Journal of Cancer Therapy
Vol. 4 No. 6A2 (2013) , Article ID: 33972 , 7 pages DOI:10.4236/jct.2013.46A2002
Feasibility and Efficacy Study of Biweekly Irinotecan Combined with Oral Tegafur/Uracil in Advanced Colorectal Cancer
![]()
1Department of Surgery, Natinal Hospital Organization Osaka National Hospital, Osaka, Japan; 2Department of Surgery, Gastroenterological Surgery, Osaka University Graduate School of Medicine, Osaka, Japan.
Email: *mikeda@onh.go.jp
Copyright © 2013 Masataka Ikeda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 6th, 2013; revised June 7th, 2013; accepted June 14th, 2013
Keywords: Colorectal Neoplasms; Pharmacokinetics; Drug Therapy; Fluorouracil; Tegafur/Uracil; UFT
ABSTRACT
Background: We evaluated the feasibility and efficacy of irinotecan (CPT-11) plus tegafur/uracil (UFT) combination chemotherapy in patients with advanced colorectal cancer. Patients and Methods: PK parameters were concurrently measured to confirm the presence of drug interactions in this treatment schedule. CPT-11 was administered intravenously at the dose of 150 mg/m2 on days 1, 15. UFT was administered at the dose of 375 mg/m2/day (B.I.D.) on days 3 - 7, 10 - 14, 17 - 21, 24 - 28 repeated every 5 weeks. Results: 31 patients were enrolled. PK parameters for CPT-11, FT, 5-FU and uracil are available from 5 patients. The overall response rate was 16.1%. The median time to treatment discontinuation was 3.9 months. There was no significant difference in PK parameters of CPT-11 between day 1 and day 15 and of UFT between day 3 and day 10. Conclusion: CPT-11 plus UFT combination chemotherapy exhibited a tolerable toxicity profile with acceptable efficacy. Pharmacokinetic analysis showed that there were no drug interactions in this treatment schedule.
1. Introduction
Intravenous 5-fluorouracil (5-FU) has been the mainstay of chemotherapy in patients with metastatic colorectal cancer (MCRC). Recent investigations on the most suitable infusion method of 5-FU have demonstrated an improved safety and efficacy profile with continuous 5-FU infusion in combination with leucovorin (LV) as compared with bolus administration [1-3]. Continuous 5-FU infusion (e.g. FOLFOX or FOLFIRI regimens) can be given on via indwelling venous catheters and portable infusion pumps. However, indwelling catheter may produce complications such as venous thrombosis [4] or catheter infections in some cases. The needs are, therefore, an alternative administration method for 5-FU, e.g., oral administration, that would yield similar blood levels to those obtained from infusional 5-FU.
UFT (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) is a mixture of tegafur (FT; a prodrug of 5-FU) and uracil at a molar ratio of 1:4 [5]. UFT in combination with oral LV has an equivalent efficacy and survival in patients with MCRC, compared with infusional 5-FU/LV [6]. These equivalence data between UFT and 5-FU are supported by the pharmacokinetic data of 5-FU and FT. When UFT is administered at the dose of 370 mg/m2/day, the area under the plasma concentration time curve (AUC) and the average concentrations for FT were similar to 5-FU continuous infusion at the dose of 250 mg/m2/day [7]. When this trial started, that oral LV had been not yet approved for manufacturing in Japan.
CPT-11 is widely used for the treatment of MCRC combined with 5-FU. CPT-11 is converted to its active metabolite SN-38 and shows its antitumor effect by inhibiting topoisomerase I. In terms of pharmacokinetics, several phases I studied did not show substantial changes in pharmacokinetic parameters of CPT-11 and active metabolite SN-38, in which CPT-11 was administered before bolus or continuous 5-FU infusion. On the contrary, some reports showed drug interactions between
CPT-11 and 5-FU. To improve the treatment convenience of 5-FU infusion, several clinical trails with CPT- 11 and oral UFT have been undertaken in Japan. Yet, there are few pharmacokinetic insights of CPT-11 and UFT combination chemotherapy.
