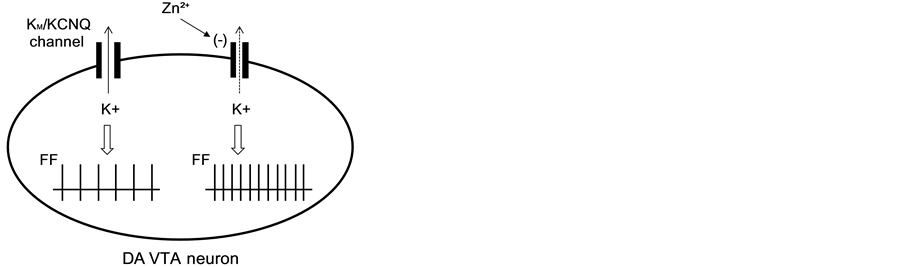Journal of Biomedical Science and Engineering
Vol.07 No.14(2014), Article ID:52283,12 pages
10.4236/jbise.2014.714105
The Effects of Zinc and Other Divalent Cations on M-Current in Ventral Tegmental Area Dopamine Neurons
Susumu Koyama1,2*, Munechika Enjoji2, Mark S. Brodie1, Sarah B. Appel1
1Department of Physiology and Biophysics, University of Illinois at Chicago, College of Medicine, Chicago, USA
2Department of Clinical Pharmacology, Faculty of Pharmaceutical Sciences, Fukuoka University, Fukuoka, Japan
Email: *susumuk@fukuoka-u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 October 2014; revised 22 November 2014; accepted 5 December 2014
ABSTRACT
Ventral tegmental area dopamine (DA VTA) neurons are important for the reinforcing effects of drugs of abuse such as ethanol and nicotine. We have previously shown that M-current (IM) regu- lates the excitability of DA VTA neurons. Zinc (Zn2+) contributes to the regulation of neuronal ex- citation as a neuromodulator. In the present study, we investigated zinc effect on the properties of IM and the spontaneous firing frequency of DA VTA neurons. The standard deactivation protocol was used to measure IM during voltage-clamp recording with a hyperpolarizing voltage step to −40 mV from a holding potential (VH) of −25 mV. Zn2+ (100 μM) inhibited IM amplitude and IM recovered completely from the inhibition after the washout of Zn2+. Zn2+ inhibited IM in a concentration- dependent manner (IC50: 5.8 μM). When hyperpolarizing voltage steps were given to −65 mV (in 10 mV increments) from a VH of −25 mV, Zn2+ (100 μM) reduced IM amplitude at each voltage and zinc inhibition of IM was not voltage-dependent. Zn2+ increased the spontaneous firing frequency of DA VTA neurons in a concentration-dependent manner, suggesting that Zn2+ causes excitation of DA VTA neurons through an action on IM. IM of DA VTA neurons was inhibited by 100 μM divalent cations in increasing order of potency: Ba2+ (16%) < Co2+ (25%) < Ni2+ (40%) < Cd2+ (59%) < Zn2+ (67%). These results suggest that Zn2+ may exert physiologically significant regulation of neuronal excitability in DA VTA neurons.
Keywords:
Divalent Cation, Dopaminergic, Nystatin-Perforated Patch Recording, Zinc

1. Introduction
The ventral tegmental area dopamine (DA VTA) neurons send axons which synapse in the nucleus accumbens (NAcb) [1] and the excitation of DA VTA neurons results in increased dopamine release in the NAcb [2] - [4] , which is important for the reinforcing effects of drugs of abuse [5] [6] . M-current (IM) is a voltage-dependent K+ current which is activated at the subthreshold range of membrane potential and contributes to the regulation of repetitive firing [7] [8] . IM is mediated by current through KCNQ type potassium channels [9] . Among the five types of channel subunits (KCNQ1 to 5) [10] , immunohistochemical studies have shown that the KCNQ2 and KCNQ4 channel proteins are present in VTA neurons [11] [12] . DA VTA neurons have intrinsic pacemaker activity and a recent study has reported that the KCNQ4 channel subunit is the major component of IM in DA VTA neurons and critical for the excitability of these neurons [13] . Thus, KCNQ channels (IM) of DA VTA neurons may be a critical factor in the mediation of the reinforcing effect of drugs of abuse.
Zinc (Zn2+) is present in the midbrain in higher concentrations than in the blood and the total amount of Zn2+ is estimated to be 4225 ng in the substantia nigra (SN) in human brain [14] . The majority of Zn2+ is associated with a Zn2+-containing enzyme in cytoplasm [15] and the remainder of Zn2+ is present in presynaptic vesicles [16] . Zn2+ is thought to be co-released with neurotransmitter from presynaptic nerve terminals [17] and to act postsynaptically by the regulation of ligand-gated ion channels and voltage-dependent ion channels [18] . Extracellular Zn2+ inhibits IM; the IC50 values are 11 μM in rodent neuroblastoma x glioma hybrid cells [19] and 300 mM in bullfrog sympathetic neurons [20] . It has been reported that Zn2+ (10 - 100 μM) accelerates evoked action potential generation, suggesting that it increases excitability of midbrain DA neurons of the SN in a concentration-dependent manner [21] . We have previously shown that IM underlies the fast and slow component of the action potential after hyperpolarization without affecting the middle component and prolongs the inter-spike interval to decrease the excitability of DA VTA neurons [22] . Therefore, it is hypothesized that relatively low concentrations of Zn2+ may inhibit IM and increase the excitability of DA VTA neurons. This hypothesis was tested in the present study, investigating whether Zn2+ would modulate the properties of IM and the excitability of DA VTA neurons through an action on IM. In addition, we examined the potency of other divalent cations, barium (Ba2+), cadmium (Cd2+), cobalt (Co2+) and nickel (Ni2+) on IM in DA VTA neurons.
