou, J. X. Jing, Y. Wang, Z. Q. Xu, Q. Yao, J. L. Yin and D. F. Ma, “Genetics and Molecular Mapping of Genes for HighTemperature Resistance to Stripe Rust in Wheat Cultivar Xiaoyan 54,” Theoretical Applied Genetics, Vol. 123, No. 3, 2011, pp. 431-438. doi:10.1007/s00122-011-1595-7
- [91] Y. Hao, Z. Chen, Y. Wang, D. Bland, J. Buck, G. BrownGuedira and J. Johnson, “Characterization of a Major QTL for Adult Plant Resistance to Stripe Rust in US Soft Red Winter Wheat,” Theoretical and Applied Genetics, Vol. 123, No. 8, 2011, pp. 1401-1411. doi:10.1007/s00122-011-1675-8
- [92] L. Zhao, J. Feng, C. Y. Zhang, X. D. Xu, X. M. Chen, Q. Sun, Q. Miao, S. C. Xu and F. Lin, “The Dissection and SSR Mapping of a High-Temperature Adult-Plant Stripe Rust Resistance Gene in American Spring Wheat Cultivar Alturas,” European Journal of Plant Pathology, Vol. 134, No. 2, 2012, pp. 281-288. doi:10.1007/s10658-012-9987-3
- [93] X. Cao, J. Zhou, X. Gong, G. Zhao, J. Jia and X. Qi, “Identification and Validation of a Major Quantitative Trait Locus for Slow-Rusting Resistance to Stripe Rust in Wheat,” Journal of Integrative Plant Biology, Vol. 54, No. 5, 2012, pp. 330-344. doi:10.1111/j.1744-7909.2012.01111.x
- [94] S. Paillard, G. Trotoux-Verplancke, M.-R. Perretant, F. Mohamadi, M. Leconte, S. Coëdel, C. de VallavieillePope and F. Dedryver, “Durable Resistance to Stripe Rust is Due to Three Specific Resistance Genes in French Bread Wheat Cultivar Apache,” Theoretical and Applied Genetics, Vol. 125, No. 5, 2012, pp. 955-965. doi:10.1007/s00122-012-1885-8
- [95] Y. Ren, Z. He, J. Li, M. Lillemo, L. Wu, B. Bai, Q. Lu, H. Zhu, G. Zhou, J. Du, Q. Lu, and X. Xia, “QTL Mapping of Adult-Plant Resistance to Stripe Rust in a Population Derived from Common Wheat Cultivars Naxos and Shanghai 3/Catbird,” Theoretical and Applied Genetics, Vol. 125, No. 6, 2012, pp. 1211-1221. doi:10.1007/s00122-012-1907-6
- [96] Y. Ren, Z. Li, Z. He, L. Wu, B. Bai, C. Lan, C. Wang, G. Zhou, H. Zhu and X. Xia, “QTL Mapping of Adult-Plant Resistances to Stripe Rust and Leaf Rust in Chinese Wheat Cultivar Bainong 64,” Theoretical and Applied Genetics, Vol. 125, No. 6, 2012, pp. 1253-1262. doi:10.1007/s00122-012-1910-y
- [97] G. M. Agenbag, Z. A. Pretorius, L. A. Boyd, C. M. Bender and R. Prins, “Identification of Adult Plant Resistance to Stripe Rust in the Wheat Cultivar Cappelle-Desprez,” Theoretical and Applied Genetics, Vol. 125, No. 1, 2012, pp. 109-120. doi:10.1007/s00122-012-1819-5
- [98] O. Chicaiza, I. A. Khan, X. Zhang, J. C. Brevis, L. Jackson, X. M. Chen and J. Dubcovsky, “Registration of Five Wheat Isogenic Lines for Leaf Rust and Stripe Rust Resistance Genes,” Crop Science, Vol. 46, No. 1, 2006, pp. 485-487. doi:10.2135/cropsci2005.04-0048
- [99] UCDavis, 2007. http://maswheat.ucdavis.edu/Achievements/cultivars2007.htm
- [100] USDA-ARS, 2005. http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=Farnum
- [101] S. S. Jones, S. R. Lyon, K. A. Balow, M. A. Gollnick, J. W. Burns, P. E. Schillingger, P. E. Reisenauer, T. D. Murray, X. M. Chen, G. C. Campbell, C. F. Morris and B. Goates, “Registration of ‘Bauermeister’ Wheat,” Crop Science Vol. 47, No. 1, 2007, pp. 430-431. doi:10.2135/cropsci2006.02.0120
- [102] K. L. Richardson, M. I. Vales, J. G. Kling, C. C. Mundt and P. M. Hayes, “Pyramiding and Dissecting Disease Resistance QTL to Barley Stripe Rust,” Theoretical and Applied Genetics, Vol. 113, No. 3, 2006, pp. 485-495. doi:10.1007/s00122-006-0314-2
- [103] C. Rossi, A. Cuesta-Marcos, I. Vales, L. Gomez-Pando, G. Orjeda, R. Wise, K. Sato, K. Hori, F. Capettini, H. Vivar, X. M. Chen and P. Hayes, “Mapping Multiple Disease Resistance Genes Using a Barley Mapping Population Evaluated in Peru, Mexico, and the USA,” Molecular Breeding, Vol. 18, No. 4, 2006, pp. 355-366. doi:10.1007/s11032-006-9043-0
- [104] A. M. Wan and X. M. Chen, “Virulence, Frequency, and Distribution of Races of Puccinia striiformis f. sp. tritici and P. striiformis f. sp. hordei Identified in the United States in 2008 and 2009,” Plant Disease, Vol. 96, No. 1, 2012, pp. 67-74. doi:10.1094/PDIS-02-11-0119
- [105] R. E. Allan, C. J. Peterson, R. F. Line, G. L. Rubenthaler and C. F. Morris, “Registration of ‘Rely’ Wheat Multiline,” Crop Science, Vol. 33, No. 1, 1993, pp. 213-214. doi:10.2135/cropsci1993.0011183X003300010059x
- [106] X. M. Chen, M. K. Moore and D. A. Wood, “Epidemics and Control of Stripe Rust on Spring Wheat in the Pacific Northwest in 2002,” Phytopathology, Vol. 93, 2003, p. S16.
