Journal of Cancer Therapy
Vol.5 No.7(2014), Article ID:46528,7 pages
DOI:10.4236/jct.2014.57074
CDX2 Overexpression in Barrett’s Esophagus and Esophageal Adenocarcinoma
Leandro Almeida Streher1, Vinícius Campos2, Guilherme da Silva Mazzini2,3*, Marcelo Binato1, Luise Meurer1, Maria Isabel Edelweiss1, Richard Ricachenevsky Gurski1,2
1School of Medicine, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
2Gastrointestinal Surgery Department, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
3Biochemistry Department, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Email: *guimazzini@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 February 2014; revised 12 March 2014; accepted 20 March 2014
ABSTRACT
Background: Patients with Barrett’s esophagus have an increased risk of developing esophageal adenocarcinoma. Our purpose was to determine CDX2 expression in esophageal mucosa and establish a correlation between this marker and the progression of disease. Methods: We analyzed biopsies and surgical specimens from 150 patients who were divided into five groups according to histopathological diagnosis: G1, normal mucosa (n = 29); G2, esophagitis (n = 19); G3, columnar epithelium without intestinal metaplasia (n = 26); G4, Barrett’s esophagus (n = 32), and G5, adenocarcinoma (n = 44). Immuno-histochemical determination of CDX2 expression was considered positive in the presence of nuclear staining. Results: No CDX2 expression was detected in the G1 or G3 groups; 5% of G2, 62.5% of G4 and 70.5% of G5 patients were CDX2 positive. There was a statistically significant difference between the G4 and G5 groups compared to the G1, G2 and G3 (p < 0.05). Conclusions: CDX2 expression was observed among patients with Barrett’s esophagus and adenocarcinoma compared to other groups. CDX2 was not expressed in the phases preceding Barrett’s esophagus, but there was no linear correlation between CDX2 expression and metaplasia-adenocarcinoma progression.
Keywords:CDX2, Barrett’s Esophagus, Intestinal Metaplasia, Esophageal Adenocarcinoma, Immunohistochemistry

1. Introduction
Barrett’s esophagus (BE), defined as the presence of columnar epithelium in the distal esophagus showing specialized intestinal metaplasia (IM), is related to gastric acid and/or duodenal reflux [1] -[5] , and is directly associated with the intensity and duration of gastro esophageal reflux disease (GERD), indicating an adaptive response of the mucosa to the damage caused by the reflux [6] . Additionally, BE predisposes patients to esophageal adenocarcinoma (EA) 30 to 125 times more frequent than in the normal population, with an average cancer risk of 0.5% per year [7] -[9] . EA is a gastrointestinal malignancy with a high mortality rate and a 5-year survival rate of approximately 11%. Metaplastic columnar epithelium may undergo morphological changes and develop into dysplasia, which is the main biological marker of progression to EA [10] [11] . Thus, the identification of a marker to detect intestinal differentiation and progression to EA would have significant clinical importance and cast some light on early diagnosis and prognosis of those patients.
The CDX2 gene belongs to the homeobox family and acts at the initial differentiation and maintenance of intestinal epithelial cells, as well as the structural formation of multiple layers with microvilli [12] . Initially, CDX2 expression was found in large and small bowel [13] , strongly and diffusely expressed in the nucleus of small and large intestinal epithelial cells, including absorptive cells, goblet cells, endocrine cells and Paneth cells. In pancreatic epithelium, however, CDX2 exhibits a focal expression [14] . Esophageal and gastric epithelial cells do not express CDX2, although CDX2 expression has been observed in esophageal and gastric IM [13] [15] -[19] .
Based on these observations, and given the limitations of conventional histological analysis of esophageal biopsies in determining the prognosis of the progression of BE to EA, the aim of the present study was to assess the expression of CDX2 in the esophageal mucosa from patients presenting with GERD, BE and EA, by imunohistochemical analysis. We also sought to determine the potential of CDX2 as an early marker of progression from GERD to EA.
