Advances in Microbiology
Vol. 2 No. 4 (2012) , Article ID: 25942 , 9 pages DOI:10.4236/aim.2012.24071
Evaluation of Morphological Changes of Aeromonas caviae Sch3 Biofilm Formation under Optimal Conditions*
1Laboratorio de Bacteriología, Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional (ENCB-IPN), Prolongación de Carpio y Plan de Ayala s/n, Mexico City, Mexico
2Departamento de Bioquímica, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (Cinvestav-IPN), Mexico City, Mexico
3Departamento de Biología Celular, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (Cinvestav-IPN), Mexico City, Mexico
4Centro de Diagnóstico y Vigilancia Epidemiológica del Distrito Federal, Instituto de Ciencia y Tecnología del Distrito Federal, Mexico City, Mexico
Email: #chelacastro@hotmail.com
Received August 23, 2012; revised September 24, 2012; accepted October 4, 2012
Keywords: Biofilm; Aeromonas caviae; Scanning Electron Microscopy; Transmission Electron Microscopy; Physical and Chemical Factors
ABSTRACT
Aeromonas is a Gram-negative bacterium that lives in aquatic habitats. It can be infective in humans. One of its remarkable attributes is the ability of biofilm formation. Many factors are involved in the construction of biofilms as has been described for Pseudomonas, Klebsiella, and Vibrio, among others. The aim of this work was to study the bacterial morphology during the establishment of biofilm through scanning electron microscopy (SEM) and transmission electron microscopy (TEM) with a modified microtiter plate assay and to determine the best conditions for the establishment of Aeromonas caviae Sch3 biolfilm in vitro. We observed several phenotypic changes, including surface appearance, size, presence of extracellular vesicles from 100 to 250 nm in diameter, and flagella. The best conditions for biofilm formation were to grow cultures at 28˚C at pH 6, as determined by the crystal violet assay. This is, to the best of our knowledge, the first study that describes the cell’s biological events involved in the establishment of biofilm formation of Aeromonas caviae Sch3 in vitro.
1. Introduction
The genus Aeromonas is constituted by waterborne Gram-negative bacteria that live in aquatic environments, including groundwater and chlorinated drinking water [1]. This genus contains a number of different taxa, where A. hydrophila, A. caviae, A. salmonicida, and A. veronii biovar veronii are the most studied [2]. These species are considered pathogenic to humans because they cause intraand extra-intestinal infections, and other severe illnesses, such as septicemia, wounds infection, and respiratory tract disease [2]. The Aeromonas mechanism of pathogenicity is not yet completely understood, although several virulence factors involved in the establishment of infection have been identified. Some of these factors are proteases, lipases, bacterial structures like flagella and pili, S layer, aerolysin, and siderophores [3,4]. In the last few years, the biofilm structure has been revealed as an important bacterial association with a significant role in exacerbating human infection, because it provides bacteria some properties that make antibiotics treatment difficult [5].
Biofilms, also known as sessile communities, are tight associations of microorganisms growing on surfaces and embedded in a matrix of extracellular polymeric substance (EPS). They have been described in Gram-positive (e.g., Staphylococcus spp. and Streptococcus spp.) and Gram-negative (e.g., Pseudomonas spp., Vibrio spp., Klebsiella spp., and Escherichia coli) bacteria. Microorganisms in a biofilm are more resistant to antimicrobial agents and innate immunity host defense than planktonic cells [6]. Biofilms constitute an intricate interplay between physical and chemical factors, and have physiological and genetic properties such as gene transfer and gene activation through bacterial communication known as quorum sensing. As a result of this complexity, biofilm-forming bacteria might express more virulent phenotypes [7].
Two of the most studied biofilm models are those generated by P. aeruginosa and V. cholerae. Depending on the experimental conditions used, biofilms develop as flat, mushroom-shaped, or loose protruding structures through a series of distinct steps where regulation of cellular migration and adhesiveness play important roles [8,9]. Several studies have allowed the identification of a number of relevant factors involved in biofilm development. These factors include molecules necessary for bacterial attachment and spreading, such as outer membrane proteins, polar and lateral flagella, polysaccharides, cell-to-cell interconnecting components, environmental clues such as pH, temperature, composition of culture media, oxygen availability, and some genetic elements (plasmids) [10-12].
Biofilms are surface-associated, multicellular communities of bacteria that form through a developmental process and they are the most common mode of bacterial growth in natural environments [6].
Several studies have now demonstrated that cells in biofilm state have phenotypic characteristics distinct from those of their planktonic counterparts with signifycant changes in the patterns of gene expression [6,13].
To date, little is known of the physiological changes that occur in biofim formation in the case of Aeromonas species. However, we known that exopolysaccharides and flagella play key roles in biofilm formation [13]. Flagella are essential for invasion and adherence to fish and human cell lines [13]. It has been shown that polar and lateral flagella heighten biofilm formation [4]. The polar flagellum is expressed constitutively and allows bacteria to move in liquid environments, whereas lateral flagella help to move on semisolid media and are responsible of swarming motility [13]. In some studies lateral flagella have been shown to be essential for cell adherence and biofilm formation [4], while in others both kinds of flagella are required for these processes [14]. A. caviae strain Sch3 was originally isolated in the United Kingdom in 1991 from the diarrheal feces of a child of less than 1 year old with gastroenteritis (nausea, vomiting, and abdominal pain). It has two distinct flagellar systems, namely a polar flagellum for swimming in liquid and multiple lateral flagella for swarming over surfaces and it can adherence to (and possibly invasion) the epithelial cell line HEp-2 [14]. However, the knowledge of the morphological changes in Aeromonas cells during biofilm formation is not yet clearly defined nor do we how certain grown conditions can improve biofilm formation by Aeromonas strains. The aim of this study was to observe the morphological variants in Aeromonas caviae Sch3 biofilm by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) and determine the effect of some physical and chemical factors, such as temperature, pH, and incubation time on its formation.
