Journal of Water Resource and Protection
Vol.5 No.5(2013), Article ID:31785,7 pages DOI:10.4236/jwarp.2013.55049
Influence of Management on the Water Quality and Sediment in Tropical Fish Farm
1Aquaculture Center, University of São Paulo State, Jaboticabal, Brazil
2Federal Institute of Espírito Santo—Ifes, Alegre, Brazil
Email: sipauba@caunesp.unesp.br
Copyright © 2013 Lúcia H. Sipaúba-Tavares et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 15, 2013; revised March 11, 2013; accepted April 11, 2013
Keywords: Water Quality; Fish Ponds; Management; Sediment
ABSTRACT
A seven-month research evaluated the management effect on the water quality and sediment of seven fish ponds. Water and sediment were collected at nine sample sites: seven in the fish ponds; one in inlet water and another in the fish farm’s effluent. The soil samples were analyzed for macroand micro-nutrients and the water samples were analyzed for physical and chemical parameters. Management and local climate conditions affected nutrient seasonality in the sediment and featured high concentrations of Al, Ca, Cu, K, Mg, C, Na, Zn and OM at the effluent with low pH, ranging between 4.4 and 6.5. Sudden decrease of DO (less than 3 mg/L) during the rainy season, with a 180 mm rainfall, and a TSS increase (approximately 10 mg/L) were reported. Use of organic manure in fish pond V6 caused higher rates of ammonia (over 1 mg/L). Due to the sediment’s acid pH (less than 4.8) and Al at 0.92 mg/L at the effluent, great care was required in the fish farm. Maintenance and procedures management in the fish farm under analysis should be given more attention since high levels of Al, Fe and acid pH and low levels of potassium and phosphorus in the sediment may produce unfavorable conditions in the water column, and may ultimately have an impact on fish.
1. Introduction
Fish farm water quality is a function of land uses such as agriculture, and feed management, fish density, water flow, polyculture and climatic conditions which in turn affect the fish ponds water and sediment. The latter is the principal compartment of the fish pond with direct influence on fish ponds’ limnology. Fish meal is an important source of nutrients in the fish pond, mainly due to nitrogen and phosphorus. In fact, only 35% of supplied nitrogen and phosphorus are retained in fish biomass [1].
A large fraction of the unused nitrogen and phosphorrus accumulate in the sediment which may store between 100 - 1000 times more nutrients than water [2]. Because of this, monitoring the water quality and sediment serves as an important function to avoid eutrophication or algal bloom often occurs in such environments enriched of nutrients. In a fish farm, the profile of nutrient flow is complex and the major sources of nutrients are fish excretion and fish feed. Besides this, the water quality and sediment in fish farm is affected by waste treatment operations. Apart from water, sediments are also responsible for nutrient transportation in the aquatic environment. A combination of measurements of water and sediment quality can provide a good indication of conditions and potential risks to the water body [3].
Sediment’s properties and processes occurring at the level and in the soil-water interface are very important when the well-being and growth of fish are taken into account. Concentrations of nutrients, organic matter and micro-organisms density in the pond sediment are several times greater than in water [4]. Although the sediment gradually releases different nutrient elements to plant or bio-available forms for the benefit of the fish food organisms, it also controls several significant bio-chemical reactions occurring in aquatic ecosystems [5].
Mineralization of accumulated organic matter under anaerobic conditions leads towards the formation of toxic metabolites to the detriment of limnological conditions of the fish ponds [6]. Since water quality is strongly influenced by feed inputs, ponds with high feeding rates frequently have severe problems with low dissolved oxygen concentrations and excessive concentrations of ammonia and nitrite than ponds with low or moderate feeding rate [7].
Prediction of sediment and water quality in a fish pond based on location in a particular soil area is subject to considerable error because sediment and water quality differed greatly among ponds in the sediment areas of different places [8]. These authors observed in westcentral Alabama (USA) fish ponds in close proximity are more likely to have similar sediment and water quality than fish ponds located farther apart. Manipulations of fish ponds to improve water quality and to enhance production require a thorough understanding of the physical, chemical and biological processes taking place [6].