2. Patients and Methods
2.1. Patients
We retrospective analyzed upper endoscopy (UE) biopsies and esophagectomy specimens from patients with GERD symptoms or EA, 30 years old or older, from 2 university hospitals from southern Brazil. All patients were treated between January 2001 and December 2005. Clinical information was obtained by clinical records analysis, with patient’s identities preservation.
The following inclusion criteria were used for the study: 1) the presence of GERD-related symptoms, such as pyrosis and/or regurgitation, at least once a week, with ether normal mucosa, esophagitis or columnar mucosa detected by UE and biopsy with or without intestinal metaplasia; 2) diagnosis of EA or adenocarcinoma of the gastroesophageal junction (Siewert types I or II) [20] .
The exclusion criteria were the following: 1) intestinal metaplasia on esophageal biopsy, but without endoscopic visualization of the columnar mucosa; 2) intestinal metaplasia of the cardia; 3) paraffin blocks with insufficient material; 4) previous oncological treatment; 5) subcardiac adenocarcinomas of the gastroesophageal junction (Siewert type III); 6) antireflux surgery prior to esophageal biopsy in patients with BE.
After review of clinical data and histopathology reports, patients were classified into 5 groups: group 1 (GERD, with normal esofagela mucosa), group 2 (esophagitis), group 3 (columnar epithelium without intestinal metaplasia), group 4 (BE) and group 5 (EA).
Endoscopic biopsies were performed by the same endoscopist, and followed the Seattle protocol [21] . The sample size was calculated based on an absolute difference of 40% between the groups, a power of 80%, and a significance level of 0.05. A minimum of approximately 22 individuals was determined to be necessary for each group.
2.2. Histological Analysis
Tissue samples were fixed in formalin 10%, stained with Hematoxylin-Eosin (H&E) and Alcian blue at pH 2.5 and submitted to histological analysis. The following diagnostic criteria modified from Paull et al. [22] were used for tissue analysis:
1) Normal squamous epithelium: no signs of reflux esophagitis, including absence of hyperplasia of basal cells >20% of epithelial thickness, in addition to the absence of intraepithelial eosinophils. Absence of infectious, dysplastic or neoplastic lesions on biopsy.
2) Cardiac mucosa: simple columnar epithelium formed only by mucous cells without parietal or goblet cells.
3) Oxyntocardiac mucosa: columnar epithelium containing a mix of glands, some constituted only by mucoid cells and some by major and parietal cells. Absence of goblet cells.
4) Intestinal metaplasia (BE): columnar epithelium with clearly defined goblet cells by H&E. Alcian-blue at pH 2.5 was used to confirm H&E findings.
5) Adenocarcinoma: detection of invasion of atypical glands into the lamina propria or into the submucosal layer.
Two blinded pathologists separately analyzed histologic samples. The esophageal biopsy or the EA fragment that best represented the changes was selected for immuno-histochemical analysis.
2.3. Immuno-Histochemical Analysis
Staining for CDX2 protein was performed by incubating samples with primary antibody (NOVOCASTRA, mouse monoclonal antibody; NCL-CDX2; clone AMT28; diluted 1:100) for 15 hours at 4˚C. The streptavidinbiotin complex was used for identification of the primary antibody, and diaminobenzidine-tetrahydrochloride was used as chromogen. Hematoxylin was used for counterstaining. Normal colon biopsy with and without antibody was used for positive and negative controls, respectively. Two independent and blinded pathologists analyzed samples. In cases of disagreement, the final score was reached by consensus. Samples were considered CDX2-positive if any amount of epithelial cells exhibited stained nuclei, as described by Groisman et al. [12] [23] .
2.4. Statistical Analysis
Pearson’s chi-square test or Fisher’s exact test, if necessary, were used to compare groups. For multiple comparisons, we used the partitioned chi-square test and chi-square test for linear trend. The kappa coefficient was used for interobserver agreement. Statistically significant difference was considered for P < 0.05. The analysis was conducted using SPSS for Windows version 12.0 (IBM Corp., USA).