2. Materials and Methods
2.1. Bacterial Strain
We used the A. caviae Sch3 strain isolated from diarrheal feces of a 5-year-old child patient. It was kindly provided by Dr. Jonathan Shaw from the University of Sheffield Medical School (United Kingdom). Genetical and biochemical characterization of A. caviae Sch3 was performed by Dr. Castro Escarpulli from the Escuela Nacional de Ciencias Biólogicas del Instituto Politécnico Nacional (Mexico).
2.2. Culture Conditions
A. caviae Sch3 strain was grown on 1.5% trypticase soy agar medium (TSA) (Bioxon, Mexico) at 37˚C for 16 or 18 h. Short-term storage of isolates was done in minimal maintenance medium [1%] [v/v] casein peptone, 0.3% [v/v] yeast extract, 1% [v/v] bacteriological agar and 0.85% [v/v] NaCl) at room temperature (RT). Long-term storage was done in Todd Hewitt broth (Oxoid, Mexico) containg 40% (v/v) glycerol at –70˚C.
2.3. Conditions of Biofilm Formation
Quantitative biofilm formation experiments were performed in a microtiter plate, as previously described by Gavín [4] with some modifications. Briefly, several colonies from an overnight culture were gently resuspended in 10 mL of trypticase soy broth (TSB) (Bioxon, Mexico) and adjusted to an optical density of 0.8 at 600 nm. Then, aliquots of bacterial suspension (5 mL) were placed in each well of a polystyrene microtiter plate (Costar) and incubated for up to 48 h at 37˚C without shaking at different values of pH (5.0, 5.5, 6.0, 6.5, 7.0, and 8.0). To determine the effect of temperature, cultures were grown at 8, 28, 37, and 42˚C for 48 h. The effects of incubation times were analyzed at 24, 48, 72, and 96 h at 28˚C (this temperature was previously determined as optimal in this study). After the establishment of the best conditions for biofilm formation, cells attached were carefully washed with phosphate-buffered saline (PBS), and fixed with 2.5% (v/v) glutaraldehyde for 10 min at RT and stained with 0.4% (w/v) crystal violet for 20 min at RT. We used the medium as control. The biofilmbound crystal violet was solubilized with 2 mL of ethanol-acetone (80/20, v/v) and the absorbance for each well (4 mL) was measured at 570 nm in a spectrophotometer Optimus 10,000 xs (Spectronic 20D Genesis). The cut-off OD was defined as the mean OD negative control. Each test was performed in triplicate.
Statistical analysis. Biofilm results were statistically evaluated by Student’s t test (test of variances) for related samples with a confidence interval of 95%, differences with a P value < 0.05 were considered statistically significant and one-way ANOVA analysis using the SPSS Predictive Analytics software version 18.0.
2.4. Scanning Electron Microscopy
Biofilms were obtained on glass coverslips (13 mm in diameter), previously treated with poly-L-lysine at 0, 24, 48, 72, and 96 h at 28˚C without shaking. After incubation, unattached cells were removed by pipetting and cells on coverslips were fixed with 2.5% glutaraldehyde and 1% OsO4 in PBS, ethanol dehydrated, critical point dried in a CO2 atmosphere in a Samdry-780A apparatus (Tousimis Research, USA), and gold coated in a Denton Vacuum Desk II (INXS, Inc., FL. USA). Coverslips containing the biofilms were attached to aluminum holders and analyzed using a SEM JEOL 65LV (JEOL, LTD. Japan). Digital images were recorded, and photocompositions were processed with Adobe Photoshop software.
2.5. Transmission Electron Microscopy
Several colonies of A. caviae Sch3 were resuspended in TSB (pH 6.0), and adjusted to a cell density of 3 × 109 cells/mL. Then, cultures were incubated at 28˚C for 48 h without shaking. Next, the bacterial suspension was placed on Formvar coated grids and negatively stained with 2% (w/v) uranyl acetate (pH 4.1) for 5 min at RT. Grids were observed under a JEOL 1400 transmission electron microscope at 80 keV (JEOL LTD, Japan). Digital images were obtained and processed with Adobe Photoshop software (USA).
3. Results
Biofilm structure varies with environmental conditions; indeed, different forms of biofilms exist (O’Toole et al. 2000) [15]. Therefore, we studied the influence of some of these factors involved in biofilm formation of A. caviae Sch3 in vitro, and then we studied the morphological changes in a mature biofilm.