2. Materials and Methods
2.1. Preparation of Dissociated Neurons
Animals used in this study were treated in strict accordance with the American Physiological Society’s Guiding Principles in the Care and Use of Animals and the US National Institutes for Health Guide for the Care and Use of Laboratory Animals; the protocol for all experimental methods was approved by the Institutional Animal Care Committee of the University of Illinois at Chicago. Both male and female Fisher 344 rats (14 - 18 days old) were decapitated and the brain quickly removed. The brain was placed in an ice-cold cutting solution (in mM: 220 sucrose, 2.5 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 NaH2PO4, 26 NaHCO3, 11 D-glucose), which was constantly bubbled with 95% O2 and 5% CO2. Transverse brain slices (350 - 400 μm thick) were made on a Vibratome (Series 1000 plus, St. Louis, MO, USA). The brain slices were incubated for 3 - 4 hr in an artificial cerebrospinal fluid (ACSF) (in mM: 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 NaH2PO4, 26 NaHCO3, 11 D-glucose, osmolarity 300 mOsm), which was constantly bubbled with 95% O2 and 5% CO2 at 26˚C. VTA neurons were dissociated as previously described [22] . Specifically, brain slices were next incubated in a HEPES-buffered solution (see below) containing papain (15 - 18 U/ml) at 32˚C for 20 - 25 min. After papain treatment, the brain slices were further incubated in the ACSF for 20 - 40 min. The VTA neurons were dissociated by a vibrating stylus apparatus for dispersing cells from the brain slices. First the brain slice was transferred to a poly-D-lysine- coated 35 mm culture dish (Becton Dickenson, Bedford, MA, USA) containing the HEPES-buffered solution. A grid of nylon threads glued to a U-shaped metal frame was used to hold the brain slice down during cell dissociation. After the VTA was visually identified, the vibrating stylus was placed in the appropriate region with a micromanipulator. The stylus was made of glass capillary tubing (1.5 mm o.d.) pulled to a fine tip, fire-polished (200 - 400 μm in diameter) and mounted on the vibrating apparatus, which horizontally vibrated the stylus tip (excursions of 100 - 200 μm at 20 - 25 Hz). Once the cell dissociation procedure was completed (4 - 7 min), the brain slice was removed from the culture dish, and the dissociated neurons settled and adhered to the bottom of the dish within 20 min.
2.2. Nystatin-Perforated Patch Recording in Dissociated Neurons
Electrophysiological measurements were made with an Axopatch-1B patch-clamp amplifier (Axon Instruments, Union City, CA, USA). Microelectrodes were fabricated on a P-97 puller (Sutter Instrument Company, Novato, CA, USA), from LE16 glass capillaries (Dagan, Minneapolis, MN, USA) and heat-polished on a microforge (Narishige, Tokyo, Japan). The tip resistances of the electrodes were 3 - 7 MΩ when filled with a pipette solution (in mM: 60 K-acetate, 60 KCl, 1 CaCl2, 2 MgCl2, 40 HEPES; pH 7.2 adjusted with KOH, final [K+]i = 131 mM; osmolality 290 mOsm). Nystatin-perforated patch recording was used to minimize the dialysis of intracellular contents and therefore prevent the rundown of IM, as previously described [22] . Nystatin was dissolved in me- thanol at a concentration of 10 mg/ml. This nystatin stock solution was diluted with the pipette solution to a final concentration of 100 - 200 μg/ml and the electrodes were backfilled with this solution. After the cell-attached configuration was attained, the access resistance was periodically monitored and capacitive transients were cancelled. When the access resistance had reached a steady level (15 - 30 MΩ), the recording was started. In case of the sudden change of the access resistance, the recording was stopped. Voltage-clamp recording was done in a HEPES-buffered solution (in mM: 145 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 11 D-glucose; pH adjusted to 7.4 with NaOH; osmolarity 300 mOsm) constantly bubbled with 100% O2. The liquid junction potential between the pipette solution and the HEPES-buffered solution was estimated to be 5 mV [23] and the results have been corrected by this amount. Membrane currents and voltage were filtered at 1 kHz by a −3 dB 4-pole filter and acquired at a sampling frequency of 10 kHz, which is higher than the Nyquist’s critical sampling rate. Data acquisition was performed with a DigiData 1322A interface and pClamp software version 9.0 (Axon Instruments Inc., Union City, CA, USA). The dissociated VTA neurons were visualized under phase-contrast optics on an inverted microscope (Diaphot 300, Nikon, Tokyo, Japan). All experiments were performed at room temperature (23˚C - 25˚C).
2.3. Drug Application for Dissociated Neurons
Neurons were continuously bathed in the external solution and drugs were dissolved at final concentration in the same solution. Drug solutions were applied via a multiple channel manifold (MLF-4; ALA Scientific Instruments, Westbury, NY, USA). Each channel of the manifold was connected to a gravity-fed reservoir with tubing (860 μm, i.d.). The output of the manifold was connected to an outflow tube (500 μm, i.d.), the tip of which was placed within 200 mm of the soma of the recorded neuron. Solutions flowed continuously through one manifold channel. Application of drug solutions was controlled by opening or closing valves connected to the reservoirs.
2.4. Preparation of Brain Slices
Following rapid removal of the brain, the tissue block containing the VTA was mounted in the Vibratome and submerged in the ice-cold cutting solution. Coronal sections (400 μm thick) were cut and the slice was placed on a mesh platform in the recording chamber. The slice was totally submerged in the ACSF maintained at a flow rate of 2 ml/min; the temperature in the recording chamber was kept at 35˚C. The ACSF was saturated with 95% O2 and 5% CO2 (pH = 7.4). Equilibration time of at least one hour was allowed after placement of the brain slice in the recording chamber before electrodes were placed in the tissue. Recording electrodes were placed in the VTA under visual control. Only those neurons which were anatomically located within the VTA and which conformed to the electrophysiological criteria for dopaminergic neurons [24] were studied. These criteria include broad action potentials and regular spontaneous firing frequency at 0.5 - 5 Hz.
2.5. Extracellular Recording in Brain Slices
Extracellular recording electrodes were fabricated from 1.5 mm diameter glass tubing and were filled with 0.9% NaCl. Tip resistance of the microelectrodes ranged from 3 to 8 MΩ. The Fintronics amplifier (Fintronics Inc., Orange, CT, USA) used in these recordings had a window discriminator, the output of which was fed to both a rectilinear pen recorder and a computer-based data acquisition system for on-line and off-line analysis of the data. The multiplexed output of the amplifier was displayed on an analog storage oscilloscope, for accurate adjustment of the window levels used to monitor single units. An IBM-PC-based data acquisition system was used to calculate, display and store the frequency of firing over 5 sec and 1 min intervals.
2.6. Drug Administration for Brain Slices
Drugs were added to the ACSF by means of a calibrated infusion pump from stock solutions 100 to 1000 times the desired final concentrations. The addition of drug solutions to the ACSF was performed in such a way as to permit the drug solution to mix completely with the ACSF before this mixture reached the recording chamber. The use of a calibrated, variable speed infusion pump permits the accurate addition of several concentrations of drugs from the same stock solution. Final concentrations were calculated from the ACSF flow rate, pump infusion speed and the concentration of drug stock solution. The small volume chamber (about 300 μl) used in this study permitted the rapid application and washout of drug solutions. Typically drugs reached equilibrium in the tissue after 2 to 3 minutes of application.
2.7. Drugs and Chemical Agents
BaCl2, CdCl2, CoCl2, HEPES, NiCl2, nystatin and ZnCl2 were purchased from Sigma (Saint Louis, MO, USA). Papain was purchased from Worthington (Lakewood, NJ, USA).