3. Results
3.1. Patients
Were initially enrolled 175 patients, with 25 patients posteriorly excluded: 7 cases of intestinal metaplasia of the cardia (absence of columnar mucosa on UE), 5 cases of subcardiac adenocarcinoma of the gastroesophageal junction (Siewert type III), and 13 cases due to insufficient material. From 150 patients finally analyzed, 29 had normal esophageal mucosa, 19 had esophagitis, 26 showed columnar epithelium without intestinal metaplasia, 32 had BE and 44 had EA.
Table 1 shows patients’ demographic data. The patient group consisted of 89 males and 61 females, with an average age of 59 years (range of 30 - 97 years). There was statistically significant difference in age between the normal UE group and the other groups (P < 0.01). Male predominance was observed in groups 4 and 5 (P < 0.05).
3.2. Upper Endoscopy Findings
The extension of columnar epithelium in patients with BE was 5.29 ± 3.39 cm. Patients with short Barrett’s esophagus (extension < 3 cm) accounted for 23.8% of the patients with BE, whereas patients with long Barrett’s esophagus (extension > 3 cm) accounted for 76.2%. Hiatal hernia was present in 90% of patients with BE, with an average size of 2.95 ± 1.9 cm (mean ± SD).
3.3. Histologic Analysis
Figure 1 shows the pattern of immuno-histochemical staining for CDX2. No patient in the normal group was positive for CDX2 expression, and 5% of patients from the esophagitis group were CDX2-positive. None of the 26 patients with esophageal columnar epithelium without intestinal metaplasia were CDX2-positive, compared
Table 1. Demographic characteristics from patients.
*Younger patients in group 1. **Male predominance in groups 4 and 5. §Statistically significant difference between groups 4 and 5 compared to groups 1, 2 and 3 (P < 0.05).
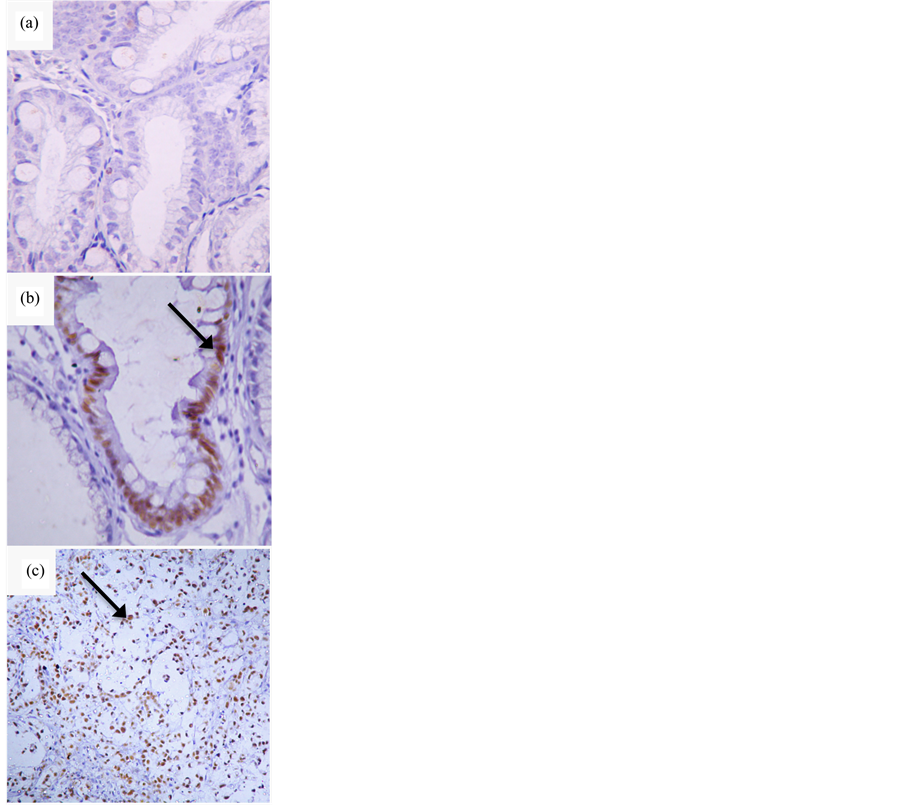
Figure 1. Imuno-histochemical determination of CDX2 expression. (a) Barrett’s esophagus without CDX2 expression (400×); (b) Barrett’s esophagus expressing CDX2 (200×); (c) Adenocarcinoma expressing CDX2 (200×). Arrows point to cell nuclei positive for CDX2.