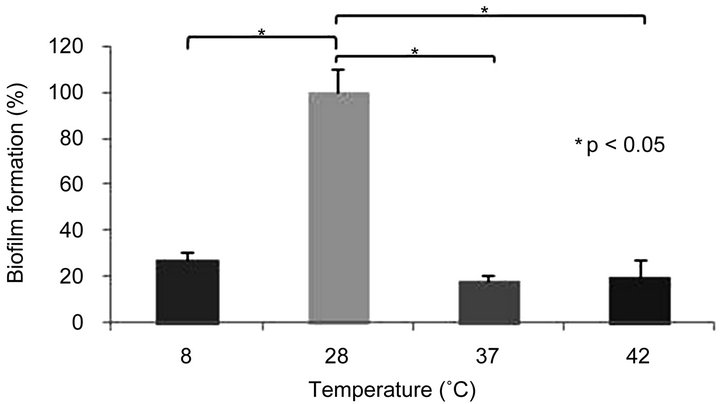
3.1. Influence of Temperature on Aeromonas caviae Sch3 Biofilm Formation
Cultures of A. caviae Sch3 were grown at 8˚C, 28˚C, 37˚C, and 42˚C for 48 h as described in Materials and methods. We considered the growth obtained at 28˚C as 100%, because this bacterium grows close to this temperature in nature [1]. Therefore, our results were normalized to this value. When cells were cultured at 8˚C, 37˚C, and 42˚C, biofilm formation dramatically decreased in 72%, 81%, and 80%, respectively, in relation to 28˚C (Figure 1(a)). We had guessed that the optimal temperature would be 37˚C, the human temperature. However, it is unknown if A. caviae Sch3 is able to form biofilms in humans.
3.2. Effect of pH on Aeromonas caviae Sch3 Biofilm Formation
Aeromonas has the capacity of growing at pH values between 6 to 8 [16]. Therefore, we tested the capability of this bacterium to form a biofilm at pH values of 5.0, 5.5, 6.0, 6.5, 7.0, and 8.0 at 28˚C for 48 h. We considered the culture obtained at pH 7.0 as 100%, and consequently, our results were normalized to this value. When cells were cultured at pH 5.0 and 5.5, we observed a reduction in biofilm formation of 53% and 28%, respectively. In contrast, at pH values of 6.0 and 6.5, the formation of biofilm increased 134% and 105%, respectively. Besides, when cells were cultured at pH 8.0, we observed an increase of only 31%. All these data indicate that the best pH value for an optimal formation of biofilm in vitro is 6.0 (Figure 1(b)).
3.3. Optimal Incubation Time for the Formation of Aeromonas caviae Sch3 Biofilm
Cultures of A. caviae were grown at pH 6.0 and 28˚C (the optimal conditions found in this work) for 24, 48, 72, and 96 h. We considered the incubation time of 48 h as 100% of biofilm production. When bacteria were cultured for 24 h, the formation of biofilm was 81% of the reference value. At longer incubation times, such as 72 h and 96 h, we observed a slight increase of 27% and 23%, respectively, in the formation of biofilm (Figure 1(c)).
3.4. Scanning Electron Microscopy of Aeromonas caviae Sch3 Biofilm Formation
Finally, we studied through SEM the characteristics of A. caviae Sch3 biofilm obtained on a polystyrene surface at optimal growth conditions in vitro at different incubation times. At 24 h, we observed few rod-shaped bacteria on the surface of the polystyrene dish (Figure 2(a)) with sizes varying between 1.2 and 4.6 mm in length. Some dividing cells are shown at an amplification of 10,000× (Figure 2(b)). When cells were observed at a larger amplification (20,000×), some cells exhibited a smooth surface while others were slightly rough. Moreover, we observed extracellular vesicular material (Figure 2(c), ar row). At 48 h, bacteria number increased (varying in size) attached to the polystyrene surface (Figure 2(d)). In Figure 2(e), most bacteria showed the smooth phenotype. At a larger magnification, some cells exhibited a rough phenotype, while others showed the smooth and semismooth phenotypes (Figure 2(f)). In addition, two cells
 (a)
(a)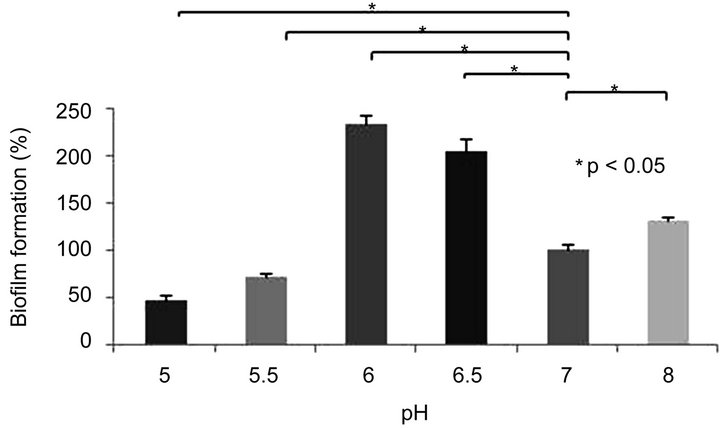 (b)
(b)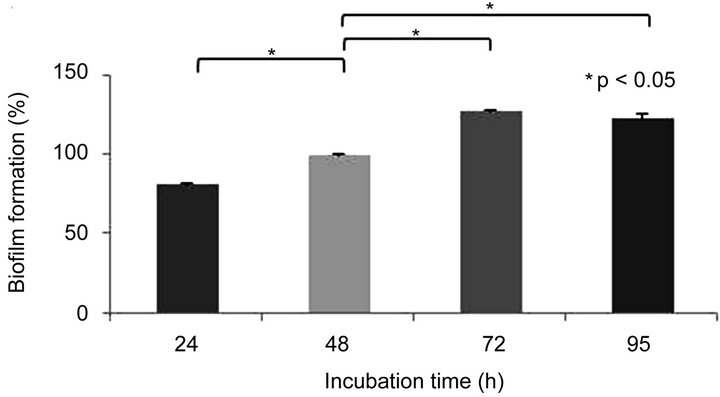 (c)
(c)
Figure 1. Biofilm formation of Aermonas caviae Sch3 at different conditions. Cells were grown in TSB medium without shaking. The amount of biofilm was determined by crystal violet method, as described in Materials and methods. (a) Effect of temperature; (b) Influence of pH; (c) Evaluation of incubation time in the biofilm formation. The analysis was done with Student t-test. Statically significance was set at P < 0.05 (*). Each bar represents the average of three replicates, and vertical lines represent standard errors.