2.8. Data Analysis and Curve Fitting
Action potentials were analyzed offline with pClamp 9.0 software (Axon Instruments Inc.). All average values are expressed as mean ± standard error of the mean (SE). Graphing and curve fitting of data was performed with Origin 7 software (OriginLab Corp., Northampton, MA, USA). Concentration-response curves for zinc were constructed by plotting percent inhibition of IM as a function of drug concentration plotted on a log scale. Smooth curves were fit to these data with the Hill equation of the form [25] :
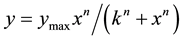
where x is the concentration, y is the percent inhibition and ymax is the maximal value of y (at saturation); in the fitting procedure ymax was constrained not to exceed 100%. The term k is the IC50 (the concentration giving half- maximal inhibition) and n (Hill slope) is the power term related to the slope of the curve. To assess the changes in spontaneous firing with drugs, drug effect was quantitated as the mean change in firing rate (normalized as the percentage of control) for 60 sec-long interval during the peak of the drug response as previously described [26] . The formula for this normalization is:
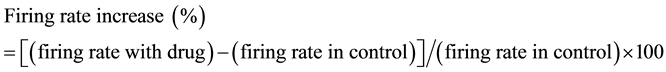
Data from dissociated cells with action potential amplitudes less than 50 mV were discarded. All average values are expressed as mean ± standard error (SE). Statistical comparison to assess significant differences was done by one-way ANOVA as appropriate followed by a Bonferroni correction. When needed, the Student-New- man-Keuls post hoc test was used to test multiple comparisons. Correlation was evaluated by linear regression with P < 0.05 being considered significant.
3. Results
3.1. Zinc (Zn2+) Inhibits M-Current (IM) in Ventral Tegmental Area Dopamine (DA VTA) Neurons
After obtaining a stable perforated patch recording, DA VTA neurons were identified in current-clamp recording based on the electrophysiological characteristics by matching spontaneous firing frequency and action potential (AP) parameters, as described previously [22] . Then, IM was measured in the same DA VTA neuron in voltage- clamp configuration.
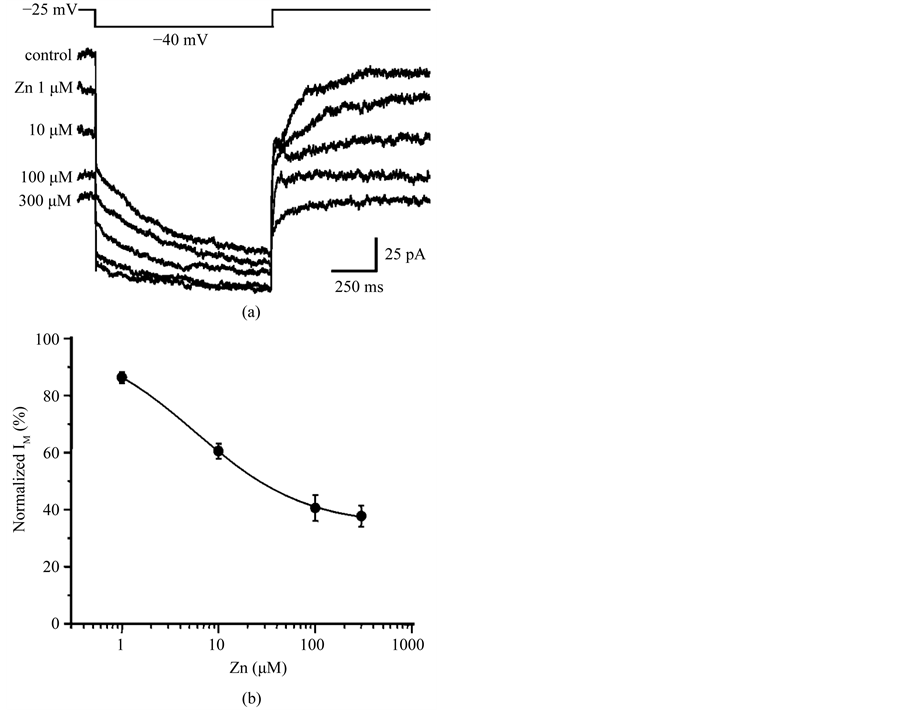
IM was measured in the standard deactivation protocol [8] with 1 sec-long hyperpolarizing voltage step from a holding potential (VH) of −25 mV to −40 mV. IM was measured as the inward relaxation current caused by deactivation of IM during the voltage step (Figure 1(a)). Figure 1(a1) shows IM before, during and after treatment with 100 mM Zn2+ in a typical DA VTA neuron. Zn2+ reduced IM amplitude and also reduced the sustained outward current present at −25 mV as indicated by the inward shift in the baseline current. Just after the termination
Figure 1. Zinc inhibition of IM in DA VTA neurons. A1: IM recorded before, during and after application of 100 mM zinc (Zn2+) in a DA VTA neuron. IM was measured in the standard deactivation protocol with 1 sec-long hyperpolarizing voltage step from a holding potential (VH) of −25 mV to −40 mV. Each IM trace was obtained by averaging 5 current recordings from the neuron. A2: IM was measured as the inward relaxation current caused by deactivation of IM (arrows between dotted lines) during the voltage step; the difference between the instantaneous current at the beginning and the steady-state current at the end of the voltage step before (left) and after Zn2+ treatment (right) was measured. The dashed line represents sustained outward current (IOUT) at a VH of −25 mV before Zn2+ treatment. B: The average time course of 100 mM Zn2+ effect on IM from DA VTA neurons (n = 6). IM was measured with 1 sec-long hyperpolarizing voltage step from a VH of −25 mV to −40 mV. This hyperpolarizing voltage step was given in every 20 sec. Each IM amplitude was normalized to the average IM amplitude obtained from the 5 events just before the application of Zn2+.
of the hyperpolarizing voltage step from −25 mV to −40 mV, a transient outward current was recorded in the presence of Zn2+ (Figure 1(a1), middle panel). This current is likely to be transient A-type K+ current (IA), because DA VTA neurons exhibit prominent IA [27] and Zn2+ shifts the voltage-dependency of steady-state IA inactivation to the depolarizing direction [28] . In the presence of zinc, IA can be activated at a membrane potential of −40 mV. Figure 1(a2) shows the measurement of the inward relaxation current caused by deactivation of IM during the voltage step; the difference between the instantaneous current at the beginning (Iin) and the steady- state current at the end of the voltage step (Iss). Figure 1(b) shows the average time course of normalized IM amplitude before, during and after application of 100 μM Zn2+ in DA VTA neurons. IM recovered completely after the washout of Zn2+ in DA VTA neurons.