to 20 (62.5%) out of the 32 patients from BE group who were positive for CDX2. Out of 44 patients from EA group, 31 were positive for CDX2 (70.5%).
Comparative analysis of the results from CDX2 expression revealed a statistically significant difference between groups 4 and 5 and groups 1, 2 and 3 (P < 0.05). No statistically significant difference between the BE and EA groups was observed. Figure 2 shows the distribution of CDX2 expression in different groups.
Although the frequency of CDX2 expression increased from 0%, 5% and 0% in groups 1 - 3 to 62.5% and
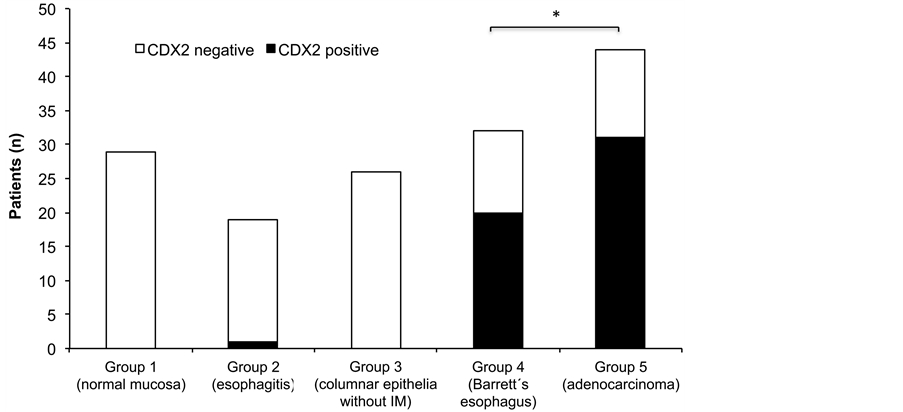
Figure 2. CDX2 expression in patients from different groups. *Statistically significant difference between groups 4 and 5 compared to groups 1, 2 and 3 (P < 0.05).
70.5% in groups 4 and 5, with the use of Fisher’s exact test, no progressive linear trend towards positive CDX2 expression was observed in the progression from esophagitis to BE to EA in our study.
The presence of CDX2 was not associated with the presence of hiatal hernia or the size of BE (data no shown). At EA group, tumor was moderately differentiated in 65% of patients, and no statistically significant correlation was found between CDX2 and the degree of tumor differentiation (data not shown).
The interobserver agreement was tested by the kappa coefficient, which corresponded to 0.97 with a statistical significance of P < 0.001 when CDX2 expression by immuno-histochemical analysis is taken into consideration; this shows a high agreement between the observers in the present study.
4. Discussion
BE is a major risk factor for EA [13] . In those cases, GERD progresses with esophagitis followed by BE and dysplasia in progressive degrees, until the eventual development into EA. Consequently, the identification of a marker for intestinal differentiation in the absence of goblet cells would be of paramount clinical importance to the early diagnosis of disease progression [24] . The present study assessed CDX2 as a marker of different epithelia at the distal esophagus in GERD patients, by immunohistochemical analysis, and found statistically significant higher expression of CDX2 in Barrett’s esophagus and adenocarcinoma, compared with normal mucosa, esophagitis and columnar epithelia without intestinal metaplasia.