that could be conjugating were seen (Figure 2(f), arrow). At 72 h of cultivation, an early stage in the biofilm formation was observed (Figure 2(g)). One characteristic identified at this time was the presence of chains of bacilli as larger as 7 to 8 mm forming a nest. Most of the cells presented no septum (Figure 2(h)). In Figure 2(i), a large amount of extracellular material containing many vesicles was detected, although some cells seemed to be damaged. After 96 h of incubation, a flat biofilm structure was found corresponding to the typical morphology of mature biofilms (Figure 2(j)) [9,17,18]. When the biofilm was analyzed at a magnification of 8500×, some
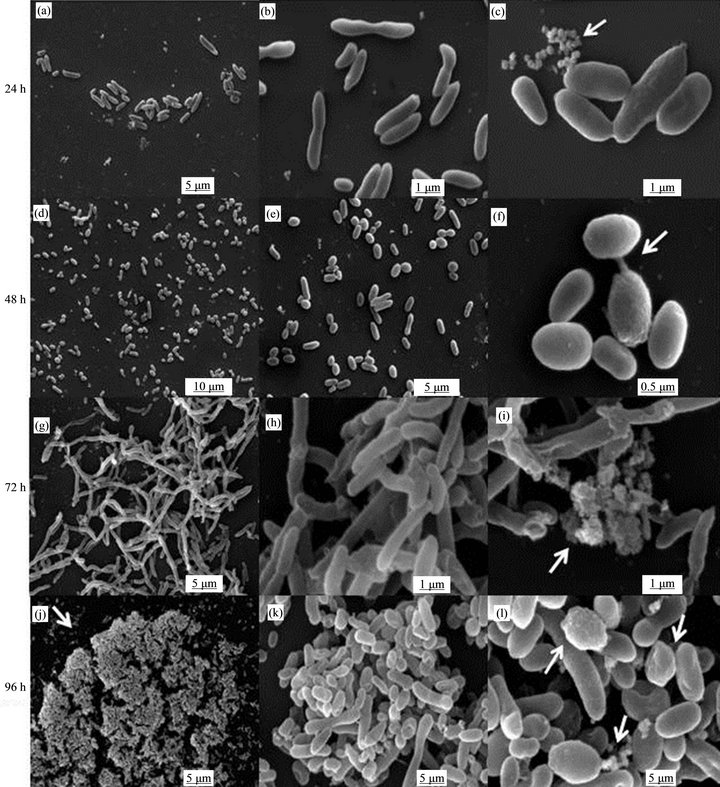
Figure 2. SEM micrographs of Aeromonas caviae Sch3 biofilm. Cells were grown on polyL-lysine-treateds attached glass coverslips at 28˚C in TSB and gently resuspended in PBS liquid medium without shaking after following incubation times: (a)-(c) 24 h (few bacterial cells attached to the surface); (d)-(f) 48 h (augment of number of attached cells); (g)-(i) 72 h (cell elongation, loose of bacterial septum); and (j)-(l) 96 hr (micro colonies, mature biofilm, multiple phenotypes). Arrows point out vesicles (c), (i), (l) or two putative conjugating bacteria (f) Mature biofilm (j) Different surface phenotypes (l). Scale bar = 0.5 μm - 5 μm.
groups of bacteria were forming microcolonies. Cells in these groups showed variation in size and shape. Most of them were from 1.9 to 2.8 mm in length, although some others measured 12.7 mm in length (Figure 2(k)). Finally, the mature biofilm at a magnification of 20,000× showed bacteria with the three surface phenotypes (smooth, semismooth, and rough) and also showed some extracellular vesicular material close to bacterial walls (Figure 2(l)).
3.5. Transmission Electron Microscopy of the Biofilm Formed by Aeromonas caviae Sch3 under Optimal Culture Conditions
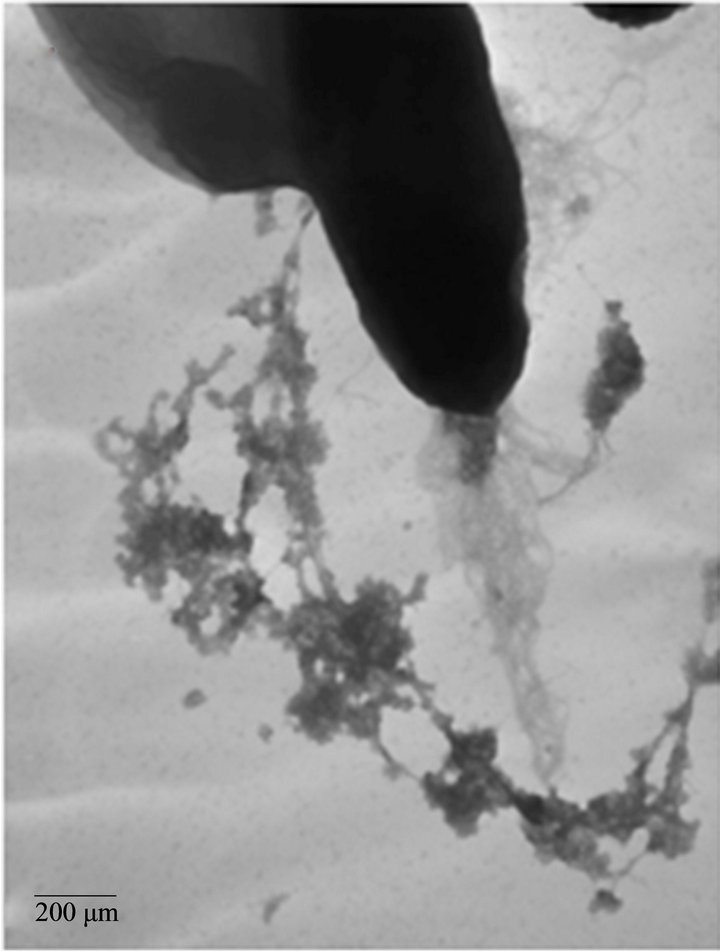
We analyzed the characteristics of A. caviae Sch3 biofilm formation under optimal conditions at 48 h by TEM. Figure 3(a) depicts one bacterial cell negatively stained by uranyl acetate. The presence of vesicular material in panel b was evident. We also corroborated the two different kinds of populations, previously observed by SEM; one of them was 4 μm in length whereas the other was only of 2 μm. Finally, panel c depicts a cell with several bacterial appendages.
