Journal of Water Resource and Protection
Vol. 3 No. 9 (2011) , Article ID: 7518 , 6 pages DOI:10.4236/jwarp.2011.39075
Arsenic(III) Remediation from Contaminated Water by Oxidation and Fe/Al Co-Precipitation
1CSIRO Process Science and Engineering, CSIRO, Bentley, Karawara, Australia
2School of Chemical and Mathematical Sciences, Murdoch University, Murdoch, Australia
E-mail: wensheng.zhang@csiro.au, {P.Singh, T.Issa}@murdoch.edu.au
Received March 14, 2011; revised June 15, 2011; accepted August 17, 2011
Keywords: Manganese oxides, Iron hydroxides, Arsenic remediation, Fe/Al coagulants, Contaminated water
ABSTRACT
Battery grade γ-MnO2 powder was investigated as an oxidant and an adsorbent in combination with Fe/Al coagulants for removal of arsenic from contaminated water. Simultaneous oxidation of As(III) and removal by coprecipitation/adsorption (one step process) was compared with pre-oxidation and subsequent removal by coprecipitation/ adsorption (two step process). The rate of As(III) oxidation with MnO2 is completed in two stages: rapid initially followed by a first order reaction. As(III) is oxidised to As(V) by the MnO2 with a release of approximately 1:1 molar Mn(II) into the solution. No significant pH effect on oxidation of As(III) was observed in the pH range 4 - 6. The rate showed a decreasing trend above pH 6. The removal of As(V) by adsorption on the MnO2 decreased significantly with increasing pH from 4 to 8. The adsorption capacity of the γ-MnO2 with particle size 90% passing 10 µm was determined to be 1.5 mg/g at pH 7. MnO2 was found to be more effective as an oxidant for As(III) in the two step process than in the one step process.
1. Introduction
Arsenic in contaminated groundwater occurs largely as arsenite (As(III)). [1] Effective and complete removal of arsenic by adsorption/coprecipitation methods requires pre-oxidation of As(III) to As(V). Oxygen or air is a cheap but kinetically slow oxidant for As(III). Various other oxidants for As(III) have been reported in the literature, including permanganate (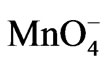 ), [2-4] ozone (O3), [5] hydrogen peroxide (H2O2), [6] chlorine (Cl2), [7-10] or hypochlorite (ClO−), [11-13] catalyzed sulphite/O2 (air) mixture, [14,15] and UV catalyzed systems. [16-19] These oxidants are effective but are either costly, or need rigid process controls for efficient oxidation. In recent years, manganese oxides, in both synthetic and natural forms, have been investigated for oxidation of As(III) [20-26].
), [2-4] ozone (O3), [5] hydrogen peroxide (H2O2), [6] chlorine (Cl2), [7-10] or hypochlorite (ClO−), [11-13] catalyzed sulphite/O2 (air) mixture, [14,15] and UV catalyzed systems. [16-19] These oxidants are effective but are either costly, or need rigid process controls for efficient oxidation. In recent years, manganese oxides, in both synthetic and natural forms, have been investigated for oxidation of As(III) [20-26].
Oscarson et al. [27] found that the oxidation of As(III) by birnessite, cryptomelane, and pyrolusite obeyed the first-order rate law with the rate constants at 298 K being 0.267, 0.189 and 0.44 × 10−3 h−1, respectively. However, Chen and Fang [28] reported that the oxidation rate of As(III) by MnO2 was rapid initially followed by a firstorder kinetics with respect to As(III) concentration. The activation energies for the oxidation reaction by the MnO2 were measured to be in the range 26.0 - 32.3 kJ/mol [27]. The oxidation process was reported to be limited by diffusion of the reactant As(III) to or the reaction products away from the surface [27-29].
Scott and Morgan [29] proposed a surface mechanism that As(III) anion forms an inner-sphere complex followed by electron transfer between the surface metal ion and As(III) anion. The adsorption of As(III) on the surface was the slowest step. The surface mechanism was supported by the observation that the rate of As(III) oxidation directly depended on the concentration of surfacebound As(III) [30]. A mechanism of production of an intermediate reaction product, Mn(III) hydroxyl (MnOOH*), was proposed by Nesbitt et al. [31].
2MnO2 + H3AsO3 = 2MnOOH* + H3AsO4(1)
2MnOOH* + H3AsO3 = 2MnO + H3AsO4 + H2O (2)
Various forms of Mn oxides as adsorbent for arsenic removal have also been investigated, including pyrolusite and cryptomelane, [32] combination of pyrolusite with granular ferric hydroxide, [30] natural manganese oxides in a packed bed or column, [33] ferruginous manganese ore (FMO), [34] Mn dioxide-coated sand (MDCS), [35, 36] and Bi-enhanced Mn oxides [37]. Chiu and Hering [30] compared the adsorption capacities for different types of Mn oxides. They found that the surface saturation for pyrolusite and cryptomelane at pH 6.5 for As(V) species were 0.75 and 1.87 mg/g, respectively. The difference in the adsorption capacity was attributed to their crystallinity and specific surface areas. Poorly crystalline birnessite and cryptomelane possess higher specific surface areas than highly ordered pyrolusite [29].