The primary purpose of the current work is to study the effects of the management currently conduced at fish farms in relation of water quality and sediment and, subsequently to purpose adequate techniques to minimize the impacts and, hence to increase the production and survival of fishes.
2. Material and Methods
2.1. Study Area and Fish Pond Management
The study was carried out at the Federal Institute of the State of Espírito Santo-IFES, Alegre, ES, Brazil, 20˚45'S and 41˚29'E, mean altitude 108 m. According to Köpen classification, the region climate is AW, subtropical, relatively dry in the winter (June to September) and rainy in the summer (October to December), with mean yearly temperature 23.4˚C. The IFES fish farm, totaling 4 ha of flooded land, comprises thirty-seven earthen ponds with independent water supply. Choice of the sampled seven fish ponds (V1 - V7) was based on morphometry, location and usage availability. Areas of the fish ponds varied between 710 (V2) and 6550 m2 (V7), with mean depth 1.5 m. Fish ponds’ water supply comes from the waterfall Braúnas, 4 km upstream the fish ponds area, and channeled to the ponds by underground tubes. Continuous water flow provided a 5% daily rate of rearing volume. The fish ponds were populated with “matrinxã” (Brycon cephalus) in V1; catfish (Ictalurus punctatus) in V2; catfish (I. punctatus) and grass carp (Ctenopharyngodon idella) in V3; fries of silver carp (Hypophthalmichthys molitrix) and “pacu” (Piaractus mesopotamicus) in V4; “pacu” fries (P. mesopotamicus), grass carp (C. idella) and silver carp (H. molitrix) in V5; “pacu” fries (P. mesopotamicus), grass carp (C. idella), silver carp (H. molitrix), bighead carp (Hypophthalmtichtlys nobilis) and catfish (I. punctatus) in V6; “pacu” fries (P. mesopotamicus), grass carp (C. idella), and bighead carp (H. nobilis) in V7, at a density of approximately 0.34 fish per m2.
Management of the ponds occurred randomly since they were fertilized according to material availability. The following management has been adopted throughout the study:
V1 V3 = diet with ration at 32% crude protein;
V4 - V5 = lime spreading; fortnightly fertilization with urea between March and July, rations with 32% crude protein were also provided;
V6 = lime spreading; fertilization with pig dung and urea; and V7 = lime spreading; fertilization with chicken dung and rations at 32% crude protein.
Livestock inner parts were provided at irregular intervals throughout the study period. Sampling for limnological parameters and sediment was conducted once a month, from June to December 2007. During this period, both dry (D) and rainy (R) seasons were experienced.
2.2. Limnological Parameters and Climatic Conditions
Water samples were collected at a depth 10 cm using a 5-L Van Dorn bottle. A total of nine sampling sites were assigned: I = inlet water; V1 to V7 = sites inside the fish ponds; E = effluent. Transparency was measured by Secchi disk, pH was measured by model Q-400A Quimis pH meter. The nutrients were determined by spectrometer, according to Koroleff [9] and Golterman et al. [10]. Chlorophyll-a was evaluated according to Nusch [11]. Conductivity, dissolved oxygen (DO), and temperature were measured in situ using a probe YSI-30 SCT water quality check, and turbidity was determined with ADAMO HD- 114. Total suspended solids (TSS), and 5-day biochemical oxygen demand (BOD5) were determined according to Boyd and Tucker [12]. Average of total precipitation was measured using pluviometer placed at the IFESAgriculture Climatology Station.
2.3. Sediment
Sediments samples were taken from the surface using a 4-cm diameter PVC core at sites inside the fish ponds (V1 - V7) and the effluent. Sediments analysis was undertaken for Ca, Cu, Mg, Mn, Na, C, organic matter (OM), P, K, Al, Fe, Zn, pH according to the methods described by Silva [13]. All samples were transported to the laboratory in cold boxes. No sediment sample was collected from the inlet water since the fish farm is supplied through this tube.
