Journal of Biomedical Science and Engineering
Vol.6 No.7A1(2013), Article ID:34674,7 pages DOI:10.4236/jbise.2013.67A1002
Field evaluation of KO-Tab 1-2-3® long lasting insecticidal net performance in Milenge, Zambia*
![]()
1Ministry of Health, National Malaria Control Centre, Lusaka, Zambia
2Department of Biomedical Sciences, School of Medicine, University of Zambia, Lusaka, Zambia
3Department of Biological Sciences, School of Natural Sciences, University of Zambia, Lusaka, Zambia
4Ministry of Health, Ndeke House, Lusaka, Zambia
Email: #emmanuel_chanda@yahoo.co.uk, alikandyata@yahoo.com, chandajavan@yahoo.com, pascykapata@gmail.com
Copyright © 2013 Emmanuel Chanda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 5 May 2013; revised 5 June 2013; accepted 15 June 2013
Keywords: Long Lasting Insecticidal Nets; Malaria Vector Control; Field Evaluation; Zambia
ABSTRACT
Insecticide treated nets (ITNs) play a pivotal role in the prevention and control of malaria. Conversely, inadequate levels of ownership, utilization and durability invariably compromise their efficacy. Operational performance of the KO-Tab 1-2-3® Long Lasting Insecticidal Nets (LLINs) was assessed using the World Health Organization (WHO) standard entomological and epidemiological procedures, and a pretested structured questionnaire. The median knock down time for Anopheles funestus s.l. was 30 minutes (95% CI 26.3 - 34.0). Post exposure mean mortality rates were 34% at one hour and 80.0% at 24 hours (OR = 0.13, P = 0.00002). Children between the ages of 1 - 4 years old exhibited higher false positives as compared to the 5 - 15 years age groups (OR = 0.10, P = 0.0006). Parasite prevalence varied between rapid diagnostic tests (RDTs) (40.4%) and microscopy (31.3%) (OR = 0.67, P = 0.2825) with 9.2% discordant RDT false positives. All malaria positive children were Plasmodium falciparum mono-infections. Hospital admissions reduced by 28% between 2005 and 2008, with case fatality rates reducing by 19% between 2006 and 2007 in children under five years. No marked heterogeneity between LLINs ownership (66%) and utilization (48.3%) was observed (OR = 0.49, P = 0.0978). There was complete (100%) community level knowledge of KO-Tab 1-2-3® LLINs. The study provides evidence of potential of KO-Tab 1-2-3® LLINs for operational scale distribution, and substantiates the need for further longitudinal studies to monitor their insecticidal and physical durability.
1. INTRODUCTION
In Zambia, malaria vector control is implemented in the context of the World Health Organization (WHO) strategic framework of integrated vector management (IVM) with Long Lasting Insecticidal Nets (LLINs) playing a pivotal role in the prevention of malaria transmission in both rural and urban areas [1,2]. Despite relatively high coverage, the insecticide durability of conventional insecticide treated nets (ITNs) is considerably variable and requires frequent re-treatment in order to remain effective [3]. The innovation of factory treated LLIN has provided a technical solution to this problem [4-9]. Bayer Environmental Science (BES) has developed a formulation technology “KO-Tab 1-2-3” which offers the prospect of conventional nets being converted into LLIN through a dipping process that can be done post-manufacture under field conditions [10]. Evaluations have shown that long lasting nets have a lower risk of failure (defined as mosquito mortality rates <50%) than conventional nets [11].
Malaria is highly endemic in Zambia with perennial transmission maintained by Anopheles gambiae s.s, Anopheles arabiensis and Anopheles funestus, due to their strong vectorial capacity [12]. Plasmodium falciparum the most virulent parasite species is responsible for 95% of all cases, while the remaining 5% is shared between P. malariae and P. ovale [13]. Accordingly, the WHO recommended interventions; case management (definitive diagnosis with rapid diagnosis tests (RDTs) and microscopy, and treatment with artemisinin-combination therapy) and vector control with LLINs and indoor residual spraying (IRS) have been scaled-up to control malaria. Approximately 24.6 million LLINs have been distributed and 6.07 million houses covered with IRS with varying levels of epidemiological impact [2].