3.2. Concentration-Dependent Inhibition of IM by Zn2+
Figure 2(a) shows that Zn2+ caused a concentration-dependent reduction of IM amplitude in a typical DA VTA neuron. Zn2+ also caused a concentration-dependent reduction of the baseline sustained outward current. The 300 μM concentration appeared to produce a inhibitory effect near to or at the maximum, since it did not inhibit IM substantially more than the 100 mM concentration. Zn2+ at 300 μM did not produce complete inhibition of IM. Figure 2(b) shows the pooled concentration-response curve which plots normalized IM amplitude versus log concentration of Zn2+ from DA VTA neurons. The Hill equation was used to fit a smooth curve to the mean data in Figure 2(b). The IC50 for Zn2+ was 5.8 mM and the Hill slope was 0.8.
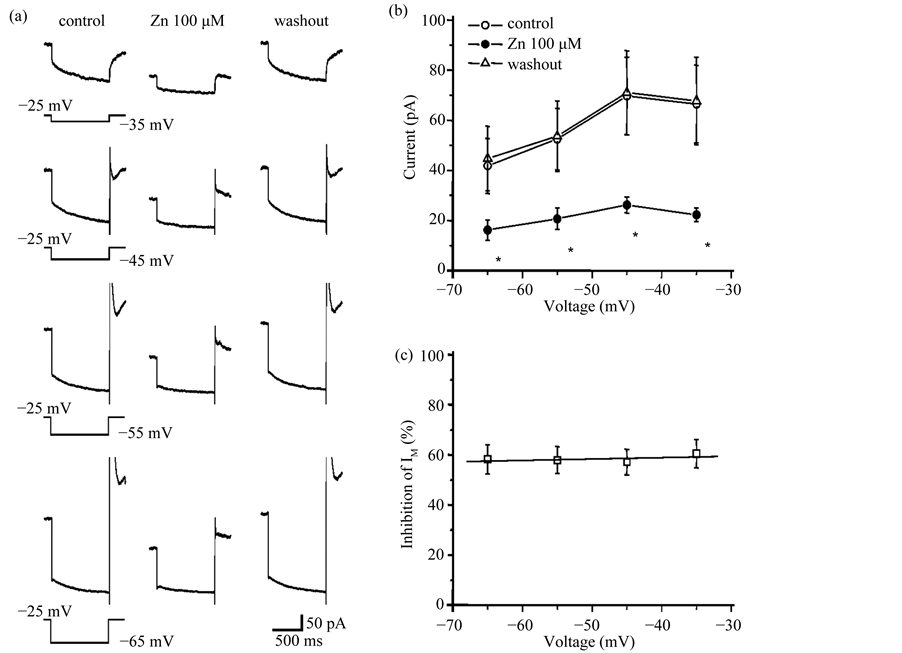
3.3. Zinc Inhibition of IM Is Not Voltage-Dependent
We then examined whether zinc inhibition of IM was voltage-dependent. Figure 3(a) shows IM induced by a series of hyperpolarizing voltage steps before, during, and after treatment with 100 mM Zn2+. Zn2+ inhibited IM amplitude measured with all four hyperpolarizing voltage steps in 6 DA VTA neurons. IA activation can be seen after the offset of the larger voltage steps in control and washout, while IA is not prominent after the offset of the
Figure 2. Concentration-dependent inhibition of IM by Zn2+ in DA VTA neurons. (a) Zinc inhibition of IM is concentration-dependent. IM traces are superimposed. The baseline outward current was shifted by Zn2+ (at a VH of −25 mV). Each IM trace was obtained by averaging 5 current recordings at each Zn2+ concentration; (b) Concentration-response curve showing mean normalized IM amplitude as a function of log Zn2+ concentration for DA VTA neurons (n = 5). The smooth curve was fitted with the Hill equation.
Figure 3. Zinc inhibition of IM is not voltage-dependent. (a) IM induced by four different hyperpolarizing voltage steps before, during and after application of 100 mM Zn2+. Hyperpolarizing voltage steps were given from a VH of −25 mV to −65 mV in 10 mV increments. Each IM trace was obtained by averaging the currents from 6 DA VTA neurons; (b) Relationship between membrane voltage and IM amplitude; control (open circles), 100 mM Zn2+ (filled circles), and washout of Zn2+ (open triangles) (n = 6); (c) Relationship between voltage and average % IM inhibition by 100 mM Zn2+ (n = 6). *P < 0.05.
larger voltage steps in the presence of Zn2+ (Figure 3(a)). This is likely due to a Zn2+-induced shift in the voltage-dependency of steady-state IA activation in the depolarizing direction [29] . Figure 3(b) shows the relationship between membrane voltage and IM amplitude before, during, and after treatment with 100 mM Zn2+ for DA VTA neurons. Zn2+ inhibited IM amplitude at any voltage examined. There was no correlation between membrane voltage and the % IM inhibition by Zn2+ (Figure 3(c)).
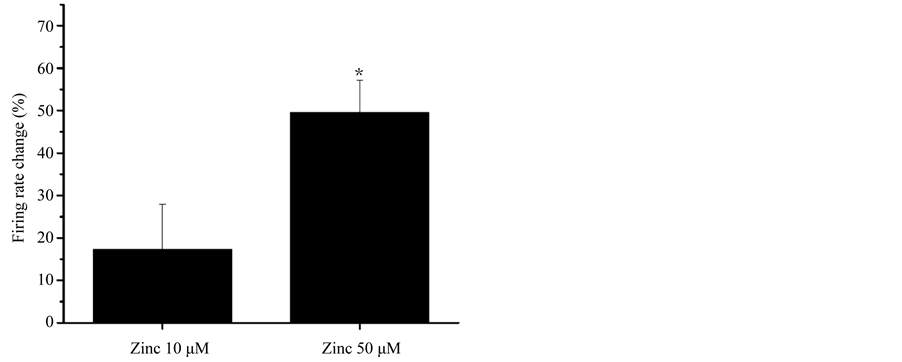
3.4. Zn2+ Increases the Spontaneous Firing Frequency of DA VTA Neurons
The effect of Zn2+ on the spontaneous firing frequency of DA VTA neurons was measured with extracellular single-unit recording of these neurons in brain slices. The percentage increase in firing frequency produced by Zn2+ was calculated. Figure 4 shows the concentration-response relationship between Zn2+ and the firing rate change by Zn2+ from DA VTA neurons. Average Zn2+-induced increase in firing frequency was 17.3% ± 10.6% with 10 mM Zn2+ and 49.5% ± 7.6% with 50 mM Zn2+ (n = 5).