2.4. Statistical Analysis
Principal Components Analysis (PCA) was used to reduce the dimensionality of the environmental variables and to rank fish ponds and seasons (dry and rainy) in relation to the sediment and water ponds. Because of the low variability between environments and months, these data on water transparence and TSS, were not included in the analysis [14].
3. Results
Nutrients in the sediment had higher concentrations of Al, Ca, Cu, C, Mg, Na, Zn, K and OM at the effluent site, coupled to lower rates of pH, which ranged between 4.4 and 6.5. In fact, they were the variables that most contributed towards the composition of the first PCA axis, separating the effluent from the other ponds (Figure 1).
The second PCA axis verified that ponds manured with urea and ration (V4 and V5) during the dry season had higher rates of Fe and P in the sediment. Besides, food manured with ration only during the rainy season (V1 and V2) had higher rates of Mn (Figure 1). Iron (Fe) in the sediment was high, generally above 148 mg/L; however, P ranged between 2 and 55 mg/L. Aluminum (Al) rates were higher at the effluent than those inside the fish ponds, varying from 0.43 mg/L to 0.92 mg/L; on the other hand, Mn ranged between 17 and 385.7 mg/L and OM between 1.2% and 3.2% (Table 1). Copper (Cu) and Zn exhibited a wide range of variation ranging from 0.7 to 5.3 mg/L for Cu and from 1.6 to 14.9 mg/L for Zn, with higher concentrations in the effluent, and the same, occurred to K, Ca, Mg, and Na (Table 1).
Ammonia and nitrate were predominant among the nitrogen compounds in the water. Nitrate, found in the fish ponds and in the effluent during the dry season, was associated with high concentrations of chlorophyll-a and turbidity, respectively 92.07 mg/L and 203 NTU (Table 2), registered during the season, with higher concentrations in V3. Nitrate, turbidity and chlorophyll-a were variables which caused grouping of ponds and effluent related to the dry season on the right hand side of the PCA first axis (Figure 2). Organic manure in V6 caused higher rates of ammonia and nitrite, 1651 mg/L and 51.9
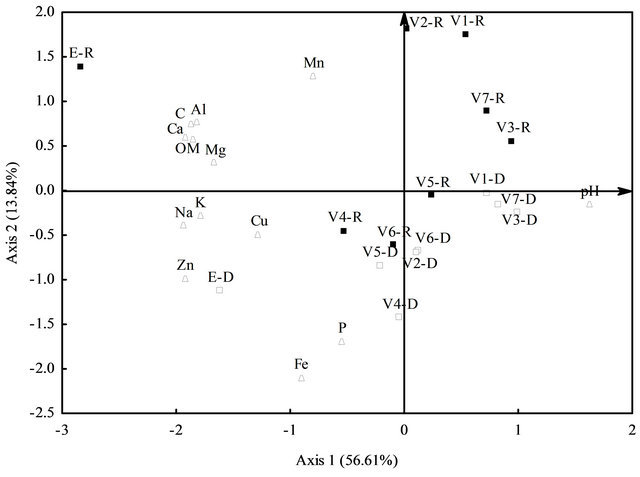
Figure 1. Interpolation of auto-values from the matrix of sediment variables. First two axes from the principal component analysis (PCA), where: V1 - V7 = fish ponds; D = dry season; R = rainy season; open triangles = sample sites in the dry season; open bars = sample sites in the rainy season; close bars = variables.
mg/L respectively, in these fish ponds during the dry season. The two variables were highly important to explain PCA second axis with regard to water (Figure 2 and Table 2).
Concentrations of total phosphorus (TP) in the water ranged between 75 mg/L in inlet water and 348 mg/L in the effluent. As a rule, orthophosphate concentrations were lower than 73 mg/L, with the exception of V2 during the rainy season, with 153 mg/L. This was associated with TP rates (238 mg/L) and to low concentrations of chlorophyll-a (5.6 mg/L) in the same period (Table 2).