This paper reports the investigation of MnO2 as an oxidant for As(III) and as adsorbent in combination with Fe/Al coagulants for As(V) removal. The adsorption capacity of MnO2 for As(V) was compared with commonly used iron and aluminium hydroxide adsorbents under similar experimental conditions. Simultaneous oxidation and coprecipitation/adsorption (one step process), and pre-oxidation followed by coprecipitation/adsorption (two step processes) were investigated for various combinations of MnO2 with in-situ formed Fe/Al hydroxides. The objectives of this work were to determine the suitability of MnO2 as an oxidant and adsorbent in combination with Fe/Al hydroxide for arsenic removal.
2. Materials and Methods
First, Battery grade g-MnO2 powder with particle size 90% passing 10 µm, supplied by Aldrich Australia, was used for all the experiments. All the other chemicals used were of AR grade without further treatment. As(III) stock solution was prepared from As2O3 in accordance with the procedure provided by Vogel [38]. As(V) stock solution was prepared by dissolving Na2HAsO4 in demonized water. Fe(III) and Al(III) stock solutions were prepared from their chloride salts. Solution pH was adjusted with dilute HCl and NaOH solutions.
All the experiments were conducted at 25˚C in 250 ml conical flasks equipped with magnetic stirring units for liquid-solid mixing. In the one step process, a dose of MnO2 and a desired volume of equal molar Fe(III)/Al(III) solution were simultaneously added to water containing known amount of As(III) or As(V). Solution pH was adjusted and maintained at the desired value throughout the experiment. Samples were taken and filtered through a 0.2 µm membrane filter. The filtrate was analysed for As(III) and As(V) by hydride generation followed by inductively coupled plasma and atomic emission spectroscopy (ICP-AES), and for total soluble Mn by ICPAES at the Marine and Freshwater Research Institute, Environmental Science, Murdoch University, Western Australia. For the two step process, the arsenic bearing solution was first treated with MnO2 followed by adsorption/precipitation with Fe(III)/Al(III) coagulants at pH 7. All the other procedures were the same as the one step process.
3. Results and Discussion
3.1. Oxidation of Arsenic(III) by MnO2
3.1.1. Stoichiometry of Oxidation of As(III)
The stoichiometry of oxidation of As(III) by MnO2 was determined by measuring residual reactants and reaction products after 2 hours contact of one gram of the MnO2 powder with initial 1 ppm As(III) solution at pH 7 in the absence of oxygen maintained by bubbling nitrogen gas through the solution. The analysis results for residual concentrations of As(III), As(V) and Mn(II) in the final solution are given in Table 1. The important observations are:
No As(III) remained in the solution, indicating that all the As(III) was oxidized to As(V).
The residual arsenic in the solution accounted for only 80% of the amount initially present in the reaction mixture.
The solid phase contained the remaining 20% of the arsenic which could be assumed to be As(V).
The solution contained Mn(II) as much as would be expected if all the reacted MnO2 were converted to Mn(II) during its reaction with As(III). Thus, the oxidation of As(III) was accompanied by a reduction of MnO2 yielding Mn(II) into solution at an approximately equal molar stoichiometry with respect to the total oxidized As(III):
Mn(IV) + As(III) = Mn(II) + As(V) (3)
3.1.2. Rate of Oxidation of As(III)
The rate of As(III) oxidation are plotted in Figure 1 and analyzed with respect to the first order rate law:
Ln[As(III)]/[As(III)] = kt(4)
where [As(III)] is the initial As(III) concentration (mg/L), [As(III)] the concentration at time t (min), k the rate constant (min–1) which is a function of MnO2 dose and tem-
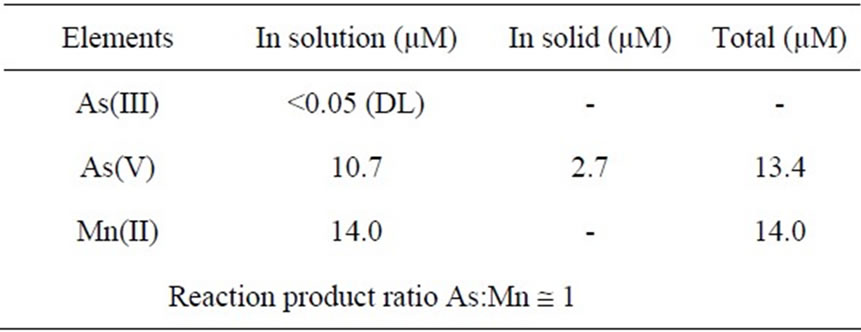
Table 1. Concentrations of reaction products in the final solutions after 2 hour contact time at pH 7 and 25˚C. Initial 1 ppm As(III), 1 g/L MnO2.