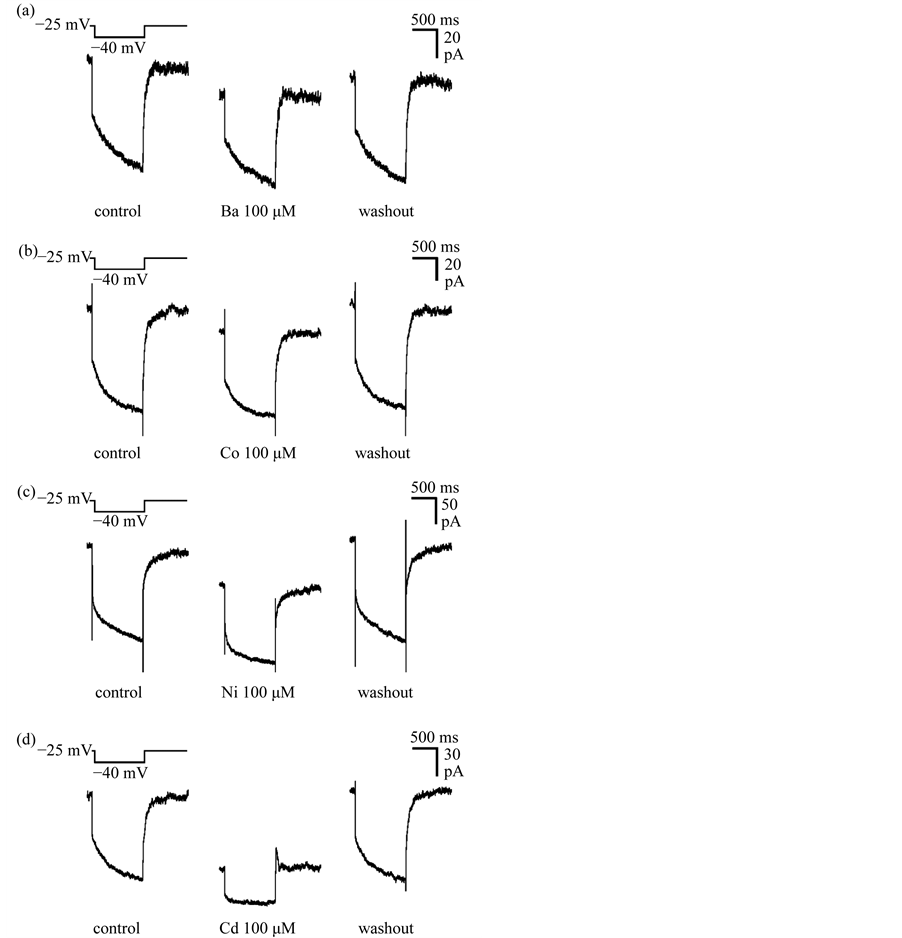
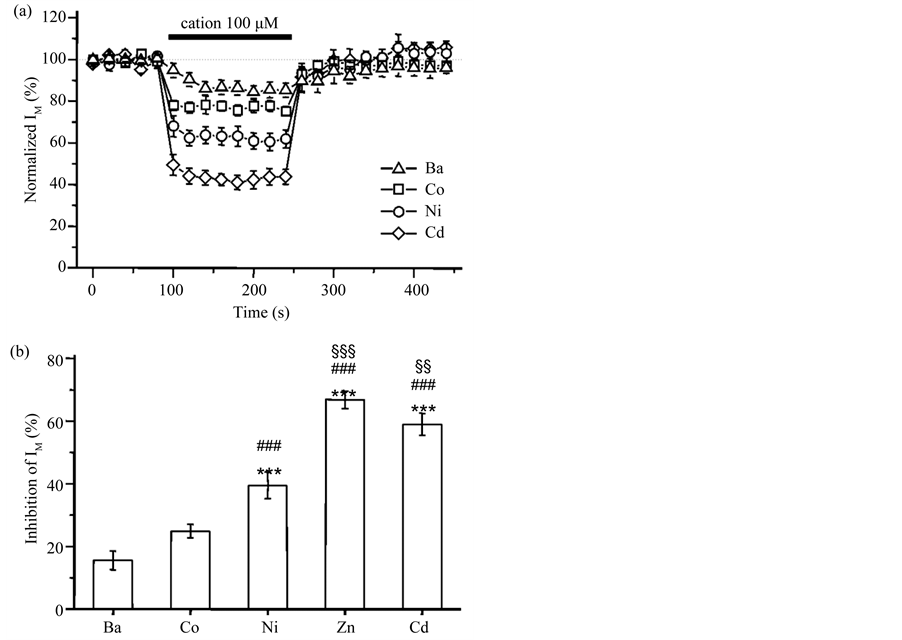
3.5. IM Inhibition by Ba2+, Co2+, Ni2+ and Cd2+
Finally, we examined whether divalent cations other than Zn2+ modulated IM (Figure 5). Ba2+, Co2+, Ni2+ and Cd2+ at a concentration of 100 mM all reduced IM amplitude and induced an inward shift of the baseline outward current in DA VTA neurons, however their potencies for IM inhibition were different (Figures 5(a)-(d)). Just after the termination of the hyperpolarizing voltage step from −25 mV to −40 mV, IA was recorded in the pres-
Figure 4. Effect of Zn2+ on the firing frequency of DA VTA neurons in brain slices. Pooled concentration-response relationship for Zn2+ (10 and 50 μM) effects on spontaneous firing rate measured in DA VTA neurons from adult rats (3 months old) (n = 5). *P < 0.05.
ence of Cd2+ (Figure 5(d), middle panel), because Cd2+, like Zn2+, shifts the voltage-dependency of steady-state IA inactivation in the depolarizing direction [28] . Figure 6(a) shows the average time course of normalized IM amplitude before, during and after application of 100 mM divalent cations in DA VTA neurons. IM of DA VTA neurons were inhibited by 100 mM extracellular divalent cations in increasing order of potency: Ba2+ < Co2+ < Ni2+ < Cd2+ < Zn2+. Average maximal inhibition of IM was 15.6% ± 3.0 % with Ba2+ (n = 7), 24.9% ± 2.2 % with Co2+ (n = 6), 39.5% ± 4.2% with Ni2+ (n = 7), 59.0% ± 3.5% with Cd2+ (n = 6), and 66.8% ± 2.8% with Zn2+ (n = 6).
4. Discussion
Extracellular Zn2+ inhibited IM in a concentration-dependent manner with IC50 value of 5.8 mM in DA VTA neurons; the IC50 value is smaller than that reported for rodent neuroblastoma x glioma hybrid cells (11 mM) [19] or bullfrog sympathetic neurons (300 mM) [20] . We estimate the maximal inhibition of IM by zinc to be about 62% with 38% of this current remaining unblocked by Zn2+ in DA VTA neurons. Since our previous study has confirmed the inward relaxation current obtained by the same voltage protocol in the present study to be IM in DA VTA neurons [22] , IM of these neurons can be classified into Zn2+-sensitive and Zn2+-insensitive components. Previous immunohistochemical studies have shown that KCNQ2 and KCNQ4 channel proteins are present in VTA neurons [11] [12] . Hansen et al. (2006) have reported that the KCNQ4 channel subunit is the main component of IM in DA VTA neurons, and found weak immunoreactivity of the KCNQ2 channel subunit and lack of KCNQ3 channel immunoreactivity in VTA neurons [13] . Since the KCNQ2 and the KCNQ4 channel subunits cannot be coassembled as a functional heteromeric channel in vitro [30] , the KCNQ2 and the KCNQ4 channels may contribute to IM independently, as homomers, in a DA VTA neuron. KCNQ4 channels may under- lie the Zn2+-sensitive component of IM and KCNQ2 channels may underlie the remaining Zn2+-insensitive com- ponent of IM. It is unlikely that the KCNQ5 channel component is a significant contributor to IM in DA VTA neurons, since KCNQ5 channels are potentiated by Zn2+ in a concentration-dependent manner [31] .