Variables BOD5, temperature, conductivity, TP and orthophosphate caused fish pond groupings and effluent during the rainy season, with inlet discarded from PCA grouping (Figure 2). When rainfall reached 180 mm (Figure 3) during the rainy season, in November, a sharp decrease of DO (3 mg/L) and an increase of TSS was registered in the water (Table 2). The pH of water was alkaline, between 7.0 and 8.2, with highest rates in the rainy season. Inlet water site of the fish farm had a simi-

Figure 2. Interpolation of auto-values from the matrix of water variables. First two axes from the principal component analysis (PCA), where: V1 - V7 = fish ponds; D = dry season; R = rainy season; open triangles = sample sites in the dry season; open bars = sample sites in the rainy season; close bars = variables.
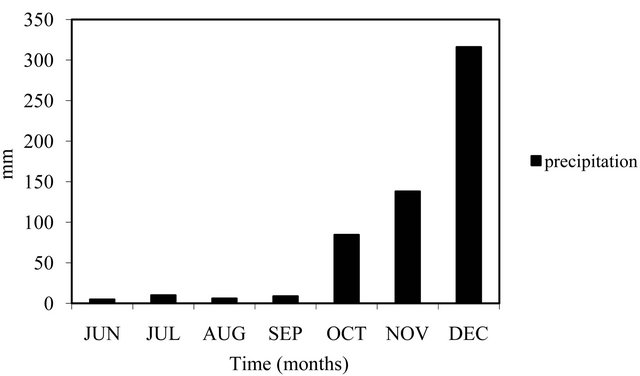
Figure 3. Monthly variation of precipitation (mm) during the study period.
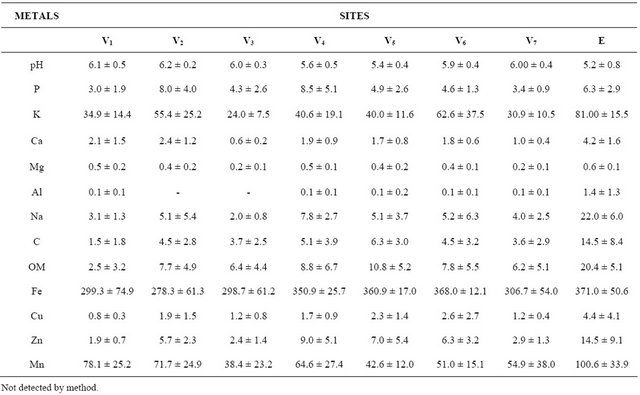
Table 1. Mean and SD concentrations of pH, organic matter (OM), and selected heavy metals in the sediment collected in different eight sites (V1 to V7 = fish ponds, and effluent = E) of the fish farm during the studied period.
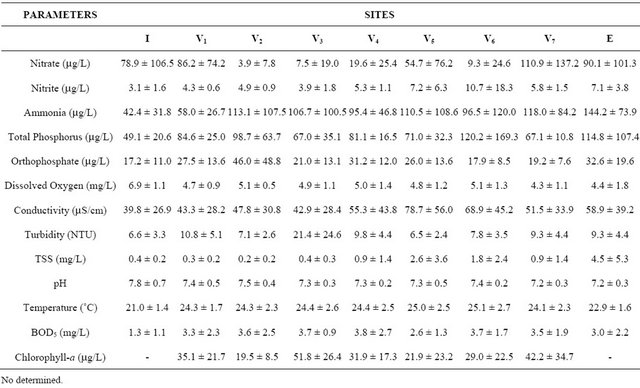
Table 2. Mean and SD of some limnological parameters of the water samples collected from nine sites (V1 to V7 = fish ponds, inlet water = I and effluent = E) in the fish farm, during the studied period.
lar pattern during the rainy season, with high concentrations of DO, alkaline pH and low concentrations of nutrients (Table 2).