perature. As can be seen in Figure 1, the oxidation of As(III) by MnO2 could be characterized by very fast kinetics within the first 30 minutes, followed by a first order rate which is indicated by the fact that Ln[As(III)]/ [As(III)] vs reaction time t is graphically linear. The rate increases with MnO2 dose, suggesting that the reaction depends on surface area or available reaction sites on the surface of MnO2. This two stage kinetic feature was also observed by Chen and Fang [28]. The slow-down in the rate at later stage of the reaction is indicative of competition for active adsorption sites between As(III) and As(V).
3.1.3. Effect of pH on Oxidation of As(III)
The pH effect was investigated by varying solution pH in the range 4 - 8 and measuring the residual As(III) in solution after 2 hour contact time for each fixed pH. The initial As(III) concentration was 6 mg/L. It was observed that about 80% of the As(III) ions was oxidized for each pH in two hours in the pH range 4 - 6. Above pH 6, a decreased trend occurred up to pH 8. This is likely to be caused by formation of manganese hydroxide on the surface which blocks some sites for reaction with As(III) on the surface.
3.2. MnO2 as Adsorbent
3.2.1. Effect of pH on As(V) Adsorption on MnO2
Figure 2 shows that the %As(V) adsorption decreases linearly when solution pH increases from pH 4 to pH 8. This effect can be explained by the surface charge characteristics of the MnO2 phase. The point of zero charge (PZC) of chemically or electrochemically prepared MnO2 materials such as α-MnO2, γ-MnO2 and δ-MnO2 lies in the pH range 1.5 - 4.15 [39,40]. Therefore, it is not surprising to observe the decreasing effect because the adsorption of As(V) species must overcome the increased
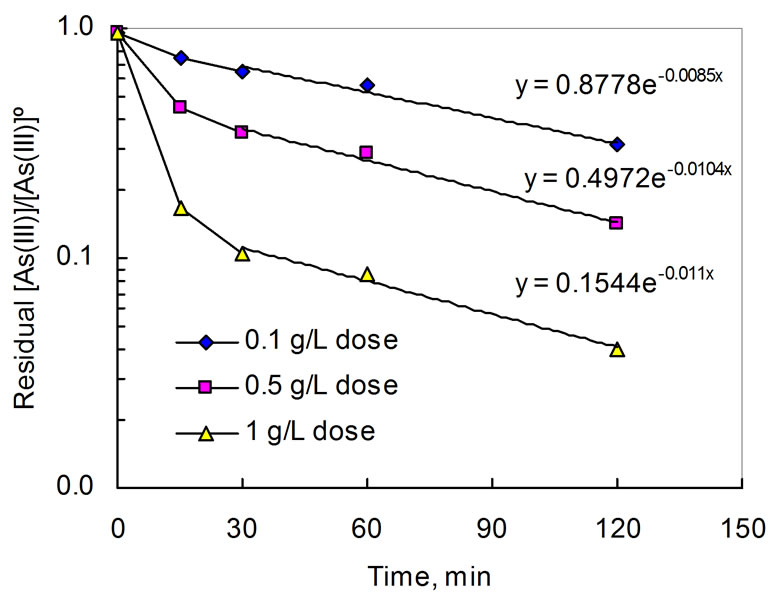
Figure 1. Ln [As(III)]/[As(III)]˚ vs time. Initial 5 mg/L As(III), pH 7, 25˚C.
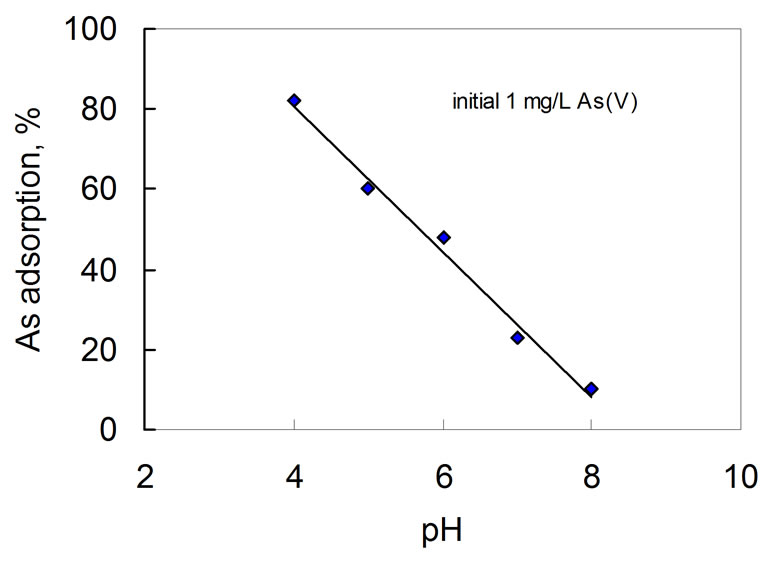
Figure 2. Effect of solution pH on adsorption of As(V) on the MnO2.