4. Discussion
Recent studies have suggested that A. hydrophila, A. caviae, and A. veronii (bv veronii) are responsible for approximately 85% of total infections in humans caused by bacteria from this genus [2]. One of the pathogenicity mechanisms of these bacteria is the formation of biofilms in their hosts, which contribute to an increase in the virulence of these microorganisms and in their resistance to antibiotics, consequently, in their survival [6,19].
The formation of the biofilm has been thoroughly studied in E. coli, P. aeruginosa, and V. cholerae [20]. Several molecules have been identified to contribute to the formation of P. aeruginosa biofilm. The alginate, Psl and Pel exopolysaccharides are structural components of the biofilm’s matrix [21-23]. Each of them is encoded in
 (a)
(a)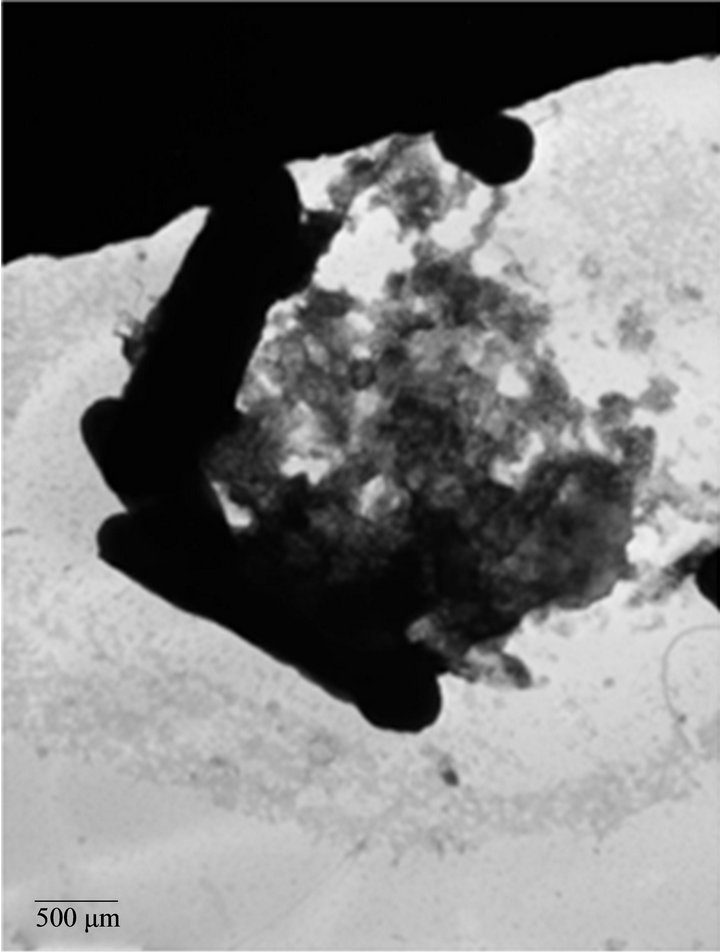 (b)
(b)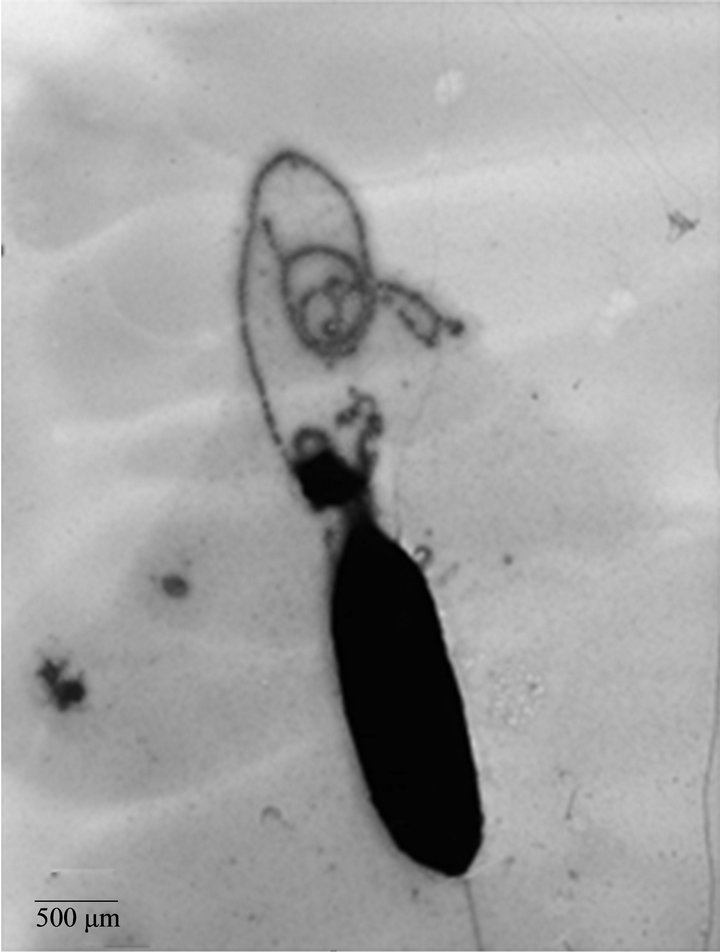 (c)
(c)
Figure 3. TEM micrographs of A. caviae Sch3 biofilm. Cells were grown at 28˚C in TSB medium for 48 h without shaking and placed on grids for negative staining with 2% (w/v) uranyl acetate. (a) Cells observed at 10,000× magnification; (b) and (c) Cells observed at 25,000× magnification. Scale bar = 200 μm - 500 μm
the algD, psl and pel operons, respectively [22,24]. Twelve genes constitute the algD operon. This operon is down-regulated by the MucA polypeptide by interacting with the AlgT/U sigma factor [23]. MucA degradation or truncation results in loss of the ability to interact with AlgT/U sigma factor, allowing it to bind to its promoter and potentiate alginate production, and, ultimately, inducing a conversion to a mucoid phenotype [25].