Previous studies have indicated low levels of ITN retreatment rates in the country [14]. While laboratory studies on do-it-yourself’ kits converting ordinary nets to LLINs have been conducted [10], no operational studies have been carried out locally to inform policy-decisions. The “do-it-yourself” long-lasting treatment approach could address the problem of having to regularly retreat conventional ITN and hence could lead to more effective malaria prevention and control [10,11] in a cost effective manner.
This study assessed the performance of KO-Tab 1-2-3® “do-it-yourself” kit converted LLIN programme in Milenge rural district and marked the beginning of transitioning from conventional ITNs to country-wide LLINs distribution in Zambia.
2. METHODOLOGY
2.1. Study Area
Milenge district is situated in the South-Eastern part of Luapula province in Zambia with an elevation of over 1192 metres above sea level. The Luapula River marks the South-Eastern boundary and the main land has a network of rivers and streams. Fishing is the major economic activity in the district with arable agriculture also being practiced. Formal employment levels remain comparatively low. This study was conducted in October 2006 in six villages within the catchment areas of two health facilities; Kapalala Rural Health Centre and Milenge East 7 Clinic, where the KO-Tab 1-2-3® “do-ityourself” kit converted LLNs were distributed.
Intervention
In February 2006, the Ministry of Health (MoH) through the National Malaria Control Programme (NMCP) embarked on a mass ITNs distribution campaign in Milenge district. During this exercise 13,900 conventional nets were distributed and treated with KO-Tab 1-2-3® “do-ityourself” kit. This was the first time the technology was used in Zambia. Prior to being distributed, all the nets were treated at the health facility. Following distribution, an evaluation was conducted to assess the operational impact of the intervention in reducing the malaria infection rates. The quality of net re-treatment and the insecticide durability was also monitored. Simple random sampling was used to generate a sample of households for estimating levels of ITN use and P. falciparum infection prevalence among children under 15 years old.
2.2. Entomological Assessment
2.2.1. Mosquito Collections and Laboratory Processing
Adult mosquitoes were collected in the morning between 06:00 h and 08:00 h by the pyrethrum spray catch (PSC) and mouth aspirated hand catch methods in randomly selected households [15]. Female Anopheles mosquitoes were identified morphologically using standard keys for anophelines of southern Africa [16,17].
2.2.2. Median Time to Knock down Bioassay Test
The median time to knock down (MTKD) bioassays were performed on nets from randomly selected households using the WHO wire ball frame technique [18]. In MTKD bioassays, 11 wild female An. funestus s.l. were introduced into a 15 cm diameter circular wire globe wrapped with the test net with a closable opening through which mosquitoes could be introduced and removed at knockdown. The time of knockdown of each mosquito was noted and the approximate time of knockdown of the median sixth mosquito was recorded. Knock down was defined as collapsed against the netting or fallen to the base of the ball frame and not moving. The time for the eleventh mosquito to be knocked down was the end point of the test. Three replicate tests were carried out on each piece of netting material, giving a total of 9 replicates for each treatment.
2.2.3. Three-Minute Exposure Bioassay Test
Three-minute exposure bioassays were carried out using WHO Plastic cones to determine the quality of impregnation and killing effect of the nets. Batches of five wild type sugar-fed An. funestus s.l. were introduced into a cone fixed to the test net and exposed for 3 (three) minutes after which they were removed and introduced to clean cone cups in which mortality was observed at 1 hr post exposure period. The exposed mosquitoes were then provided with a cotton wool pad soaked in 10% sugar solution and held for 24 h after which mortality was scored. This was repeated ten (times) on different parts of the netting material.