repulsion force as pH rose above the PZC. Additionally, the proportion of the more negatively charged As(V) species increases as the solution pH increases, e.g. 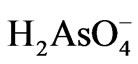 ions dominate in pH range 2 - 6.5. The
ions dominate in pH range 2 - 6.5. The 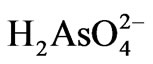 ions on the other hand dominate in pH range 6.5 - 11.8. This should also contribute to the observed pH effect.
ions on the other hand dominate in pH range 6.5 - 11.8. This should also contribute to the observed pH effect.
3.2.2. Effect of As(V) Concentration
The effect of As(V) concentration on As(V) adsorption was investigated by varying initial As(V) concentration at a fixed dose of 1 g/L MnO2 at pH 7. As shown in Figure 3, the results reasonably fit the Langmuir isotherm model:
Ce/Qe = 1/bQ˚ + Ce/Q(5)
where, Ce (mmol/L) is the equilibrium concentration in the solution, Qe (mmol/g) is the amount adsorbed on the adsorbent at equilibrium, Q˚ and b are the Langmuir constants related to adsorption capacity and binding energy of adsorption respectively. From the slope of best fit, the adsorption capacity of the γ-MnO2 is determined to be 1.5 mg/g.
For comparison, the adsorption capacities of commonly used iron and aluminum hydroxide adsorbents were also similarly studied and the reagents summarized in Table 2. The published data from the literature are also included in the table. As seen, the adsorption capacity of MnO2 increased in the order b-MnO2 < g-MnO2 < a-MnO2 << δ-MnO2. Clearly, the capacity depended on the form of MnO2 and its preparation method which determine the crystalline properties and surface area. For example, the amorphous δ-MnO2 possesses highest surface area and thus highest adsorption capacity compared with other well crystalline forms of MnO2. However, the capacity of the amorphous δ-MnO2 is found to be significantly lower than the amorphous iron hydroxide (ferrihydrite). The capacity of As(V) removal also depends on the method used. The removal of As(V) by adsorp-
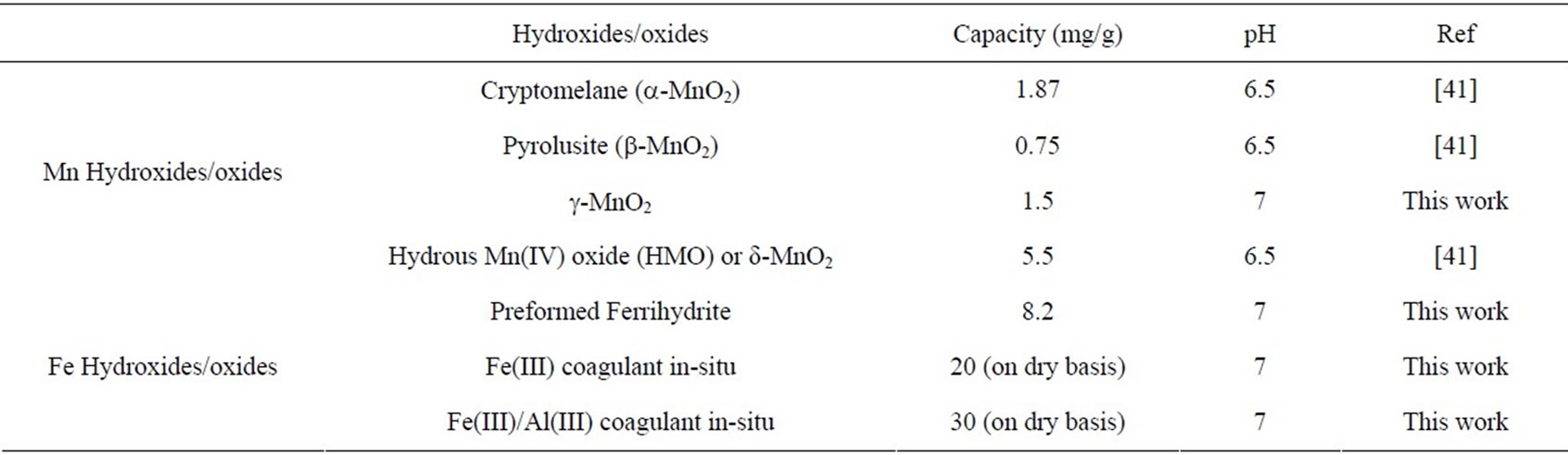
Table 2. Comparison of adsorption capacity of Mn oxides with iron oxides.