4. Discussion
Shallow artificial systems are directly affected by management and local climate conditions. High nutrient rates in the effluent’s sediment of the fish farm have been associated with inadequate managements of hauling and total emptying of the fish ponds at a later date. It was actually a source of pollution owing to high nutrient charges released in the receiving stream without any treatment. In animal manure-fertilized fish pond, nutrients, such as ammonia and phosphorus, maintained high concentrations in the water column, whereas phosphorus averaged above 2.0 mg/L in the sediment. Nutrients added to pond water from fertilizers, unconsumed feed, fish feces and fish metabolites account for organic material. Suspended solids, phosphorus and ammonia are of the greatest concern for their potential impacts on pond effluents and on the environment [2].
Orthophosphate availability along the water column is the result of its interaction with iron and complete mixing. High Fe concentrations (between 148 and 425 mg/L) were reported throughout the experiment, caused by the type of soil found in the region (red latosol). High concentrations of nitrate, ferric iron, sulfate, and other oxidized inorganic compounds in pore water favor decomposition, since they may be used by many microorganisms as alternative electron acceptors (oxidants) in the absence of oxygen [12]. Low P concentrations in the sediment between November and December (rainy season) were associated with a slight decrease in Fe concentrations during the period. Increase in P concentrations in the sediment may be associated to P affinity for ironbearing mineral in the soil [15].
Further, OM affected P rates in the sediment. Organic matter in submerged latosolic soils by restricting fixation of added phosphorus into iron and aluminum phosphate forms occurs owing to reduction reactions and even chelating effects which inhibit phosphorus transformation into insoluble forms [5]. The general recommendation of [16] is that OM concentration in pond sediment should vary between 1% and 3%. However, in ponds where fish are fed, OM concentrations below 1% are acceptable. High OM content in the effluent (above de 1.2%) may be associated with manure deposited by pig dung and chicken dung as fertilizers (V6 - V7). However, sediment is a major sink for P in most aquatic environments, including fish ponds.
Bhadha and Jawitz [15] showed that soils of wetland, OM and P at the top few centimeters of the soil were highest when compared to the rest of the soil profile. At least both were partially derived from manure inputs. Furthermore, geographical proprieties such as particle size distribution associated with total surface area might be dominant factors in determining P retention [17].
Current study shows that strong fluctuations in P and K rates in the sediment were directly related to chlorophyll-a rates. The phosphorus and potassium are important to increase primary productivity and high contents of K appeared to be an asset to P. Organic matter plays a vital role in maintaining an improving sediment quality. It is a key quality factor that determines the degree of nutrient retention in sediment. Pond sediment is rich in nitrogen, phosphorus and potassium, and other macroand micro-nutrients. If the nutrient availability in pond sediment is known, the sediment may successfully provide nutrients together with inorganic fertilizer [2].
The management of randomized emptying had a direct result on the TSS of the water column, with mean rates below 4.5 mg/L, during the rainy season. Besides, there is the effect of the water’s continuous flow in the fish farm.
The water column’s pH did not vary throughout the experiment. This fact contrasts the acid pH of the sediment associated to OM, which may have impaired drastic changes in pH with high buffer capacity. Although the best pH for pond sediments seems to lie between 6.5 and 7.5 [7], it was below 6.6 in current research. Aluminum is toxic since when pH is above 6.8 due to the formation of . Some smolts aluminum is toxic even at low concentrations when pH is below 6.2 [18]. Precautions should be taken in the evaluation of fish farms under analysis: Al rates increased and varied between 0.53 and 0.92 mg/L when sediment’s pH varied between 4.4 and 4.8 in the effluent.
. Some smolts aluminum is toxic even at low concentrations when pH is below 6.2 [18]. Precautions should be taken in the evaluation of fish farms under analysis: Al rates increased and varied between 0.53 and 0.92 mg/L when sediment’s pH varied between 4.4 and 4.8 in the effluent.
Manganese concentration represented the first highest metals in the sediment. Manganese functions as an essential constituent for bone structure, but it is toxic only when presented in higher amount, but at low level is considered as micronutrient [3].