- [107] G. A. Strobel and E. L. Sharp, “Protein of Wheat Associated with Infection Type of Puccinia striiformis,” Phytopathology, Vol. 55, No. 4, 1965, pp. 413-414.
- [108] A. Laroche, F. Eudes, M. M. Frick, R. Huel, C. L. Nykiforuk, R. L. Conner, Q. Chen, A. Kuzyk, H. Soltanloo and D. Gaudet, “Yr10, the Wheat Stripe Rust Resistance Gene from Moro,” Abstracts of the Symposium “Stripe Rust of Wheat: A Plan for Recovery,” The ASA-CSSA-SSA International Annual Meetings, Salt Lake City, 6-10 November 2005, p. 232-2. http://a-c-s.confex.com/a-c-s/2005am/techprogram/P4919.htm
- [109] S. H. Hulbert, J. Bai, J. P. Fellers, M. G. Pacheco and R. L. Bowden, “Gene Expression Patterns in Near Isogenic Lines for Wheat Rust Resistance Gene Lr34/Yr18,” Phytopathology, Vol. 97, No. 9, 2007, pp. 1083-1093. doi:10.1094/PHYTO-97-9-1083
- [110] T. E. Coram, G. Brown-Guedira and X. M. Chen, “Using Transcriptomics to Understand the Wheat Genome,” CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, Vol. 3, No. 83, 2008, pp. 1-9.
- [111] T. E. Coram, M. L. Settles and X. M. Chen, “Transcriptome Analysis of High-Temperature Adult-Plant Resistance Conditioned by Yr39 during the Wheat-Puccinia striiformis f. sp. tritici Interaction,” Molecular Plant Pathology, Vol. 9, No. 4, 2008, pp. 479-493. doi:10.1111/j.1364-3703.2008.00476.x
- [112] T. E. Coram, M. N. Wang and X. M. Chen, “Transcriptome Analysis of the Wheat-Puccinia striiformis f. sp. tritici Interaction,” Molecular Plant Pathology, Vol. 9, No. 2, 2008, pp. 157-169. doi:10.1111/j.1364-3703.2007.00453.x
- [113] T. E. Coram, X. L. Huang, G. M. Zhan, M. L. Settles and X. M. Chen, “Meta-Analysis of Transcripts Associated with Race-Specific Resistance to Stripe Rust in Wheat Demonstrates Common Induction of Blue Copper-Binding Protein, Heat-Stress Transcription Factor, PathogenInduced WIR1A Protein, and Ent-Kaurene Synthase Transcripts,” Functional and Integrative Genomics, Vol. 10, No. 3, 2010, pp. 383-392. doi:10.1007/s10142-009-0148-5
- [114] X. M. Chen, T. Coram, X. L. Huang, M. N. Wang and A. Dolezal, “Towards an Understanding of the Molecular Mechanisms of Durable and Non-Durable Resistance to Stripe Rust in Wheat,” Oral Presentations, Poster Abstracts, Participants and Program of BGRI 2011 Technical Workshop, St. Paul, 13-16 June 2011, pp. 70-81.
- [115] C. Harter, J. Pavel, F. Coccia, E. Draken, S. Wegehingel, H. Tschochner and F. Wieland, “Nonclathrin Coat Protein γ, a Subunit of Coatomer, Binds to the Cytoplasmic Dilysine Motif of Membrane Proteins of the Early Secretory Pathway,” Proceedings of National Academy of Sciences of the USA, Vol. 93, No. 5, 1996, pp. 1902-1906.
- [116] J. Moldenhauer, Z. Pretorius, B. M. Moerschbacher, R. Prins and A. J. van der Westhuizen, “Histopathology and PR-Protein Markers Provide Insight into Adult Plant Resistance to Stripe Rust of Wheat,” Molecular Plant Pathology, Vol. 9, No. 2, 2008, pp. 137-145. doi:10.1111/j.1364-3703.2007.00449.x
- [117] S. Mallard, S. Nègre, S. Pouya, D. Gaudet, Z. X. Lu and F. Dedryver, “Adult Plant Resistance-Related Gene Expression in ‘Camp Remy’ Wheat Inoculated with Puccinia striiformis,” Molecular Plant Pathology, Vol. 9, No. 2, 2008, pp. 213-225. doi:10.1111/j.1364-3703.2007.00459.x
- [118] X. L. Huang, J. B. Ma, X. M. Chen, X. J. Wang, K. Ding, D. J. Han, Z. P. Qu, L. L. Huang and Z. S. Kang, “Genes Involved in Adult Plant Resistance to Stripe Rust in Wheat Cultivar Xingzi 9104,” Physiological and Molecular Plant Pathology, Vol. 81, 2013, pp. 26-32. doi:10.1016/j.pmpp.2012.10.004