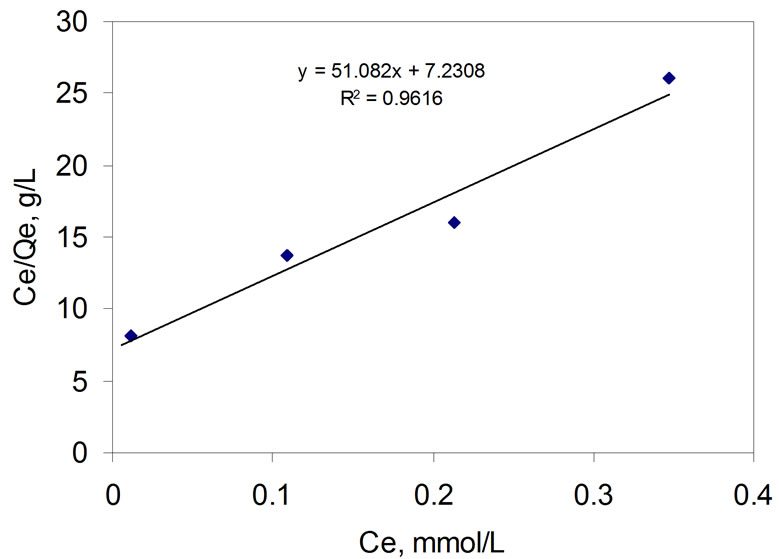
Figure 3. Langmuir plot for adsorption of As(V) on the MnO2 at pH 7.
tion/precipitation in-situ with Fe(III) or Fe(III)/Al(III) coagulants is much more effective and efficient than the preformed ferrihydrite (Table 2).
3.3. Removal of As(III) by MnO2 and Fe(III)/Al(III) Coagulants
The efficiency of As(III) removal by adsorption on Fe/Al hydroxides was investigated in two ways:
One step process: this involved simultaneous addition of MnO2 oxidant and the Fe or Fe/Al coagulant followed by pH adjustment.
Two step process: this involved pre-oxidation of As(III) by MnO2 and subsequent removal of As(V) by coprecipitation/adsorption on in-situ formed Fe or Fe/Al hydroxides.
• One step process As shown in Figure 4, the residual arsenic in solution decreased exponentially from 0.2 mg/L to 0.02 mg/L as the MnO2 dose increased from 0.1 g/L to 0.5 g/L. A dose of 1 g/L of MnO2 was needed to lower the residual arsenic to 0.01 mg/L (standard drinking water limit by US Environmental Protection Agency). This reflects the relative inefficiency of the one step process for removal
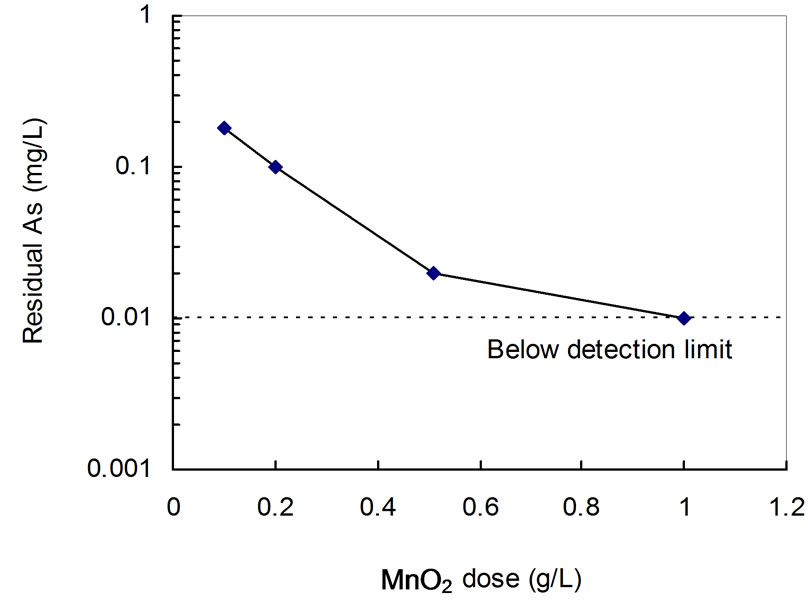
Figure 4. Effect of MnO2 dose on removal of arsenic by coprecipitation with Fe/Al coagulant. Initial 1 mg/L As(III), pH 7, molar ratio (Fe:Al = 1:1):As = 50:1.
of very low level of arsenic.