The importance of Ca, Mg, and K concentrations in sediments for pond water quality and fish production has not been elucidated, although high concentrations of major cations are beneficial [8]. The concentration of the Cu exhibited wide range of variation between fish ponds during the rainy and dry seasons. Copper can combine with other contaminants such as ammonia, mercury and zinc to produce an additive toxic effect on fish [3]. Low concentrations of Ca and Mg directly impact the acid pH of the fish pond’s sediment. This is due to the fact that calcium and magnesium are liming components used to increase soil pH and reduce soil acidity [19].
The metals and nutrients in the sediment of fish ponds vary considerably among different studies [5,8,16], possible due differences in chemical characteristics and nutrients of water, feeding patterns, kid of soil and also the seasons in which studies were carried out.
Mc Intosh [20] indicated that many of the environment problems in aquaculture are the direct result of farm mismanagement. These effects may be minimized if ponds are well managed and good sediment and water quality conditions prevail. It is difficult to find ponds with similar sediment and water quality parameters. Differences in water quality parameters and sediments between inlet water, fish ponds and effluent were found within the same fish farm. Successful management of tropical fish ponds for biologically optimal fish growth requires supply of necessary ponds inputs which have to include balance nutrients via fertilization and supplementary feeding.
5. Conclusion
Maintenance and procedures management in the fish farm under analysis should be given more attention since high levels of Al, Fe and acid pH and low rates of potassium and phosphorus in the sediment may produce unfavorable conditions in the water column, and may ultimately have an impact on fish. The higher mean value of conductivity, TSS, ammonia, nitrate, orthophosphate in the water, and trace metals in the sediment collected from different sites into the fish farm prove the presence of large quantities of organic and inorganic materials in the fish ponds, this was expected due to the fact that the pond management occurred randomly. The rational use of water should be adopted mainly in the following procedures: 1) emptying of fish ponds should be done when strictly necessary; 2) animal manure should be substituted by inorganic fertilizers since they dissolve quickly in water and consequently their fast incorporation in the trophic chain occurs; 3) improvement of the polyculture combination so that each fish may exploit different trophic levels within the water column which affects directly the water quality and sediment. Organic and inorganic charge for the receiving body is thus reduced. The evidence brought in this paper demonstrates the importance of the appropriate technologies to optimize the fish production in this fish farm.
6. Acknowledgements
The authors would like to thank the Federal Institute of Espírito Santo—Ifes for its logistical support of the experiment. We also acknowledge LAFARSOL (CCA-UFES) for helping the laboratory analysis.
REFERENCES
- Y. Avnimelech, “Activated Suspension Ponds: A New Concept in Water Treatment,” Hatchery Magazine, Vol. 1, No. 2, 2000, pp. 24-30.
- M. M. Rahman, Q. Jo, Y. G. Gong, S. A. Miller and M. Y. Hossain, “A Comparative Study of Common Carp (Cyprinus carpio L.) and Calbasu (Labeo calbasu Hamilton) on Bottom Soil Re-Suspension, Water Quality, Nutrient Accumulations, Food Intake and Growth of Fish in Simulated Rohu (Labeo rohita Hamilton) Ponds,” Aquaculture, Vol. 285, No. 1-4, 2008, pp. 78-83. doi:10.1016/j.aquaculture.2008.08.002
- A. G. M. Osman and W. Kloas, “Water Quality and Heavy Metal Monitoring in Water, Sediments, and Tissues of African Catfish Clarias gariepinus (Burchell, 1822) from the River Nile, Egypt,” Journal of Environmental Protection, Vol. 1, No. 4, 2010, pp. 389-400. doi:10.4236/jep.2010.14045
- Y. Avnimelech and G. Ritvo, “Shrimp and Fish Pond Soils: Processes and Management,” Aquaculture, Vol. 220, No. 1-4, 2003, pp. 549-567. doi:10.1016/S0044-8486(02)00641-5
- G. N Chattopadhyay, R. Mukherjee and A. Banerjee, “Phosphorus Management for Fish Ponds Located in Red and Lateritic Soil Zones,” Better Crops International, Vol. 17, No. 2, 2003, pp. 18-21.