The effect of some physical, chemical, and genetical factors in the Aeromonas biofilm has not been studied in detail.
The genus Aeromonas has a remarkable capability to tolerate high pH values, for example, alkaline peptone water culture medium (pH 8.5 - 8.8), but these bacteria can grow also at pH values of 4.6 [26]. It is well known that tolerance to acidic conditions depends on the species of Aeromonas [27]. In our biofilm formation model, when bacteria were grown at pH 6.0 and 6.5, we observed an increase in biofilm formation (twice higher than that obtained at pH 7.0). At pH 5.0 or 5.5, the biofilm production was equal or lower than half the value obtained in the control, whereas at pH 8.0, it presented a modest increase. This result is different from those observed with P. aeruginosa, K. pneumoniae, and V. cholerae biofilm models, where maximum production was achieved between 7.5 and 8.5, although they also grew at a higher level at pH of 5.5 to 6.5 than that obtained at pH 7.0 [12]. With respect to the temperature effect we found that formation of biofilm was highly efficient at 28˚C. In other models, the temperatures assayed were only 30 and 37˚C showing no significant difference in biofilm production [12,28,29].
Our SEM results of the A. caviae Sch3 biofilm at optimal conditions revealed the presence of vesicular material with sizes ranging between 100 nm and 250 nm (Figure 3), which has not been previously reported. We do not know the composition of these vesicles. In Gram negative bacteria like P. aeruginosa, extracellular vesicles or outer membrane vesicles (OMVs) are composed of outer membrane proteins OprD, OprE, OprF, OprG, OprH, OprI, PagL, and PcoB, as well as lipopolysaccharides, phospholipids, and DNA [30]. These elements have been implicated in cell-cell communication, attachment, aggregation and biofilm formation [31]. One advantage of their compartmentalization in vesicles is that they can exert their function far away from the place, where they were produced [31]. OMVs can nucleate and maintain cohesion of biofilms in P. aeruginosa and Helicobacter pylori [32]. However, the production of these vesicles is not exclusive of sessile cultures, but planktonic cells can produce them also in different sizes and numbers [31,33].
In this study we observed changes in the bacterial morphology. Three phenotypes were related to the bacterial surface: smooth, semi-rough, and rough. Different sizes were also detected, finding bacterial elongation as large as 7 μm, with a consequent increase in bacterial surface that could facilitate attachment to the surface or to other bacteria. These characteristics could help in the establishment or maintenance of the biofilm [34-37]; their role remains to be determined. Biofilm development involves a series of steps starting with physicochemical interactions between microbial cells and substrate, followed by cell adhesion, multiplication, and differentiation, leading to the formation of mature biofilm. Some appendages were observed through TEM. Most of them were polar flagella. Very few lateral flagella were detected. In Aeromonas, polar and lateral flagella have been described as essential for biofilm formation [4,38]. However, more studies are needed to reveal their role in A. caviae Sch3 biofilm formation.
5. Conclusion
In order to unveil the process involved in biofilm development in Aeromonas caviae Sch3, we established an in vitro model under controlled laboratory conditions. The best conditions for the formation of the biofilm were a pH value of 6.0 and a temperature of 28˚C, which allowed us to know some microscopic characteristics of this biofilm, such as different changes in bacterial morphology, presence of vesicular material of 100 to 250 nm in size, and polar flagella.
6. Acknowledgements
We thank Dr. Jonathan Shaw for providing the Aeromonas caviae Sch3 strain used in this work. We also thank Sirenia Gónzalez from the Electron Microscopy facility and Mónica Mondragón from the Biochemistry Department, both from CINVESTAV-IPN for their technical help and EM technical assistance. We also acknowledge CONACyT for financial support to Dr. Juan Pedro LunaArias and for the scholarship granted to Erika Beatriz Angeles-Morales. This work was also supported by research and graduate office grants SIP 20100252 and SIP 20110191 from COFAA and EDI from the Instituto Politecnico Nacional to Dr. Graciela Castro Escarpulli.