2.3. Parasite Prevalence Survey
Malaria parasite prevalence among children under fifteen years of age was determined by using microscopy and RDTs. Blood was collected via finger prick from randomly selected children with prior consent to participate by parents. The first drop of blood from the finger was wiped clean and subsequent two drops were screened for parasite species and gametocytes by microscopy using 4% Giemsa thick and thin blood smears for 30 minutes [19] and RDTs (ICT Malaria Test®, R and R marketing, Cape Town, South Africa). Blood slides were considered negative if no asexual parasites were found in 200 high-power fields. High-density parasitemia was defined as presence of ≥5000 parasites/µl [20]. Quality control was conducted for all positive slides and 10% of the negative slides [21]. The age range of subjects was stratified into three age categories: 1 - 4, 2 - 10 and 5 - 15. Children testing positive by RDTs were immediately offered free treatment with artemisin in-based combination therapy (ACT) according to the national malaria control programme treatment policy [22].
2.4. Health Facility Records
Malaria-related indicators, i.e., outpatient attendances, hospital admissions, malaria attributed mortality and case fatality rates, from all public health facilities in Milenge District were obtained from the Health Management and Information System (HMIS) records of the Ministry of Health in Zambia. The existing HMIS records were about 95% complete for the period 2005-2008. Data were validated and missing information retrieved by retrospective review of source documents from all health facilities.
2.5. Knowledge and Attitude Survey
A pre-tested structured questionnaire was administered to 31 randomly selected respondents in the study area to determine the proportion of net ownership and their utilization and the knowledge levels on LLINs in the population.
2.6. Data Management and Statistical Analysis
Randomization was calculated for both study sites. Data was collected and entered in Excel spread sheets (Microsoft Corporation) and statistically analyzed by employing Epi Info version 3.2.2. The Chi-square (χ2) test and logistic regression were used to determine the proportional differences in parasite prevalence and discordant test results (RDT positive, microscopy negative) between age categories, and those of mosquito mortalities.
2.7. Ethical Consideration
No specific ethical approval was sought for this study as it was part of the NMCP routine monitoring activities. All parasitological activities were conducted by trained health facility and District Health Management Team personnel. A freely administered informed consent was given to respondents and householders for participation in the study.
3. RESULTS@NolistTemp#3.1. Entomological Assessments
3.1.1. Mosquito Collections
Malaria vector mosquito collections were conducted from a total of 33 households in the two study areas. A total of 386 female Anopheles mosquitoes were collected and identified as An. gambiae s.l. (7%) and An. funestus s.l. (93%). The Anopheles mosquito densities per household ranged from 0 to 25 and 51.3% (95% CI 46.3 - 56.3) were found to have had a blood meal.
3.1.2. Median Time to Knock down Bioassay Test
The median knock down time in minutes for the sixth mosquito was 30 (95% CI 26.3 - 34.0) with the average knock down time ranging from 17 (95% CI 15.1 - 21.5) for the first mosquito to 46 (95% CI 38.9 - 53.1) for the eleventh mosquito (Figure 1).
3.1.3. Three-Minute Exposure Bioassay Test
Mosquito mortality rate post exposure was measured as proportion of deaths among the 10 replicates of 5 mosquitoes each (N = 50) for each net. There was a significant difference in mean mortality between one hour 34% (95% CI 22.4 - 47.9) and 24 hour 80.0% (95% CI 67.0 - 88.8) post exposure OR = 0.13 (95% CI 0.05 - 0.32, P = 0.00002).
3.2. Parasitological Assessments
A total of 272 randomly selected children aged between 1 and 15 years were tested. All 85 microscopy detected infections were caused by P. falciparum. Parasite density of more than 5000/µl of blood was observed. There was no marked disparity in estimated point parasite prevalence between RDTs 40.4% (95% CI: 34.8 - 46.4) and microscopy 31.3% (95% CI: 26.0 - 37.0), OR = 0.67 (95% CI 47.1 - 95.3, P = 0.2825) with discordant RDT false positive percentage of 9.2% (95% CI: 06.3 - 13.2) (Table 1). All 85 microscopy-determined infections were confirmed as RDT positive. A total of 23% (N = 110) of the total RDT positive children were negative by microscopy. Logistic regression controlling for age indicate that children between the ages of 1 - 4 yrs exhibited a higher false positives than the 5 - 15 yrs age groups OR = 0.10 (95% CI: 0.02 - 0.45, P = 0.0006) (Table 1).