In this study, we set out a feasibility study of CPT-11 in combination with UFT to seek the safety and efficacy profile. To validate the applicable administration dose and schedule, we examined pharmacokinetic parameters of SN-38, FT, uracil and 5-FU.
2. Patients and Methods
Patient eligibility. The eligibility criteria included patients with histologically confirmed diagnosis of colorectal adenocarcinoma with metastatic disease; additional criteria included 1) previous chemotherapy within 1 regimen; 2) an interval of at least 4 weeks after completion of the previous chemotherapy; 3) age 20 to 75 years; 4) Eastern Cooperative Oncology Group (ECOG scale) performance status of 1 or better; 5) a life expectancy of at least 8 weeks; 6) adequate bone marrow, liver, kidney, heart and lung functions (hemoglobin concentration ≥ 10.0 g/dL; white blood cell (WBC) count ≥ 4000/mm3; absolute neutrophil count (ANC) ≥ 2000/mm3, platelet count ≥ 10 × 104/mm3; serum total bilirubin ≤ 1.5 mg/dL; AST ≤ 75 U/L and ALT ≤ 80 U/L, serum creatinine (Cr) ≤ 1.2 mg/dL); and 7) ability for oral intake. Patients were excluded from the study if they had second malignancies, serious complications such as active infection, diarrhea, bowel obstruction, massive ascites or pleural effusion, active gastrointestinal bleeding, uncontrolled diabetes mellitus, underlying cardiac or pulmonary disease, CNS metastasis, or life-threatening drug toxicity.
Study design. This trial is prospective Open-label multicenter randomaized PhaseII trial conducted by the Department of Surgery, Gatroenterological Surgery, Graduate School of Medicine University. This study was conducted according to the Declaration of Helsinki and approved by the Ethics and Scientific Committee of each participating institution.
Treatment schedule. CPT-11 was given at the dose of 150 mg/m2 with 90 - min intravenous infusion of on days 1 and 15. Enteric granule of oral UFT was administered B.I.D. on days 3 - 7, 10 - 14, 17 - 21 and 24 - 28. The dose of UFT was 400 mg for body surface area (BSA) < 1.20 m2, 500 mg for BSA 1.20 - 1.47 m2, and 600 mg for BSA ≥ 1.47 m2. This schedule was repeated every 5 weeks until disease progression, unacceptable toxicity, grade 3/4 AST/ALT elevation or the patient’s refusal for the treatment continuation. Toxicity was assessed before starting each cycle, using the NCI-CTC. Irinotecan infusion doses were reduced to the 80% dosage in subsequent cycles, in case of neutropenia <2000/mm3, plateles < 20,000, and grade 3/4 non-hematotoxicity excluding smatotitis nausea, vomititing, anorexia,aropecia. If grade 3/4 stomatitis was seen in the previous cycle, the dose of UFT was reduced by 100 mg/m2/day. To prevent nausea and vomiting, betamethasone 8 mg and granisetron 3 mg were administered on Days 1 and 15. Patients also received ursodeoxycholic acid 300 mg, metoclopramide 15 mg, magnesium oxide 1.5 g and sodium bicarbonate 3 g after every meal on Days 1 - 4 and Days 15 - 18 with oral alkalization.
Assessment of toxicity. Pretreatment evaluation included a complete medical history and physical examination. Full blood analysis, tumor markers (CEA and CA19-9), chest x-ray, and electrocardiogram were also performed before the treatment. Toxicity was assessed by the National Cancer Institute Common Toxicity Criteria (version 2.0). Especially for the evaluation of anorexia, nausea, vomiting, stomatitis, diarrhea and abdominal pain, the evaluation criteria were translated into an easy-to-understand grading on a record sheet for the subjects to grade these gastrointestinal adverse events on a daily basis.
Assessment of Efficacy. Time to treatment discontinuation was calculated from the initiation of therapy to the date of treatment change. The Kaplan-Meier method was used to plot the time to treatment discontinuation curves. Image inspection was carried out before entry into the study and at least after every two cycles of treatment. The tumor response was evaluated according to the RECIST criteria [8].