REFERENCES
- J. L. Parker and J. G. Shaw, “Aeromonas spp. Clinical Microbiology and Disease,” Journal of Infection, Vol. 62, 2011, pp. 109-118. doi:10.1016/j.jinf.2010.12.003
- J. M. Janda and S. L. Abbott, “The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection,” Clinical Microbiology Reviews, Vol. 23, No. 1, 2010, pp. 35-73. doi:10.1128/CMR.00039-09
- A. K. Chopra and C. W. Houston, “Enterotoxins in Aeromonas-Associated Gastroenteritis,” Microbes and Infection, Vol. 1, 1999, pp. 1129-1137. doi:10.1016/S1286-4579(99)00202-6
- R. Gavín, S. Merino, M. Altarriba, R. Canals, J. G. Shaw and J. M. Tomás, “Lateral Flagella Are Required for Increased Cell Adherence, Invasion and Biofilm Formation by Aeromonas spp,” FEMS Microbiology Letters, Vol. 224, 2003, pp. 77-83. doi:10.1016/S0882-4010(03)00047-0
- L. Chen and Y. M. Wen, “The Role of Bacterial Biofilm in Persistent Infections and Control Strategies,” International Journal of Oral Science, Vol. 3, 2011, pp. 66-73. doi:10.4248/IJOS11022
- J. W. Costerton, P. S. Stewart and E. P. Greenberg, “Bacterial Biofilms: A Common Cause of Persistent Infections,” Science, Vol. 284, 1999, pp. 1318-1322. doi:10.1126/science.284.5418.1318
- B. Lee, J. A. Haagensen, O. Ciofu, J. B. Andersen, N. Høibyand S. Molin, “Heterogeneity of Biofilms Formed by Nonmucoid Pseudomonas aeruginosa Isolates from Patients with Cystic Fibrosis,” Journal of Clinical Microbiology, Vol. 43, 2005, pp. 5247-5255. doi:10.1128/JCM.43.10.5247-5255.2005
- H. Kobayashi, H. Watanabe, N. Ohgaki and H. Takeda, “Bacterial Adhesion and Biofilm,” Nihon Rinsho, Vol. 52, No. 2, 1994, pp. 332-338.
- H. Mikkelsen, Z. Duck, K. S. Lilley and M. Welch, “Interrelationships between Colonies, Biofilms, and Planktonic Cells of Pseudomonas aeruginosa,” Journal of Bacteriology, Vol. 189, 2007, pp. 2411-2416. doi:10.1128/JB.01687-06
- K. Harjai, R. K. Khandwahaa, R. Mittal, V. Yadav, V. Gupta and S. Sharma, “Effect of pH on Production of Virulence Factors by bIofilm Cells of Pseudomonas aeruginosa,” Folia Microbiologica, Vol. 50, 2005, pp. 99-102. doi:10.1007/BF02931455
- C. C. Goller and T. Romeo, “Environmental Influences on Biofilm Development,” Current Topics in Microbiology and Immunology, Vol. 322, 2008, pp. 37-66
- A. Hostacká, I. Ciznár and M. Stefkovicová, “Temperature and pH Affect the Production of Bacterial Biofilm,” Folia Microbiologica, Vol.55, 2010, pp. 75-78.
- S. M. Kirov, “Bacteria That Express Lateral Flagella Enable Dissection of the Multifunctional Roles of Flagella in Pathogenesis,” FEMS Microbiology Letters, Vol. 224, 2003, pp. 151-159. doi:10.1016/S0378-1097(03)00445-2
- S. M. Kirov, B. C. Tassell, A. B. Semmler, L. A. O’Donovan, A. A. Rabaan and J. G. Shaw, “Lateral Flagella and Swarming Motility in Aeromonas Species,” Journal of Bacteriology, Vol. 184, No. 2, 2002, pp. 547- 555. doi:10.1128/JB.184.2.547-555.2002
- G. O’Toole, H. B. Kaplan and R. Kolter, “Biofilm Formation as Microbial Development,” Annual Review of Microbiology, Vol. 54, 2000, pp. 49-79.