Figure 1. Mosquito knock down time in minutes by household.
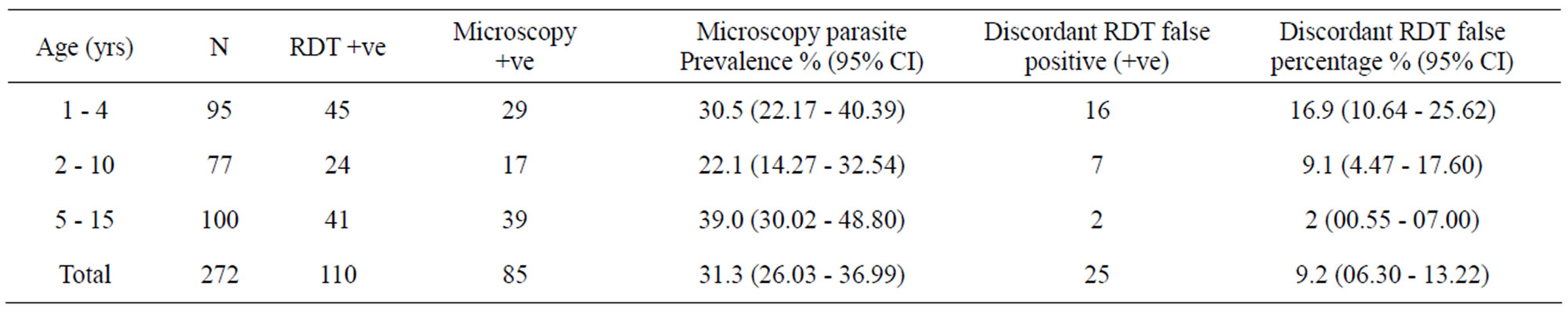
Table 1. Parasite prevalence by microscopy and RDTs and discordant RDTs false percentage.
3.3. Health Facility Surveillance
Malaria-related hospital admissions, outpatient diagnoses, outpatient incidence, malaria-attributed mortality and case fatality rates in children under five between 2005 and 2008 are shown in Table 2. There was no intervenetion effect on Outpatient diagnoses between 2005 and 2008. Hospital admission and outpatient incidence reduced by 28% and 3% between 2005 and 2008 respectively. Malaria-attributed deaths and case fatality rates reduced by 12% and 19% between 2006 and 2007 correspondingly.
3.4. Knowledge and Attitude Survey
Of the respondents, 55% were male, 19% were employed and 75% had primary level education. There was 100% knowledge of what an LLIN was. There was no marked difference between possession of at least one net 66% (95% CI: 47.4 - 80.1) and net use the previous night 48.3% (95% CI: 31.4 - 65.6), OR = 0.49 (95% CI: 0.17 - 1.41, P = 0.0978). While 100% cited the health facility and the radio as their source of information on ITNs use, 86% reported visiting the health facility when they suspect to have malaria.
4. DISCUSSION
Malaria remains a major public health problem in Milenge district and the present findings should be interpreted within the context of high malaria transmission areas. In this study, KO-Tab 1-2-3® LLINs provided effective killing with 80.0% (95% CI 67.0 - 88.8) mean mortality of wild caught An. funestus s.l. at 24 h post exposure. With 51% of the collected indoor-resting female Anopheles mosquitoes being blood-fed, and An. funestus s.l. predominating, sibling malaria vector species within this group could be the ones perpetuating malaria transmission in Milenge. These findings are in agreement with those of previous studies (Table 3). The high level of parasite prevalence has consistently been reported in this region by population-based surveys [13]. This is corroborated by the high point parasite prevalence of 31.3% (95% CI: 26.0 - 37.0) and high-density parasitemia (≥5000/µl) observed in this study. No marked heterogeneity was demonstrated in pertinent malaria related epidemiological parameters using routine surveillance data following LLINs distribution. Malariarelated hospital admissions and outpatient incidence reduced by 28% and 3% between 2005 and 2008 respectively (Table 2). The observed reduction in malaria-attributed deaths and case fatality rates by 12% and 19% between 2006 and 2007 correspondingly, has implications (Table 2). This could be as a result of waning efficacy of the KO-Tab 1-2-3® technology converted LLINs. However, data obtained from routine health facility records have inherent potential pitfalls and need to be interpreted cautiously [23]. While LLINs provide personal protection, and, in settings with sustained high levels of coverage and anthropophilic vectors, they can reduce transmission and protect an entire community [11], in the present study both possession of at least one net 66% (95% CI: 47.4 - 80.1) and net use the previous night 48.3% (95% CI: 31.4 - 65.6) were quite low.