• Two step processes The results can be summarized as follows:
Fe/Al alone is an effective adsorbent for As(V) (<0.01 mg/L, Expt. Code 5) but poor for As(III) (0.24 mg/L, Expt. Code 4).
MnO2 alone is effective for As(III) oxidation (Expt. Code 6) but relatively poor adsorbent for As(V) (Expt. Code 7) compared with Fe/Al adsorbent.
Combination of MnO2 with Fe/Al in the one step process with initial As(III) effectively lowered the residual As to 0.01 - 0.02 mg/L (Expt. Code 2).
The two step process is more effective for arsenic removal (<0.01 mg/L residual As, Expt. Code 1) than the one step process (0.01 - 0.02 mg/L residual As, Expt. Code 2), but requires additional 2 hours for pre-oxidation.
Soluble Mn(II) was effectively removed from the system in both one-step and two-step processes.
The arsenic removal is not affected when MnO2, Fe/Al, and As(V) are co-present in the one step process (Expt. Code 3).
The As(III) removal by the one step process is relatively poor compared with the two step process. Two factors are likely to affect the removal process. First, the in-situ formed amorphous Fe/Al ‘hydroxide’ may partially cover the surface of MnO2, which slows down the diffusion process of As(III) to its surface. Second, the As(III) initially adsorbed on the surface of Fe/Al “hydroxide” is desorbed by As(V) ions, establishing a new equilibrium. As(III), which is known to occur as unionised H3AsO3 in the pH range 2 - 9 is weakly bonded on the surface of ferrihydrite compared with As(V) which occur as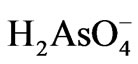 ,
,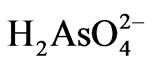 . The As(V) species is strongly bonded on the surface via specific inner sphere adsorption mechanism. Furthermore, the As(III) adsorbed on the surface of ferrihydrite may need further treatment to oxidize the As(III) to As(V) for safe disposal. The cost for this treatment may not justify using the one step process in real applications where safe disposal is the prime objective.
. The As(V) species is strongly bonded on the surface via specific inner sphere adsorption mechanism. Furthermore, the As(III) adsorbed on the surface of ferrihydrite may need further treatment to oxidize the As(III) to As(V) for safe disposal. The cost for this treatment may not justify using the one step process in real applications where safe disposal is the prime objective.
4. Conclusions
As(III) is oxidized to As(V) by the γ-MnO2 with almost equal molar stoichiometric release of Mn(II) into solution. The rate of the oxidation is characterised by an initial fast kinetics followed by a first order rate reaction. No significant variation in the oxidation rate occurs in the pH range 4 - 8, except for a decreased trend at pH above 6, is observed. The adsorption of As(V) with the γ-MnO2 is favored in low pH 4 and decreases rapidly where the pH rises to 8. The adsorption capacity of MnO2 (90% passing 10 µm) is 1.5 mg (As(V)/g at pH 7. The MnO2 is more efficient when used as an oxidant in the two step process than in the one step process. All the soluble Mn(II) ions are removed in the solution in both the processes.
5. Acknowledgment
The authors are thankful to DEST, Australia for financial support under INDO-AUS Strategic Research Fund Scheme.
REFERENCES
- G. H. Khoe, M. T. Emett, M. Zaw and P. Prasad, “Arsenic Removal from Tubewell Water in Bangladesh, Report,” Australian Nuclear Science & Technology Organisation CRC for Waste Management & Pollution Control ANSTO/C585, CRC/1956R, August 1999.
- W. Driehaus and M. Jekel, “Oxidation Process for Trivalent Arsenic,” DVGW-Schriftenr, Wasser, Vol. 82, 1993, pp. 55-69.
- T. Phommavong, T. Viraraghavan and K. S. Subramanian, “Removal of Arsenic with KMnO4. Oxidation and Manganese Greensand Filtration,” Proceedings of the Annual Conference on West Canadian Water Wastewater Association, 48th, 1996.
- Y. Li, M. Xi, F. L. Kong and C. Y. Yu, “Experimental Study on the Removal of Arsenic in Waste Water from Semiconductor Manufacturing,” Journal of Water Resource and Protection, Vol. 1, 2009, pp. 48-51.
- T. Nishimura and Y. Umetsu, “Removal of As(III), Arsenic(V) with Manganese from Aqueous Solution by Ozonation,” Impurity Control and Disposal in Hydrometallurgical Processes, Toronto, 21-24 August 1994, pp. 91-100.