- S. Merino, X. Rubires, A. Aguilar and J. M. Tomás, “The Role of Flagella and Motility in the Adherence and Invasion to Fish Cell Lines by Aeromonas hydrophila Serogroup O: 34 Strains,” Microbiology Letters, Vol. 151, 1997, pp. 213-217. doi:10.1111/j.1574-6968.1997.tb12572.x
- C. Van Delden and B. H. Iglewski, “Cell-to-Cell Signaling and Pseudomonas aeruginosa Infections,” Emerging Infectious, Vol. 4, 1998, pp. 551-560. doi:10.3201/eid0404.980405
- E. P. Greenberg, “Bacterial Communication and Group Behavior,” Journal of clinical investigation, Vol. 112, 2003, pp. 1288-1290. doi:10.1172/JCI200320099
- J. Y. Hu, Y. Fan, Y. H. Lin, H. B. Zhang, S. L. Ong, N. Dong, J. L. Xu, W. J. Ng and L. H. Zhang, “Microbial Diversity and Prevalence of Virulent Pathogens in Biofilms Developed in a Water Reclamation System,” Research in Microbiology, Vol. 154, 2003, pp. 623-629. doi:10.1016/j.resmic.2003.09.004
- J. W. Costerton, Z. Lewandowski, D. Caldwell, D. R. Korber and H. M. Lappin-Scott, “Microbial Biofilms,” Annual Review of Microbiology, Vol. 49, 1995, pp. 711- 745. doi:10.1017/CBO9780511525353.005
- J. C. Davies, “Pseudomonas aeruginosa in Cystic Fibrosis: Pathogenesis and Persistence,” Paediatric Respiratory Reviews, Vol. 3, 2002, pp. 128-134. doi:10.1016/S1526-0550(02)00003-3
- C. Ryder, M. Byrd and D. J. Wozniak, “Role of Polysaccharides in Pseudomonas aeruginosa Biofilm Development,” Current Opinion in Microbiology, Vol. 10, 2007, pp. 644-648. doi:10.1016/j.mib.2007.09.010
- E. E. Mann and D. J. Wozniak, “Pseudomonas Biofilm Matrix Composition and Niche Biology,” FEMS Microbiology Reviews, 2012, in press. doi:10.1111/j.1574-6976.2011.00322.x
- M. Whiteley, M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory and E. P. Greenberg, “Gene Expression in Pseudomonas aeruginosa Biofilms,” Nature, Vol. 413, 2001, pp. 860-864. doi:10.1038/35101627
- A. P. Stapper, G. Narasimhan, D. E. Ohman, J. Barakat, M. Hentzer, S. Molin, A. Kharazmi, N. Høiby and K. Mathee, “Alginate Production Affects Pseudomonas aeruginosa Biofilm Development and Architecture, but Is Not Essential for Biofilm Formation,” Journal of Medical Microbiology, Vol. 53, 2004, pp. 679-690. doi:10.1099/jmm.0.45539-0
- S. A. Palumbo, M. M. Bencivengo, F. Del Corral, A. C. Williams and R. L. Buchanan, “Characterization of the Aeromonas hydrophila Group Isolated from Retail Foods of Animal Origin,” Journal of Clinical Microbiology, Vol. 27, 1989, pp. 854-859.
- H. Namdari and V. J. Cabelli, “The Suicide Phenomenon in Motile Aeromonads,” Applied and Environmental Microbiology, Vol. 55, 1990, pp. 543-547.
- S. M. September, F. A. Els, S. N. Venter and V. S. Brözel, “Prevalence of Bacterial Pathogens in Biofilms of Drinking Water Distribution Systems,” Journal of Water and Health, Vol. 5, 2007, pp. 219-227.
- G. Di Bonaventura, R. Piccolomini, D. Paludi, V. D’Orio, A. Vergara, M. Conter and A. Ianieri, “Influence of Temperature on Biofilm Formation by Listeria monocytogenes on Various Food-Contact Surfaces: Relationship with Motility and Cell Surface Hydrophobicity,” Journal of Applied Microbiology, Vol. 104, 2008, pp. 1552-1561. doi:10.1111/j.1365-2672.2007.03688.x
- S. Nakamura, Y. Higashiyama, K. Izumikawa, M. H. Seki, Kakeya, Y. Yamamoto, K. Yanagihara, Y. Miyazaki, Y. Mizuta and S. Kohno, “The Roles of the Quorum-Sensing System in the Release of Extracellular DNA, Lipopolysaccharide, and Membrane Vesicles from Pseudomonas aeruginosa,” Japanese Journal of Infectious Diseases, Vol. 61, 2008, pp. 375-378.
- A. Kulp and M. J. Kuehn, “Biogenesis of Secreted Bacterial Outer Membrane Vesicles,” Annual Review of Microbiology, Vol. 64, 2010, pp. 163-184. doi:10.1146/annurev.micro.091208.073413
- S. R. Schooling, A. Hubley and T. J. Beveridge, “Interactions of DNA with Biofilm-Derived Membrane Vesicles,” Journal of Bacteriology, Vol. 191, 2009, pp. 4097-4102. doi:10.1128/JB.00717-08
- L. Mashburn-Warren, R. J. McLean and M. Whiteley, “Gram-Negative Outer Membrane Vesicles: Beyond the Cell Surface,” Geobiology, Vol. 6, 2008, pp. 214-219. doi:10.1111/j.1472-4669.2008.00157.x
- M. E. Shirtliff, J. T. Mader and A. K. Camper, “Molecular Interactions in Biofilms,” Chemistry & Biology, Vol. 9, 2002, pp. 859-871. doi:10.1016/S1074-5521(02)00198-9
- P. Stoodley, K. Sauer, D. G. Davies and J. W. Costerton, “Biofilms as Complex Differentiated Communities,” Annual Review of Microbiology, Vol. 56, 2002, pp. 187-209. doi:10.1146/annurev.micro.56.012302.160705
- P. S. Stewart and M. J. Franklin, “Physiological Heterogeneity in Biofilms,” Nature Reviews Microbiology, Vol. 6, 2008, pp. 199-210. doi:10.1038/nrmicro1838
- M. Alhede, K. N. Kragh, K. Qvortrup, M. Allesen-Holm, M. Van Gennip, L. D. Christensen, P. Ø. Jensen, A. K. Nielsen, M. Parsek, D. Wozniak, S. Molin, T. TolkerNielsen, N. Høiby, M. Givskov and T. Bjarnsholt, “Phenotypes of Non-Attached Pseudomonas aeruginosa Aggregates Resemble Surface Attached Biofilm,” PLoS One, Vol. 6, 2011, pp. 927-943. doi:10.1371/journal.pone.0027943
- R. Canals, S. Vilches, M. Wilhelms, J. G. Shaw, S. Merino and J. M. Tomás, “Non-Structural Flagella Genes Affecting Both Polar and Lateral Flagella-Mediated Motility in Aeromonas hydrophila,” Microbiology, Vol. 153, 2007, pp. 1165-1175. doi:10.1099/mic.0.2006/000687-0
NOTES
*The authors declare that they have no competing interests.
#Corresponding author.