This study was not devoid of limitations. Wild Anopheles mosquitoes were used to assess the threeminute exposure bioassay test instead of a laboratory reared susceptible strain of malaria vectors. An. funestus, the most abundant malaria vector in Milenge is notoriously difficult to rear in the laboratory. In addition, WHO cones are rather awkward and time-consuming for conducting tests of three minutes duration and present a large, non-insecticidal surface area if the test insecticide happens to be repellent [10]. More reliable results would have been obtained if WHO susceptibility test kits were chosen as the exposure chamber for netting because use of these involves less mosquito handling, are easier to do and enable more precise exposures [18]. In this study, children between the ages of 1 - 4 yrs exhibited higher false positives as compared to the 5 - 15 yrs age groups (Table 1).The unusual overlapping 2 - 10 years age group (Table 1) reflects the results for children whose guardians could not state the exact age of the child but an approximate one falling within this range. Despite yielding more positive cases of infection than microscopy, RDTs have the potential to give false positives due to the detection of HRP-2 antigens following a previously treated infection for up to several weeks [24]. If unchecked, the discordant RDT false positive results could potentially lead to misuse of anti-malarial drugs. The
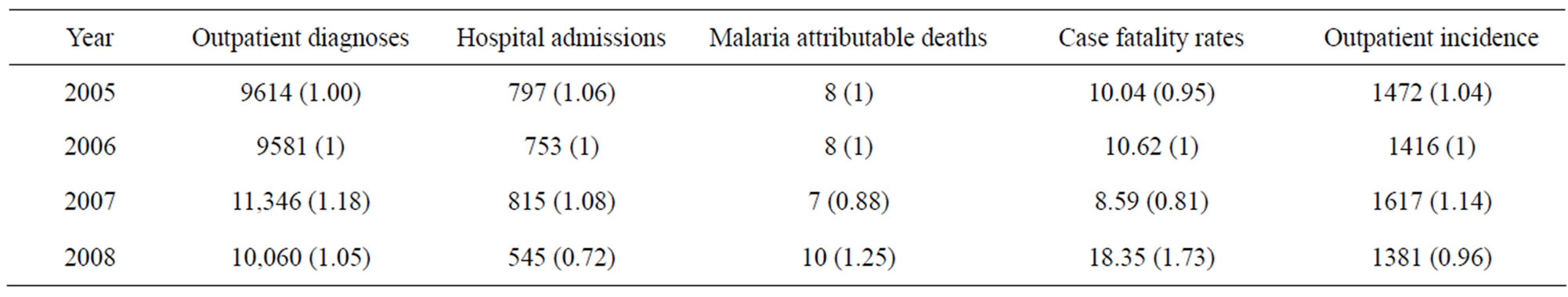
Table 2. Outpatient diagnoses, outpatient incidence, hospital admissions, malaria attributable deaths and case fatality rates in children under five years of age.

Table 3. Comparison of results of the present study with those of previous related studies.
sample size drawn for the Knowledge and Attitude survey was too small to allow for generalization of the findings as relates to the perceptions of the communities.
Insecticide treated nets are an effective preventive mechanism against malaria. If these nets are to remain effective, the insecticide should continue to provide a killing and/or a knock down (KD) effect above the cut off point (80% Killing effect/Mortality and/or 95% KD) for a number of months or after a number of washes [18]. The findings in this study provide useful information to the stakeholders for implementing decisions on the LLINs campaigns in this area of Zambia. KO-Tab 1-2-3® is a new technology, which allows the conversion of ordinary nets into long lasting treatment post manufacturing using ‘do it yourself’ products such as KO-Tab 1-2-3® [10]. With low levels of re-treatment [14], adoption of the new LLINs technology provided an alternative to the costly annual re-treatment campaigns. The study provided the empirical basis for the policy decision by the NMCP to switch from deploying conventional bundled nets to country wide distribution of LLINs in Zambia.