In DA VTA neurons, zinc inhibition of IM was not voltage-dependent and the Hill slope was near 1 (0.8) for the concentration-dependent zinc inhibition of IM. These observations suggest a single Zn2+ site of action and one that is different from the voltage-sensitive region of the M-channels. Furthermore, it seems likely that the site of action of zinc in DA VAT neurons is not the M-channel pore, since the mechanism for voltage-dependent IM inhibition with divalent cation (Ba2+) has been reported to be KCNQ channel pore blocking [32] . The voltage-dependency for the action of divalent cations on KCNQ/M-current differ among experimental preparations. In neuroblastoma x glioma hybrid cells [19] and the rod photoreceptor of tiger salamander [33] , IM inhibition by Ba2+ or Cd2+ shows voltage-dependency. Expressed KCNQ2/KCNQ3 heteromeric channels are inhibited by Ba2+ in a voltage-independent manner [34] .
Figure 5. IM inhibition by divalent cations in DA VTA neurons. (a) IM recorded before, during and after application of 100 μM barium (Ba2+) in a DA VTA neuron. IM was measured in the standard deactivation protocol with 1 sec-long hyperpolarizing voltage step from a VH of −25 mV to −40 mV. Each IM was obtained by averaging 5 currents for the neuron; Effects of other divalent cations on IM in DA VTA neurons were recorded by the same protocol and illustrated in the same method as described above: 100 μM cobalt (Co2+) (b); 100 μM nickel (Ni2+) (c); and 100 μM cadmium (Cd2+) (d).
Our extracellular recording study revealed that a relatively low concentration of Zn2+ (50 mM) significantly increased the spontaneous firing frequency of DA VTA neurons. Taken together with the present voltage-clamp analysis, zinc inhibited IM and subsequently increased the excitability of DA VTA neurons, leading to increase in firing frequency of these neurons (Figure 7). Consistent with our present study, it has been reported that Zn2+ (10 - 100 mM) increases the firing frequency during evoked spike trains in midbrain DA neurons of the SN in a
Figure 6. Different potencies of divalent cations for IM inhibition. (a) The average time course of 100 μM cation effect on IM in DA VTA neurons: Ba2+ (open triangles, n = 7), Co2+ (open squares, n = 6), Ni2+ (open circles, n = 7) and Cd2+ (open diamonds, n = 6). IM was measured with 1 sec-long hyperpolarizing voltage step from a VH of −25 mV to −40 mV. This hyperpolarizing voltage step was given in every 20 sec. All IM amplitude was normalized to the average IM amplitude obtained from the 5 events just before the application of Ba2+, Co2+, Ni2+ and Cd2+; (b) The mean maximal inhibition of IM by 100 μM Ba2+, Co2+, Ni2+, Cd2+ and Zn2+. ***P < 0.001 to Ba2+; ###P < 0.001 to Co2+; §§P < 0.01; §§§P < 0.001 to Ni2+.
Figure 7. Schematic illustration for the role of zinc in DA VTA neurons. In the control status, KM/KCNQ channels open and outflow of K+ ions from the cytoplasm hyperpolarizes membrane potentials, leading to inhibitory effect on DA VTA neurons. Zinc inhibits KM/KCNQ channel opening and the subsequent decrease in the outflow of K+ ions from the cytoplasm depolarizes membrane potentials, leading to excitation of DA VTA neurons with increase in FF. KM, M-type K+ channel; FF, firing frequency.
concentration-dependent manner [21] . Several types of voltage-dependent ion currents contribute to the spontaneous activity of midbrain DA neurons. A high voltage-activated (HVA) Ca2+ current (ICa) underlies the spontaneous oscillatory potential [35] . A low voltage-activated (LVA) transient ICa is tightly coupled to a small conductance Ca2+-activated K+ (SK) current which underlies the middle component of the after hyperpolarization [36] . A transient A-type K+ current (IA) is critical for the regulation of inter-action potential trajectory by generating a time- and voltage-dependent repolarization delay [26] [37] . IM underlies the fast and slow components without affecting the middle component of AHP and prolongs inter-action potential intervals [22] . Many studies have reported that Zn2+ modulates all the types of ion currents described above. Zn2+ inhibits HVA ICa with the IC50 value of 21 μM and slows current activation [38] . Zn2+ inhibits LVA ICa with the IC50 value from 11 to 55 μM [39] [40] . Zn2+ (10 - 1000 μM) shifts both steady-state activation and inactivation of IA to a depolarizing direction and slows current activation [27] [28] [41] . Thus, the Zn2+-induced excitation of midbrain DA neurons is likely to be the sum of zinc effects on HVA ICa, LVA ICa, IA and IM. Since IM is more sensitive to Zn2+ (IC50 = 5.8 μM) than HVA ICa, LVA ICa or IA and Zn2+ inhibition of IM was not voltage-dependent, Zn2+ can inhibit IM potently at any membrane potential during the spontaneous activity of DA VTA neurons and increase the excitability of these neurons as shown in the present study.
IM of DA VTA neurons were inhibited by 100 μM extracellular divalent cations with the following of order of potency: Zn2+ > Cd2+ > Ni2+ > Co2+ > Ba2+. Similarly to the results with Zn2+, the onset of IM inhibition by Ba2+, Cd2+, Co2+ or Ni2+ was fast and IM recovered completely after the washout of these divalent cations. In neuro- blastoma x glioma hybrid cells, Cd2+, Ni2+ and Zn2+ exhibit a similar order of potency for the inhibition of IM but Ba2+ is twice as potent as Co2+ [19] . In the rod photoreceptor of tiger salamander, IM is inhibited by Ba2+ with the IC50 value of 7.6 mM without apparent sensitivity to 5 mM Co2+ and Zn2+ [32] . Again, the specific KCNQ subunits, and their combination in functional channels, in each tissue may dictate the sensitivity to inhibition by these divalent cations.
Considering the fact that the concentration of Zn2+ is relatively high in the brain, we suggest that Zn2+ exerts physiologically significant regulation of neuronal excitability through an action on IM. It has been shown that Zn2+ can be co-released with glutamate from presynaptic terminals, and the concentration of Zn2+ in the synapse has been estimated to be between 10 and 100 µM [42] [43] . Our results indicate that the concentration of Zn2+ that is achieved during synaptic transmission could be expected to have effects on IM in addition to directly affecting glutamate neurotransmission. Therefore, inhibition of IM may be a critical aspect of the neuromodulatory action of Zn2+.