Sample collection and processing for pharmacokinetic evaluation. Blood samples were obtained from 11 patients during the first cycle chemotherapy. For the measurement of SN-38 (n = 11) and CPT-11 (n = 5), blood samples were obtained before and at 0, 1, 2, 8, 12 and 24 hr after the drug administration on days 1 and 15. For the measurement of FT, uracil and 5-FU (n = 5 respectively), blood samples were obtained before and at 1, 2, 4, and 8 hr after the morning drug administration on days 3 and 10. The blood was collected from the antecubital vein at a volume of 5 mL each point and preserved in heparinized tubes. The samples were then centrifuged at 3000 rpm for 10 min at 4˚C, and the isolated plasma samples were stored at −20˚C.
Drug assay and pharmacokinetic parameters. Concentrations of CPT-11 and SN-38 were simultaneously determined by reversed-phase HPLC using solid-phase extraction and fluorometric detection. The quantitation limit was 1 μg/L for CPT-11 and 0.5 μg/L for SN-38. Individual plasma concentrations of drugs were fitted in a nonlinear regression analysis model using computer software (WINNONLIN®; Pharsight Corporation, Mountain View, CA). Where applicable, the calculation of the pharmacokinetic parameters included the AUC from time 0 to 24 hours, terminal half-life, and total body clearance was carried out. The maximal concentration and time to reach maximum concentration (Tmax) were obtained from visual inspection of the plasma profiles. Analysis of FT and 5-FU was conducted using HPLC and gas chromatography-negative ion chemical ionization mass spectrometry, according to the method of Matsushima et al. [9]. Their maximum plasma concentrations (Cmax) were determined from the observed highest concentrations after administration of UFT. The AUC was calculated from 0 to 8 h (AUC 0 - 8 h) on days 3 and 10 according to the trapezoidal rule, using the same program as used in the calculation of the AUC for CPT-11 and SN-38.
Statistical Analysis
Measured values of the plasma levels on consecutive days of administration were plotted on a simulation curve that had been prepared based on the single drug administration results. Comparison of the two groups was conducted by two-sided paired t-test. Paired t-test was used to assess significant differences in pharmacokinetics of normalized plasma concentrations of SN-38, FT, LV and 5-FU.
3. Results
Patient characteristics. From September 2001 to September 2003, 31 patients were enrolled. The patient characteristics are listed in Table 1. 9 patients had synchronous metastatic disease at the time of diagnosis, while the remaining 22 patients developed recurrent metastatic disease after curative surgery. The lung, liver and lymph nodes were the major sites of metastasis, and 4 patients with pelvic local recurrences developing after curative resection for rectal cancer were included in this study. 22 patients had undergone curative resection. 4 of them had received no adjuvant therapy and 5 patients had received adjuvant therapy (oral fluoropyrimidine in 4 patients, and fluoropyrimidine plus mitomycin C in 1 patient). 8 patients had received prior chemotherapy for recurrent disease before registration in the study.
Treatment administration. A total of 110 cycles of chemotherapy was administered, with a median of 3.5 cycles per patient. The dose of CPT-11 had to be reduced in 3 patients due to the development of hematologic toxicity in 2 patients and of non-hematologic toxicity in 1 patient. However, there were no patients requiring dose reduction of UFT. The average absolute dose intensities administered were 54 mg/m2/week for CPT-11 (90.4% of the planned dose). 86.9% of the planned dose was administered for UFT.