We decided to use immunohistochemistry since it has been increasingly used to investigate CDX2 expression. Although less expensive than protein chain reaction (PCR) and easily reproducible, this method requires experienced pathologists due to interobserver variability, as it uses qualitative criteria. Also, specimen collection by esophageal biopsy was preferred, as this technique is easy to perform and is part of the assessment protocol of GERD patients. In addition, we employed the Seattle protocol for esophageal biopsy to minimize the variability of columnar epithelium biopsies or loss of a possible area with IM [21] .
Previous studies have documented the immunohistochemical expression of CDX2 in esophagus [12] [23] . Also, CDX2 expression was determined by PCR in different esophageal epithelia [25] . In a study published by Eda et al. in 2003, 15 patients (34 biopsies) with BE, esophagitis and normal esophageal mucosa were assessed by immunohistochemistry and PCR. CDX2 expression was positive in 100% of patients with BE and in 67% of cases of esophagitis. CDX2 was not detected in the normal esophageal epithelium cases. This was the first study that showed positive CDX2 expression in BE and in esophagitis, and the authors considered the possibility of CDX2 expression as an early event in progression to BE [14] .
Additionally, a study directed to evaluate the regression of BE with proton pump inhibitors treatment showed that, in patients with lower degree of CDX2 expression, together with other markers, the chance for reversibility of BE was higher [26] . This study corroborates the role of CDX2 in esophageal epithelial pathologic differentiation and suggests biomarkers could predict the reversibility of this process.
Despite certain limitations, our study was conducted with a great number of patients, reinforcing the feasibility of imuno-histochemical detection of CDX2 in clinical practice, to evaluate pathologic differentiation of esophageal mucosa. Our results presented over 70% of the EA cases with CDX2-positive biopsies. It has been difficult to prove whether BE was present before EA in these cases, since most of EA cases are at an advanced stage and the area of the peritumoral mucosa is usually compromised by the neoplasm. However, the presence of CDX2 in EA in a frequency statistically similar to those patients with BE raises the possibility to suggest that those cases of EA with CDX2 positive biopsies progressed from BE.
It also should be noted that none of the patients with columnar epithelium without metaplasia were CDX2- positive. The hypothesis that CDX2 could be an early marker to select a subset of patients with greater risk for BE, was not confirmed by our results. Further studies, with follow-up of GERD patients, could determine if a subgroup of patients with columnar epithelium in distal esophagus will express CDX2 before the conventional histologic detection of intestinal metaplasia with goblet cells.
5. Conclusion
We demonstrated that CDX2 is present in UE biopsies from patients with BE and EA, reinforcing the possibility of its use as an imuno-histochemical marker. The employment of diagnostic methods (e.g. high-resolution endoscopy) that can better define suspected areas in the columnar mucosa might enhance the sensitivity of assessment of these biopsies and perhaps shed some light upon this controversy. Moreover, our study supports the possible role of CDX2 in esophageal carcinogenesis, encouraging further clinical and experimental studies using this molecular marker.
Conflicts of Interests
The authors declare there are no conflicts of interests to disclose.
Acknowledgements
The authors would like to thank the financial support from Fundo de Incentivo à Pesquisa e Eventos (FIPE/ HCPA).