The mortality rate of mosquitoes in cones observed in this study was comparable to those reported under experimental conditions. Graham et al. [9] observed 81.8% mortality and Gonzales et al. [25] observed 87.1% mortality in cones but less than the 100% mortality in cylinders was observed by Yates et al. [10]. Studies have equally shown that LLNs may be more effective in the long term than conventional ITNs [10,11]. Although RDT discordance, or HRP-2 false positivity, was high in children with a reported history of ant malarial treatment, which is consistent with previous research [26], it was higher in younger children contrary to the finding by Keating et al. [24]. Utilisation of LLINs still remains relatively low [14]. However, the high levels of knowledge on LLINs observed in this study have been demonstrated before [13]. There is need to invest in extensive Information Education and Communication/Behaviour Change Communication campaigns to raise the awareness of communities on the importance of using ITNs for malaria prevention.
The currently available but limited insecticide-based vector control tools are being threatened by the development of insecticide resistance in malaria vectors [27]. This is further aggravated by the changing behaviour of the malaria vectors towards out door biting following extensive control efforts [28]. In the quest of endeavouring to find effective and sustainable control interventions, research has been invigorated by the chemical industry [29]. The KO-Tab 1-2-3® technology, constitutes an innovation that provides a potential solution to the expensive and cumbersome annual re-treatment campaigns conducted by malaria endemic countries. The findings in this study substantiate the need for exhaustive insecticide and physical net durability investigations, including vector bionomics and disease epidemiology, in order to generate empirical evidence for decision making. Cognizant of the reality that low coverage of LLINs can not generate the desired impact especially that utilisation is still minimal [30], there is need to step up their distribution to meet the minimum 80% coverage target to afford community effect and the inherent desired impact.
5. CONCLUSION
The study provides evidence of potential of KO-Tab 1-2-3® LLINs for operational scale distribution, and substantiates the need for further longitudinal studies to monitor their insecticidal and physical durability for rational decision making and optimal use of available resources.
6. AUTHORS’ CONTRIBUTION
EC: Co-designed the study, collected and analysed the data, and drafted the manuscript. AK: participated in data collection and reviewing of the manuscript. JC: Assisted with data analysis and reviewing of the manuscript. PCK: Participated in data collection, interpretation and critical review of the manuscript. All authors read and approved the final manuscript.
7. ACKNOWLEDGEMENTS
We would like to acknowledge the invaluable financial and material support from UNICEF-Zambia. Thank you to the officers at the National Malaria Control Programme and Milenge District Health Management Team for coordinating and implementing the intervention respectively.
REFERENCES
- Chanda, E., Masaninga, F., Coleman, M., Sikaala, C., Katebe, C., MacDonald, M., Baboo, K.S., Govere, J. and Manga, L. (2008) Integrated vector management: The Zambian experience. Malaria Journal, 7, 164. doi:10.1186/1475-2875-7-164
- Chanda, E., Mukonka, V.M., Kamuliwo, M., Macdonald, M. and Haque, U. (2013) Operational scale entomological intervention for malaria control: Strategies, achievements and challenges in Zambia. Malaria Journal, 12, 10. doi:10.1186/1475-2875-12-10
- Mwangi, T.W., Ross, A., Marsh, K. and Snow, R.W. (2003) The effects of untreated bednets on malaria infection and morbidity on the Kenyan coast. Transactions of the Royal Society of Tropical Medicine and Hygiene, 97, 369-372.