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA05846 (to S.B.A.). This study was partly supported by Grants-in-Aid for Scientific Research (C) (Nos. 22500685 and 25350166 to S.K.) from the Japan Society for the Promotion of Science (JSPS) and the Mishima Kaiun Memorial Foundation, Japan (to S.K.). This study was also supported by funds (No. 106006 to S.K.) from the General Research Institute of Fukuoka University.
Competing Interests
The authors have declared that no competing interests exist.
References
- Oades, R.D. and Halliday, G.M. (1987) Ventral Tegmental (A10) System: Neurobiology. 1. Anatomy and Connectivity. Brain Research, 434, 117-165. http://dx.doi.org/10.1016/0165-0173(87)90011-7
- Di Chiara, G. and Imperato, A. (1988) Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. Proceedings of the National Academy of Sciences of the USA, 85, 5274-5278. http://dx.doi.org/10.1073/pnas.85.14.5274
- Weiss, F., Lorang, M.T., Bloom, F.E. and Koob, G.F. (1993) Oral Alcohol Self-Administration Stimulates Dopamine Release in the Rat Nucleus Accumbens: Genetic and Motivational Determinants. Journal of Pharmacology and Experimental Therapeutics 267, 250-258.
- Wise, R.A. (2002) Brain Reward Circuitry: Insights from Unsensed Incentives. Neuron, 36, 229-240. http://dx.doi.org/10.1016/S0896-6273(02)00965-0
- Corrigall, W.A., Coen, K.M. and Adamson, K.L. (1994) Self-Administered Nicotine Activates the Mesolimbic Dopamine System through the Ventral Tegmental Area. Brain Research, 653, 278-284. http://dx.doi.org/10.1016/0006-8993(94)90401-4
- Pfeffer, A.O. and Samson, H.H. (1988) Haloperidol and Apomorphine Effects on Ethanol Reinforcement in Free Feeding Rats. Pharmacology Biochemistry and Behavior, 29, 343-350. http://dx.doi.org/10.1016/0091-3057(88)90167-0
- Aiken, S.P., Lampe, B.J., Murphy, P.A. and Brown, B.S. (1995) Reduction of Spike Frequency Adaptation and Block- ade of M-Current in Rat CA1 Pyramidal Neurones by Linopirdine (DuP 996), a Neurotransmitter Release Enhancer. British Journal of Pharmacology, 115, 1163-1168. http://dx.doi.org/10.1111/j.1476-5381.1995.tb15019.x
- Brown, D.A. and Adams, P.R. (1980) Muscarinic Suppression of a Novel Voltage-Sensitive K+ Current in a Vertebrate Neurone. Nature, 283, 673-676. http://dx.doi.org/10.1038/283673a0
- Wang, H.S., Pan, Z., Shi, W., Brown, B.S., Wymore, R.S., Cohen, I.S., Dixon, J.E. and McKinnon, D. (1998) KCNQ2 and KCNQ3 Potassium Channel Subunits: Molecular Correlates of the M-Channel. Science, 282, 1890-1893. http://dx.doi.org/10.1126/science.282.5395.1890
- Robbins, J. (2001) KCNQ Potassium Channels: Physiology, Pathophysiology, and Pharmacology. Pharmacology & Therapeutics, 90, 1-9. http://dx.doi.org/10.1016/S0163-7258(01)00116-4
- Cooper, E.C., Harrington, E., Jan, Y.N. and Jan, L.Y. (2001) M Channel KCNQ2 Subunits Are Localized to Key Sites for Control of Neuronal Network Oscillations and Synchronization in Mouse Brain. Journal of Neuroscience, 21, 9529-9540.
- Kharkovets, T., Hardelin, J.P., Safieddine, S., Schweizer, M., El-Amraoui, A., Petit, C. and Jentsch, T.J. (2000) KCNQ4, a K+ Channel Mutated in a Form of Dominant Deafness, Is Expressed in the Inner Ear and the Central Auditory Pathway. Proceedings of the National Academy of Sciences of the United States of America, 97, 4333-4338. http://dx.doi.org/10.1073/pnas.97.8.4333
- Hansen, H.H., Ebbesen, C., Mathiesen, C., Weikop, P., Ronn, L.C., Waroux, O., Scuvee-Moreau, J., Seutin, V. and Mikkelsen, J.D. (2006) The KCNQ Channel Opener Retigabine Inhibits the Activity of Mesencephalic Dopaminergic Systems of the Rat. Journal of Pharmacology and Experimental Therapeutics, 318, 1006-1019. http://dx.doi.org/10.1124/jpet.106.106757
- Zecca, L., Pietra, R., Goj, C., Mecacci, C., Radice, D. and Sabbioni, E. (1994) Iron and Other Metals in Neuromelanin, Substantia Nigra, and Putamen of Human Brain. Journal of Neurochemistry, 62, 1097-1101. http://dx.doi.org/10.1046/j.1471-4159.1994.62031097.x
- Romans, A.Y., Graichen, M.E., Lochmuller, C.H. and Henkens, R.W. (1987) Kinetics and Mechanism of Dissociation of Zinc Ion from Carbonic Anhydrase. Bioinorganic Chemistry, 9, 217-229. http://dx.doi.org/10.1016/S0006-3061(78)80007-6
- Perez-Clausell, J. and Danscher, G. (1985) Intravesicular Localization of Zinc in Rat Telencephalic Boutons. A Histochemical Study. Brain Research, 337, 91-98. http://dx.doi.org/10.1016/0006-8993(85)91612-9
- Assaf, S.Y. and Chung, S.H. (1984) Release of Endogenous Zn2+ from Brain Tissue during Activity. Nature, 308, 734- 736. http://dx.doi.org/10.1038/308734a0
- Harrison, N.L. and Gibbons, S.J. (1994) Zn2+: An Endogenous Modulator of Ligand- and Voltage-Gated Ion Channels. Neuropharmacology, 33, 935-952. http://dx.doi.org/10.1016/0028-3908(94)90152-X
- Robbins, J., Trouslard, J., Marsh, S.J. and Brown, D.A. (1992) Kinetic and Pharmacological Properties of the M-Cur- rent in Rodent Neuroblastoma x Glioma Hybrid Cells. Journal of Physiology, 451, 159-185.