References
- Lord, R.V. (1999) Norman Barrett, “Doyen of Esophageal Surgery”. Annals of Surgery, 229, 428-439. http://dx.doi.org/10.1097/00000658-199903000-00018
- DeMeester, S.R. and DeMeester, T.R. (2000) Columnar Mucosa and Intestinal Metaplasia of the Esophagus: Fifty Years of Controversy. Annals of Surgery, 231, 303-321. http://dx.doi.org/10.1097/00000658-200003000-00003
- Caygill, C.P., Watson, A., Lao-Sirieix, P. and Fitzgerald, R.C. (2004) Barrett’s Oesophagus and Adenocarcinoma. World Journal of Surgical Oncology, 7, 12. http://dx.doi.org/10.1186/1477-7819-2-12
- Sampliner, R.E. (2002) Updated Guidelines for the Diagnosis, Surveillance, and Therapy of Barrett’s Esophagus. American Journal of Gastroenterology, 97, 1888-1895. http://dx.doi.org/10.1111/j.1572-0241.2002.05910.x
- Sampliner, R.E. (1998) Practice Guidelines on the Diagnosis, Surveillance, and Therapy of Barrett’s Esophagus. The Practice Parameters Committee of the American College of Gastroenterology. American Journal of Gastroenterology, 93, 1028-1032. http://dx.doi.org/10.1111/j.1572-0241.1998.00362.x
- Conio, M., Lapertosa, G., Blanchi, S. and Filiberti, R. (2003) Barrett’s Esophagus: An Update. Critical Reviews in Oncology/Hematology, 46, 187-206. http://dx.doi.org/10.1016/S1040-8428(02)00123-3
- Jenkins, G.J., Doak, S.H., Parry, J.M., D’Souza, F.R., Griffiths, A.P. and Baxter, J.N. (2002) Genetic Pathways Involved in the Progression of Barrett’s Metaplasia to Adenocarcinoma. British Journal of Surgery, 89, 824-837. http://dx.doi.org/10.1046/j.1365-2168.2002.02107.x
- Ronkainen, J., Aro, P., Storskrubb, T., Johansson, S.E., Lind, T., Bolling-Sternevald, E., Vieth, M., Stolte, M., Talley, N.J. and Agréus, L. (2005) Prevalence of Barrett’s Esophagus in the General Population: An Endoscopic Study. Gastroenterology, 129, 1825-1831. http://dx.doi.org/10.1053/j.gastro.2005.08.053
- Dulai, G.S., Guha, S., Kahn, K.L., Gornbein, J. and Weinstein, W.M. (2002) Preoperative Prevalence of Barrett’s Esophagus in Esophageal Adenocarcinoma: A Systematic Review. Gastroenterology, 122, 26-33. http://dx.doi.org/10.1053/gast.2002.30297
- Rodrigues, M.A.M. (2004) Barret’s Esophagus and Dysplasia: Diagnostic Criteria. Jornal Brasileiro de Patologia e Medicina Laboratorial, 40, 185-191. http://dx.doi.org/10.1590/S1676-24442004000300009
- Schmidt, M.K., Meurer, L., Volkweis, B.S., Edelweiss, M.I., Schirmer, C.C., Kruel, C.D. and Gurski, R.R. (2007) c-Myc Overexpression Is Strongly Associated with Metaplasia-Dysplasia-Adenocarcinoma Sequence in the Esophagus. Diseases of the Esophagus, 20, 212-216. http://dx.doi.org/10.1111/j.1442-2050.2007.00673.x
- Phillips, R.W., Frierson Jr., H.F. and Moskaluk, C.A. (2003) Cdx2 as a Marker of Epithelial Intestinal Differentiation in the Esophagus. American Journal of Surgical Pathology, 27, 1442-1447. http://dx.doi.org/10.1097/00000478-200311000-00006
- Steininger, H., Pfofe, D.A., Muller, H., Haag-Sunjic, G. and Fratianu, V. (2005) Expression of CDX2 and MUC2 in Barrett’s Mucosa. Pathology—Research and Practice, 201, 573-577. http://dx.doi.org/10.1016/j.prp.2005.03.010
- Eda, A., Osawa, H., Satoh, K., Yanaka, I., Kihira, K., Ishino, Y., Mutoh, H. and Sugano, K. (2003) Aberrant Expression of CDX2 in Barrett’s Epithelium and Inflammatory Esophageal Mucosa. Journal of Gastroenterology, 38, 14-22. http://dx.doi.org/10.1007/s005350300001
- Moskaluk, C.A., Zhang, H., Powell, S.M., Cerilli, L.A., Hampton, G.M. and Frierson Jr., H.F. (2003) Cdx2 Protein Expression in Normal and Malignant Human Tissues: An Immunohistochemical Survey Using Tissue Microarrays. Modern Pathology, 16, 913-919. http://dx.doi.org/10.1097/01.MP.0000086073.92773.55
- Werling, R.W., Yaziji, H., Bacchi, C.E. and Gown, A.M. (2003) CDX2, a Highly Sensitive and Specific Marker of Adenocarcinomas of Intestinal Origin: An Immunohistochemical Survey of 476 Primary and Metastatic Carcinomas. American Journal of Surgical Pathology, 27, 303-310. http://dx.doi.org/10.1097/00000478-200303000-00003
- Seno, H., Oshima, M., Taniguchi, M.A., Usami, K., Ishikawa, T.O., Chiba, T. and Taketo, M.M. (2002) CDX2 Expression in the Stomach with Intestinal Metaplasia and Intestinal-Type Cancer: Prognostic Implications. International Journal of Oncology, 21, 769-774.