- N'Guessan, R., Darriet, F., Doannio, J.M.C., Chandre, F. and Carnevale, P. (2001) Olyset net efficacy against pyrethroid-resistant Anopheles and Culex after 3 years’ field usein Côte D’Ivoire. Medical and Veterinary Entomology, 15, 97-104. doi:10.1046/j.1365-2915.2001.00284.x
-
 Muller, O., Ido, K. and Traore, C. (2002) Evaluation of a prototype long-lasting insecticide-treated mosquito net under field conditions in rural Burkina Faso. Transactions of the Royal Society of Tropical Medicine and Hygiene, 96, 483-484. doi:10.1016/S0035-9203(02)90411-6
Muller, O., Ido, K. and Traore, C. (2002) Evaluation of a prototype long-lasting insecticide-treated mosquito net under field conditions in rural Burkina Faso. Transactions of the Royal Society of Tropical Medicine and Hygiene, 96, 483-484. doi:10.1016/S0035-9203(02)90411-6 - Kroeger, A., Skovmand, O., Phan, Q.C. and Boewono, D.T. (2004) Combined field and laboratory evaluation of a long-term impregnated bednet, PermaNet®. Transactions of the Royal Society of Tropical Medicine and Hygiene, 98, 152-155. doi:10.1016/S0035-9203(03)00038-5
- Tami, A., Mubyazi, G., Talbert, A., Mshinda, H., Duchon, S. and Lengeler, C. (2004) Evaluation of olyset insecticide-treated nets distributed seven years previously in Tanzania. Malaria Journal, 3, 1-9. doi:10.1186/1475-2875-3-19
- Graham, K.L., Kayedi, M.H., Maxwell, C., Kaur, H., Rehman, H., Malima, R., Curtis, C.F., Lines, J.L. and Rowland, M.W. (2005) Multi-country field trials comparing wash-resistance of PermaNet™ and conventional insecticide-treated nets against anopheline and culicine mosquitoes. Medical and Veterinary Entomology, 19, 72- 83. doi:10.1111/j.0269-283X.2005.00543.x
- Yates, A., N’Guessan, R., Harparkash, K., Akogbeto, M. and Rowland, M. (2005) Evaluation of KO-Tab 1-2-3@: A wash-resistant “dip it yourself” insecticide formulation for long lasting treatment of mosquito nets. Malaria Journal, 4, 52. doi:10.1186/1475-2875-4-52
- Lindablade, K.A., Dotson, E., Hawley, W.A., et al. (2005) Evaluation of long-lasting insecticide nets after 2 years of household use. Tropical Medicine & International Health, 10, 1141-1150. doi:10.1111/j.1365-3156.2005.01501.x
- Chanda, E., Phiri, F.N., Chanda, J., Ramdeen, V., Kamuliwo, M. and Baboo, K.S. (2012) Impact of entomological interventions on malaria vector bionomics in low transmission settings in Zambia. Journal of Public Health and Epidemiology, 4, 189-196.
- MoH (2000) National malaria situation analysis. Ministry of Health, Lusaka.
- MoH (2006) Zambia national malaria indicator survey report. Ministry of Health, Lusaka.
- MoH (2008) Zambia national malaria indicator survey report. Ministry of Health, Lusaka.
- WHO (1975) Manual on practical entomology in malaria. part II: Methods and techniques. World Heath Organization, Geneva.
- Gillies, M.T. and Coetzee, M. (1987) A supplement to the Anophelinae of Africa South of the Sahara (Afro-Tropical region). Vol. 55, South African Institute for Medical Research, Johannesburg.
- Gillies, M.T. and De Meillon, B.A. (1968) The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). Vol. 54, 2nd Edition, South African Institute for Medical Research, Johannesburg.
- WHO (1998) Test procedures for insecticide resistance, bio efficacy and persistence of insecticides on treated surfaces. World Health Organization, Geneva.
- WHO (1991) Basic laboratory methods in medical parasitology. In: Basic Malaria Microscopy, Part I, World Heath Organization, Geneva.
- Bloland, P.B., Boriga, D.A., Ruebush, T.K., McCormick, J.B., Roberts, J.M., et al. (1999) Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. The American Journal of Tropical Medicine and Hygiene, 60, 641-648.
- WHO (2003) Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. World Health Organization, Geneva.