- M. Pettine and F. J. Millero, “Effect of Metals on the Oxidation of As(III) with H2O2,” Marine Chemistry, Vol. 70, No. 1-3, 2000, pp. 223-234. doi:10.1016/S0304-4203(00)00028-1
- T. Chen, S. J. Kang, K. P. Olmstead, M. D. Rathsack and J. R. Porter, “Removal of Arsenic from BTEX-Contaminated Groundwater,” Water Environment Federation Technical Conference 1996 (WEFTEC’96), 69th Annual Conference & Exposition, 1996.
- E. Nieminski and D. Evans, “Pilot Testing of Trace Metals Removal with Ozone at Snowbird Ski Resort,” Ozone: Science & Engineering, vol. 17, 1995, pp. 297-309.
- Gaid, I. Raguenes and P. Ravarini, “Arsenic Removal at Baudricourt (Vosges, France) Drinkable Water Plant,” Industry Water Pollution, vol. 205, 1997, pp. 54-58.
- S. P. Pande, L. S. Deshpande, P. M. Patni and S. L. Lutade, “Arsenic Removal Studies in Some Groundwaters of West Bengal, India,” Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering, vol. A32, No. 7, 1997, pp. 1981-1987.
- R. K. Sinha, A. M. Bandyopadhyaya, B. Roy, G. Poddar, I. Chakravarty, A. Majumdar and K. J. Nath, “Use of Some Iron Salts on Total Removal of Arsenic from Arsenical Tubewell Water of West Bengal,” Journal of the Institution of Chemists, Vol. 65, No. 1, 1993, pp. 30-31.
- K. Sano and S. Hosudo, “Removal of Arsenic from Water,” Jpn. Kokai Tokkyo Koho., Kotobuki Kakoki Kk, Japan, 1995, p. 5.
- H. Naito, “Apparatus and Method for Removing Tace Arsenic from Groundwater or Pond Water,” Jpn. Kokai Tokkyo Koho, NBL K. K., Japan, 1997, 6 Pages.
- W. Zhang, P. Singh and D. M. Muir, “Kinetics of Oxidation of As(III) with SO2/O2 and UV Light,” In: C. A. Young, Ed., Proceedings of Minor Elements 2000: Processing and Environmental Aspects of As, Sb, Se, Te, and Bi, SME, Littleton CO, 2000, pp. 333-343.
- T. Nishimura, Q. Wang and Y. Umetsu, “Removal of Arsenic from Process Liquors by Oxidation of Iron(II), Arsenic(III) and Sulfur(IV) with Oxygen,” In: J. E. Dutrizac and G. B. Harris, Eds., Iron Control and Disposal, CIM, Montreal Quebec, 1996, pp. 535-548.
- J. Bednar, J. F. Ranville, T. R. Wildeman, J. R. Garbarino, P. J. Lamothe and K. S. Smith, “Remediation Approaches Using Photooxidation of Inorganic and Organic Arsenic Species,” ACS National Meeting, American Chemical Society, Division Environmental Chemistry, vol. 41, 2001, pp. 253-256.
- M. T. Emett and G. H. Khoe, “Photochemical Oxidation of Arsenic by Oxygen and Iron in Acidic Solutions,” Water Research, vol. 35, No. 3, 2001, pp. 649-656. doi:10.1016/S0043-1354(00)00294-3
- G. H. Khoe, M. Zaw, P. S. Prasad and M. T. Emett, “Photo-Assisted Oxidation of Inorganic Species in Aqueous Solutions,” PCT Int. Appl. Wo, CRC for Waste Management & Pollution Control Limited, Australia; Australian Nuclear Science and Technology Organisation, 1999, 30 p.
- S. J. Hug, L. Canonica, M. Wegelin, D. Gechter and U. von Gunten, “Solar Oxidation and Removal of Arsenic at Circumneutral pH in Iron Containing Waters,” Environmental Science & Technology, Vol. 35, No. 10, 2001, pp. 2114-2121.doi:10.1021/es001551s
- L. Carlson and U. Schwertmann, “Iron and Manganese Oxides in Finnish Ground Water Treatment Plants,” Water Research, Vol. 21, No. 2, 1987, pp. 165-170. doi:10.1016/0043-1354(87)90045-5
- M. Ogawa, M. Kato, T. Okuyama and T. Saito, “Removal of As from Water by Manganese Dioxide,” Shigen Kankyo Taisaku, Vol. 37, 2001, pp. 1459-1468.
- T. Takamatsu, M. Kawashima and M. Koyama, “The Role of Manganese(2+)-Rich Hydrous Manganese Oxide in the Accumulation of Arsenic in Lake Sediments,” Water Research, Vol. 19, No. 8, 1985, pp. 1029-1032. doi:10.1016/0043-1354(85)90372-0
- D. W. Oscarson, P. M. Huang, C. Defosse and A. Herbillon, “Oxidative Power of Manganese(IV) and Iron(III) Oxides with Respect to Arsenic(III) in Terrestrial and Aquatic Environments,” Nature, Vol. 291, No. 5810, 1981, pp. 50-51.