- Bai, Y.Q., Yamamoto, H., Akiyama, Y., Tanaka, H., Takizawa, T., Koike, M., Kenji Yagi, O., Saitoh, K., Takeshita, K., Iwai, T. and Yuasa, Y. (2002) Ectopic Expression of Homeodomain Protein CDX2 in Intestinal Metaplasia and Carcinomas of the Stomach. Cancer Letters, 176, 47-55. http://dx.doi.org/10.1016/S0304-3835(01)00753-4
- Almeida, R., Silva, E., Santos-Silva, F., Silberg, D.G., Wang, J., De Bolós, C. and David, L. (2003) Expression of Intestine-Specific Transcription Factors, CDX1 and CDX2, in Intestinal Metaplasia and Gastric Carcinomas. Journal of Pathology, 199, 36-40. http://dx.doi.org/10.1002/path.1246
- Stein, H.J., Feith, M. and Siewert, J.R. (2000) Cancer of the Esophagogastric Junction. Surgical Oncology, 9, 35-41. http://dx.doi.org/10.1016/S0960-7404(00)00021-9
- Levine, D.S., Blount, P.L., Rudolph, R.E. and Reid, B.J. (2000) Safety of a Systematic Endoscopic Biopsy Protocol in Patients with Barrett’s Esophagus. American Journal of Gastroenterology, 95, 1152-1157. http://dx.doi.org/10.1111/j.1572-0241.2000.02002.x
- Paull, A., Trier, J.S., Dalton, M.D., Camp, R.C., Loeb, P. and Goyal, R.K. (1976) The Histologic Spectrum of Barrett’s Esophagus. New England Journal of Medicine, 26, 476-480. http://dx.doi.org/10.1056/NEJM197608262950904
- Groisman, G.M., Amar, M. and Meir, A. (2004) Expression of the Intestinal Marker Cdx2 in the Columnar-Lined Esophagus with and without Intestinal (Barrett’s) Metaplasia. Modern Pathology, 17, 1282-1288. http://dx.doi.org/10.1038/modpathol.3800182
- Saporiti, M.R., Almada e Souza, R.C., Pisani, J.C., Amarante, H.M., Carmes, E.R. and Sakamoto, D.G. (2003) Methylene Blue Chromoendoscopy for Barrett’s Esophagus Diagnosis. Arquivos de Gastroenterologia, 40, 139-147.
- Vallbohmer, D., Demeester, S.R., Peters, J.H., Oh, D.S., Kuramochi, H., Shimizu, D., Hagen, J.A., Danenberg, K.D., Danenberg, P.V., DeMeester, T.R. and Chandrasoma, P.T. (2006) Cdx-2 Expression in Squamous and Metaplastic Columnar Epithelia of the Esophagus. Diseases of the Esophagus, 19, 260-266. http://dx.doi.org/10.1111/j.1442-2050.2006.00586.x
NOTES

*Corresponding author.