- MoH (2006) National Malaria Strategic Plan 2006-2011: A Road Map for RBM Impact in Zambia. Ministry of Health, Lusaka.

- Bhattarai, A., Ali, A.S., Kachur, S.P., Martensson, A., Abbas, A.K., et al. (2007) Impact of artemisinin-based combination therapy and insecticide treated nets on malaria burden in Zanzibar. PLOS Medicine, 4, e309. doi:10.1371/journal.pmed.0040309
- Keating, J., Miller, J.M., Bennett, A., Moonga, H.B. and Eisele, T.P. (2009) Plasmodium falciparum parasite infection prevalence from a household survey in Zambia using microscopy and a rapid diagnostic test: implications for monitoring and evaluation. Acta Tropica, 112, 277- 282. doi:10.1016/j.actatropica.2009.08.011
- WHO (2004) Review of: Vectobac WG, PermaNet, Gokilaht-S 5EC. Report of the 7th WHOPES Working Group Meeting WHO/HQ, Geneva, 2-4 December 2003.
-
 Willcox, M.L., Sanogo, F., Graz, B., Forster, M., Dakouo, F., Sidibe, O., Falquet, J., Giani, S., Diakite, C. and Diallo, D. (2009) Rapid diagnostic tests for the home-based management of malaria, in a high-transmission area. Annals of Tropical Medicine and Parasitology, 103, 3-16. doi:10.1179/136485909X384983
Willcox, M.L., Sanogo, F., Graz, B., Forster, M., Dakouo, F., Sidibe, O., Falquet, J., Giani, S., Diakite, C. and Diallo, D. (2009) Rapid diagnostic tests for the home-based management of malaria, in a high-transmission area. Annals of Tropical Medicine and Parasitology, 103, 3-16. doi:10.1179/136485909X384983 - Hemingway, J., Field, L. and Vontas, J. (2002) An overview of insecticide resistance. Science, 298, 96-97. doi:10.1126/science.1078052
- Stevenson, J., St. Laurent, B., Lobo, N.F., Cooke, M.K., Kahindi, S.C., Oriango, R.M., Harbach, R.E., Cox, J. and Drakeley. C. (2012) Novel vectors of malaria parasites in the western highlands of Kenya. Emerging Infectious Diseases, 18, 1547-1549. doi:10.3201/eid1809.120283
- Hemingway, J., Beaty, B.J., Rowland, M., Scott, T.W. and Sharp, B.L. (2006) The innovative vector control consortium: Improved control of mosquito-borne diseases. Trends in Parasitology, 22, 308-312. doi:10.1016/j.pt.2006.05.003
- Rehman, A.M., Coleman, M., Schwabe, C., Baltazar, G., Matias, A., et al. (2011) How much does malaria vector control quality matter: The epidemiological impact of holed nets and inadequate indoor residual spraying. PLoS ONE, 6, e19205. doi:10.1371/journal.pone.0019205
- Badolo, A., Guelbeogo, W.M., Tiono, A.B., Traoré, A., Sagnon, N.F. and Sirima, S.B. (2012) Experimental hut evaluation of Fendona 6SC®-treated bednets and Interceptor® long-lasting nets against Anopheles gambiae s.l. in Burkina Faso. Journal of Vector Borne Diseases, 49: 234-241.
- Oxborougha, R.M., Weira, V., Irish, S., Kaura, H., N’ Guessana, R., Bokob, P., Odjob, A., Metonnoub, C., Yatesa, A., Akogbetob, M. and Rowland, MW. (2009) Is KO Tab 1-2-3® long lasting on non-polyester mosquito nets? Acta Tropica, 112, 49-53. doi:10.1016/j.actatropica.2009.06.005
- Horn, K., Boecker, T., Grofmeyer, D., Nentwig, G. and Stoecker, R. (2005) Technology for conversion of conventional mosquito nets in the field into long lasting bed nets. Proceedings of the Fifth International Conference on Urban Pests, Malaysia, 287-294.
NOTES
*Competing Interests: The authors declare that they have no competing interests.
#Corresponding author.