- B. A. Manning, S. E. Fendorf and D. L. Suarez, “Arsenic(III) Complexation and Oxidation Reactions on Environmental Surfaces,” Environmental Science & Technology, Vol. 36, No. 5, 2002, pp. 976-981.
- B. A. Manning, S. E. Fendorf and D. L. Suarez, “Arsenic(III) Complexation and Oxidation Reactions on Soil,” ACS Symposium Series, Vol. 835, 2003, pp. 55-69
- B. A. Manning, E. Fendorf Scott, B. Bostick, and L. Suarez Donald, “Arsenic(III) Oxidation and Arsenic(V) Adsorption Reactions on Synthetic Birnessite,” Environmental Science & Technology, Vol. 36, 2002, pp. 976- 981.
- D. W. Oscarson, P. M. Huang, W. K. Liaw and U. T. Hammer, “Kinetics of Oxidation of Arsenite by Various Manganese Dioxides,” Soil Science Society of America Journal, Vol. 47, 1983, pp. 644-648. doi:10.2136/sssaj1983.03615995004700040007x
- H. Chen and S. Fang, “Study on Oxidation of As(III) to As(V) by MnO2 in wastewater,” Gaoxiao Huaxue Gongcheng Xuebao, Vol. 14, 2000, pp. 48-52.
- M. J. Scott and J. J. Morgan, “Reactions at Oxide Surfaces. 1. Oxidation of As(III) by Synthetic Birnessite,” Environmental Science and Technology, Vol. 29, 1995, pp. 1898-1905. doi:10.1021/es00008a006
- V. Q. Chiu and J. G. Hering, “Oxidation State of Arsenic on Manganite (.Gamma.-MnOOH) Surfaces,” 217th ACS National Meeting, Anaheim, 21-25 March 1999.
- H. W. Nesbitt, G. W. Canning and G. M. Bancroft, “XPS Study of Reductive Dissolution of 7 A-Birnessite by H3AsO3, with Constraints on Reaction Mechanism,” Geochimica et Cosmochimica Acta, Vol. 62, No. 12, 1998, pp. 2097-2110.
- S. Ouvrard, M.-O. Simonnot and M. Sardin, “Reactive Behavior of Natural Manganese Oxides toward the Adsorption of Phosphate and Arsenate,” Industrial & Engineering Chemistry Research, Vol. 41, No. 11, 2002, pp. 2785-2791.
- S. Chakravarty, V. Dureja, G. Bhattacharyya, S. Maity and S. Bhattacharjee, “Removal of Arsenic from Groundwater Using Low Cost Ferruginous Manganese Ore,” Water Research, Vol. 36, No. 3, 2002, pp. 625-632. doi:10.1016/S0043-1354(01)00234-2
- S. Bajpai and M. Chaudhuri, “Removal of Arsenic from Ground Water by Manganese Dioxide-Coated Sand,” Journal of Environmental Engineering, Vol. 125, No. 8, 1999, pp. 782-784.
- T. Kasai, H. Koyanaka, J. Aizawa and Y. Fujimoto, “Removal of Arsenic Ion from Aqueous Solution with Manganese Oxide,” Nippon Bunri Daigaku Kiyo, Vol. 28, No. 2, 2000, pp. 81-86.
- T. Kasai and H. Koyanaka, “Removal of Arsenic Ion with Manganese Oxide Compounds,” Transactions of the Materials Research Society of Japan, Vol. 27, 2002, pp. 463- 466.
- J. J. Wen and L. H. Odell, “Case Studies on Concurrent Arsenic Removal in Pyrolusite Iron and Manganese Treatment,” Proceedings—Water Quality Technology Conference, 1999, pp. TU11.5.1-TU11.5.14.
- A. Vogel, “Text Book of Quantitative Inorganic Analysis,” 3rd Edition, Longman, London, 1961.
- S. B. Kanungo and D. M. Mahapatra, “Interfacial Properties of Some Hydrous Manganese Dioxides in 1-1 Electrolyte Solution,” Journal of Colloid and Interface Science, Vol. 131, 1989, pp. 103-111. doi:10.1016/0021-9797(89)90150-1
- S. Ardizzone and S. Trasatti, “Interfacial Properties of Oxides with Technological Impact in Electrochemistry,” Advances in Colloid and Interface Science, Vol. 64, 1996, pp. 173-251. doi:10.1016/0001-8686(95)00286-3
- P. Thanabalasingam and W. F. Pickering, “Effect of pH on Interaction between Arsenic(III) or Arsenic(V) and Manganese(IV) Oxide,” Water, Air, and Soil Pollution, Vol. 29, No. 2, 1986, pp. 205-16. doi:10.1007/BF00208409