- B. Hari, B. Madhusoodana-Kurup, J. T. Varghese, J. W. Schrama and M. C. J. Verdegem, “The Effect of Carbohydrate Addition on Water Quality and the Nitrogen Budget in Extensive Shrimp Culture Systems,” Aquaculture, Vol. 252, No. 2-4, 2006, pp. 248-263. doi:10.1016/j.aquaculture.2005.06.044
- C. E. Boyd and C. S. Tucker, “Pond Aquaculture Water Quality Management,” Kluwer Academic Publishers, Boston, 1998. doi:10.1007/978-1-4615-5407-3
- K. Silapajarn, C. E. Boyd and O. Silapajarn, “Physical and Chemical Characteristics of Pond Water and Bottom Soil in Channel Catfish Ponds in West-Central Alabama,” Agricultural Experiment Station, Auburn University, Alabama, 2004.
- F. Koroleff, “Determination of Nutrients,” In: E. Grashof, and E. Kremling, Eds. Methods of Seawater Analysis, Verlag Chemie Wenhein, New York, 1976.
- H. L. Golterman, R. S. Clymo and M. A. M. Ohnstad, “Methods for Physical and Chemical Analysis of Freshwater,” Blackwell Scientific Publication, London, 1978.
- E. A. Nusch, “Comparison of Different Methods for Chlorophyll and Phaeopigments Determination,” Archiv für Hydrobiologie, Vol. 14, 1980, pp. 4-36.
- C. E. Boyd and C. S. Tucker, “Water Quality and Pond Soil Analyses for Aquaculture,” Agricultural Experiment Station, Auburn University, Alabama, 1992.
- F. C. Silva, “Handbook of soils, Plants and Fertilizers Analysis,” Embrapa Communications and Information to Transference, Brasília, 1999.
- P. Legendre and L. Legendre, “Numerical Ecology,” Elsevier Science B.V., Amsterdam, 1998.
- J. H. Bhadha and J. W. Jawitz, “Characterizing Deep Soils from an Impacted Subtropical Isolated Wetland: Implications for Phosphorus Storage,” Journal of Soils and Sediments, Vol. 10, No. 3, 2010, pp. 514-525. doi:10.1007/s11368-009-0151-4
- C. E. Boyd, C. W. Wood and T. Thunjai, “Aquaculture Pond Bottom Soil Quality Management,” Pond Dynamoics/Aquaculture Collaborative Research Support Program, Oregon State University, Cowallis, 2002.
- Q. Wang and Y. Li, “Phosphorus Adsorption and Desorption Behavior on Sediments of Different Origins,” Journal of Soils and Sediments, Vol. 10, No. 6, 2010, pp. 1159-1173. doi:10.1007/s11368-010-0211-9
- S. Fivelstad, R. Waagbo, S. F. Aeitz, A. C. D. Hosfeld, A. B. Olsen and S. Stefansson, “A Major Water Quality Problem in Smolt Farms: Combined Effects of Carbon Dioxide, Reduced pH and Aluminum on Atlantic Salmon (Salmo salar L.) Smolts: Physiology and Growth,” Aquaculture, Vol. 215, No. 1, 2003, pp. 339-357. doi:10.1016/S0044-8486(02)00197-7
- G. C. Sigua, J. Griffin, W. J. Kang and S. W. Coleman, “Wetland Conversion to Beef Cattle Pasture: Changes in Soil Properties,” Journal of Soils and Sediments, Vol. 4, No. 1, 2004, pp. 4-10. doi:10.1007/BF02990822
- D. Mc Intosh, “The Tragedy of the Commons: Perspectives of Sustainable Aquaculture,” World Aquaculture Magazine, Vol. 34, No. 4, 2002, pp. 21-22.