- Hirasawa, T., Kudo, Y. and Tokimasa, T. (1998) Actions of Zinc on Rapidly Inactivating A-Type and Non-Inactivating M-Type Potassium Currents in Bullfrog Sympathetic Neurons. Neuroscience Letters, 255, 5-8. http://dx.doi.org/10.1016/S0304-3940(98)00683-1
- Chung, J.M., Chang, S.Y., Kim, Y.I. and Shin, H.C. (2000) Zinc Increases the Excitability of Dopaminergic Neurons in Rat Substantia Nigra. Neuroscience Letters, 286, 183-186. http://dx.doi.org/10.1016/S0304-3940(00)01120-4
- Koyama, S. and Appel, S.B. (2006) Characterization of M-Current in Ventral Tegmental Area Dopamine Neurons. Journal of Neurophysiology, 96, 535-543. http://dx.doi.org/10.1152/jn.00574.2005
- Neher, E. (1992) Correction for Liquid Junction Potential in Patch Clamp Experiments. Methods in Enzymology, 207, 123-131. http://dx.doi.org/10.1016/0076-6879(92)07008-C
- Mueller, A.L. and Brodie, M.S. (1989) Intracellular Recording from Putative Dopamine-Containing Neurons in the Ventral Tegmental Area of Tsai in a Brain Slice Preparation. Journal of Neuroscience Methods, 28, 15-22. http://dx.doi.org/10.1016/0165-0270(89)90005-8
- Zot, H.G., Hasbun, J.E. and Van Minh, N. (2012) Second-Chance Signal Transduction Explains Cooperative Flagellar Switching. PLoS ONE, 7, e41098. http://dx.doi.org/10.1371/journal.pone.0041098
- Brodie, M.S., Shefner, S.A. and Dunwiddie, T.V. (1990) Ethanol Increases the Firing Rate of Dopamine Neurons of the Rat Ventral Tegmental Area in Vitro. Brain Research, 508, 65-69. http://dx.doi.org/10.1016/0006-8993(90)91118-Z
- Koyama, S. and Appel, S.B. (2006) A-Type K+ Current of Dopamine and GABA Neurons in the Ventral Tegmental Area. Journal of Neurophysiology, 96, 544-554. http://dx.doi.org/10.1152/jn.01318.2005
- Kuo, C.C. and Chen, F.P. (1999) Zn2+ Modulation of Neuronal Transient K+ Current: Fast and Selective Binding to the Deactivated Channels. Biophysical Journal, 77, 2552-2562. http://dx.doi.org/10.1016/S0006-3495(99)77090-6
- Harrison, N.L., Radke, H.K., Talukder, G. and Ffrench-Mullen, J.M. (1993) Zinc Modulates Transient Outward Current Gating in Hippocampal Neurons. Receptors &Channels, 1, 153-163.
- Kubisch, C., Schroeder, B.C., Friedrich, T., Lütjohann, B., El-Amraoui, A., Marlin, S., Petit, C. and Jentsch, T.J. (1999) KCNQ4, a Novel Potassium Channel Expressed in Sensory Outer Hair Cells, Is Mutated in Dominant Deafness. Cell, 96, 437-446. http://dx.doi.org/10.1016/S0092-8674(00)80556-5
- Jensen, H.S., Callo, K., Jespersen, T., Jensen, B.S. and Olesen, S.P. (2005) The KCNQ5 Potassium Channel from Mouse: A Broadly Expressed M-Current Like Potassium Channel Modulated by Zinc, pH and Volume Changes. Molecular Brain Research, 139, 52-62. http://dx.doi.org/10.1016/j.molbrainres.2005.05.007
- Gibor, G., Yakubovich, D., Peretz, A. and Attali, B. (2004) External Barium Affects the Gating of KCNQ1 Potassium Channels and Produces a Pore Block via Two Discrete Sites. Journal of General Physiology, 124, 83-102. http://dx.doi.org/10.1085/jgp.200409068
- Wollmuth, L.P. (1994) Mechanism of Ba2+ Block of M-Like K Channels of Rod Photoreceptors of Tiger Salamanders. Journal of General Physiology, 103, 45-66. http://dx.doi.org/10.1085/jgp.103.1.45
- Prole, D.L. and Marrion, N.V. (2004) Ionic Permeation and Conduction Properties of Neuronal KCNQ2/KCNQ3 Potassium Channels. Biophysical Journal, 86, 1454-1469. http://dx.doi.org/10.1016/S0006-3495(04)74214-9
- Kang, Y. and Kitai, S.T. (1993) Calcium Spike Underlying Rhythmic Firing in Dopaminergic Neurons of the Rat Substantia Nigra. Neuroscience Research, 18, 195-207. http://dx.doi.org/10.1016/0168-0102(93)90055-U
- Wolfart, J. and Roeper, J. (2002) Selective Coupling of T-Type Calcium Channels to SK Potassium Channels Prevents Intrinsic Bursting in Dopaminergic Midbrain Neurons. Journal of Neuroscience, 22, 3404-3413.
- Liss, B., Franz, O., Sewing, S., Bruns, R., Neuhoff, H. and Roeper, J. (2001) Tuning Pacemaker Frequency of Individual Dopaminergic Neurons by Kv4.3L and KChip3.1 Transcription. EMBO Journal, 20, 5715-5724. http://dx.doi.org/10.1093/emboj/20.20.5715
- Magistretti, J., Castelli, L., Taglietti, V. and Tanzi, F. (2003) Dual Effect of Zn2+ on Multiple Types of Voltage-Dependent Ca2+ Currents in Rat Palaeocortical Neurons. Neuroscience, 117, 249-264. http://dx.doi.org/10.1016/S0306-4522(02)00865-5
- Noh, J.H. and Chung, J.M. (2003) Zinc Reduces Low-Threshold Ca2+ Currents of Rat Thalamic Relay Neurons. Neuroscience Research, 47, 261-265. http://dx.doi.org/10.1016/S0168-0102(03)00198-6
- Todorovic, S.M. and Lingle, C.J. (1998) Pharmacological Properties of T-Type Ca2+ Current in Adult Rat Sensory Neurons: Effects of Anticonvulsant and Anesthetic Agents. Journal of Neurophysiology, 79, 240-252.
- Huang, R.C., Peng, Y.W. and Yau, K.W. (1993) Zinc Modulation of a Transient Potassium Current and Histochemical Localization of the Metal in Neurons of the Suprachiasmatic Nucleus. Proceedings of the National Academy of Sciences of the United States of America, 90, 11806-11810. http://dx.doi.org/10.1073/pnas.90.24.11806
- Vogt, K., Mellor, J., Tong, G. and Nicoll, R. (2000) The Actions of Synaptically Released Zinc at Hippocampal Mossy Fiber Synapses. Neuron, 26, 187-196. http://dx.doi.org/10.1016/S0896-6273(00)81149-6
- Li, Y., Hough, C.J., Suh, S.W., Sarvey, J.M. and Frederickson, C.J. (2001) Rapid Translocation of Zn2+ from Presynaptic Terminals into Postsynaptic Hippocampal Neurons after Physiological Stimulation. Journal of Neurophysiology, 86, 2597-2604.
NOTES

*Corresponding author.