Efficacy. 29 out of 31 patients were evaluable for the tumor response (Table 2). One patients had a complete
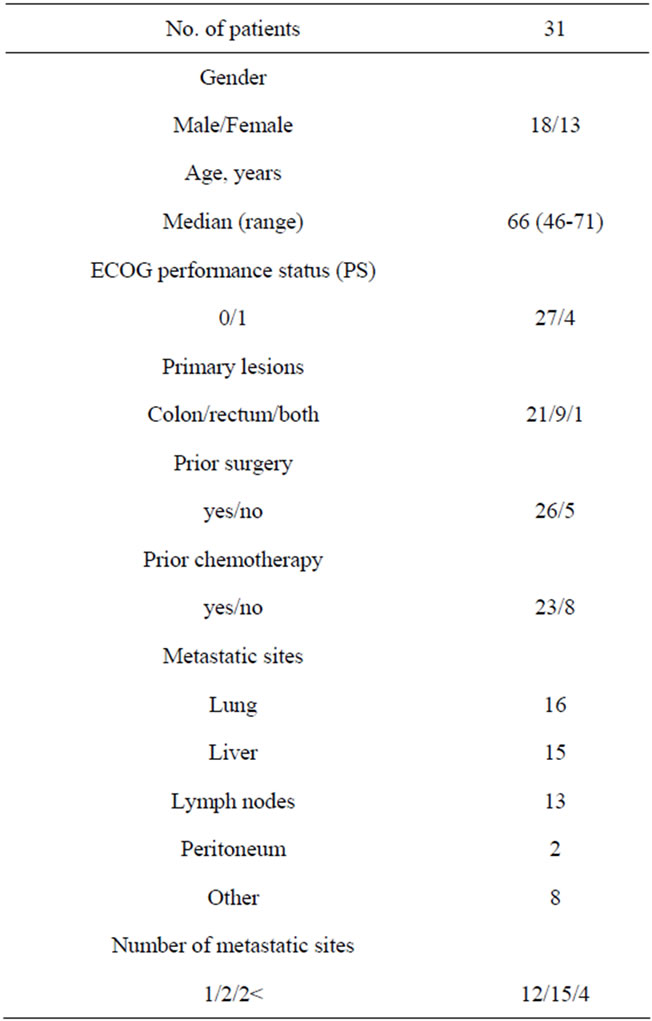
Table 1. Characteristics of patients.
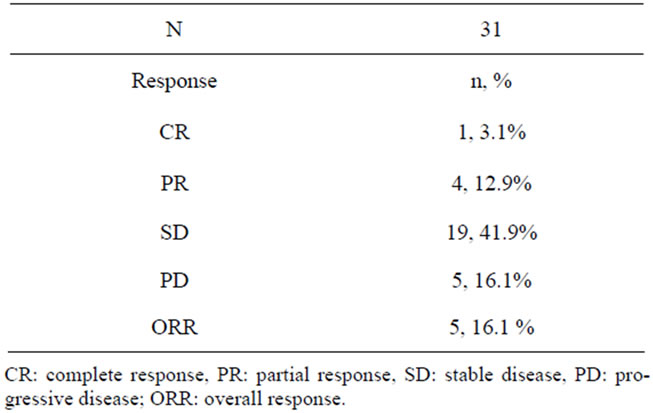
Table 2. Response.
response (CR).The overall response rate was 16% (5/31 patients; 95% CI 5.5 - 33.7). In patients without a history of prior chemotherapy, the response rate was 25% (2/8 patients; 95% CI 3.2 - 65.1). While, the response rate was only 13% in patients with a history of prior chemotherapy (3/23 patients; 95% CI 2.8 - 33.6). Complete response was obtained in 1 patient who had extensive lymphadenopathy. Partial responses were obtained in 4 patients. The time to treatment discontinuation was 3.9 months (Figure 1). The patient who showed complete response still remains alive without disease progression for more than 5 years.
Toxicity. All patients were evaluable for the toxicity (Tables 3(a) and (b)). No patients developed febrile neutropenia. We record over 10% incidences of grade 3/4 toxicity for vomiting (26%), anorexia (16%), diarrhea (13%) and neutropenia (19%). There were a total of 7 dose reduction cases at the planned dose. 3 patients required dose reduction from grade 3/4 neutropenia and 1 patient from grade 3 diarrhea. Apart from them, 1 patient required dose reduction for 3 times, twice from neutropenia and once from grade 3 diarrhea. There were no early deaths within 30 days from the last treatment date.
Pharmacokinetics of SN-38 and CPT-11. The mean Cmax, Tmax, AUC and MRT of SN-38 determined on day 1 were 51.3 (ng/mL), 1.8 (hr), 319.2 (ng hr/mL) and 8.1 (hr), respectively (Table 4). The mean Cmax, Tmax, AUC and MRT of SN-38 on day 15 were 48.0 (ng/mL), 2.0 (hr), 384.0 (ng hr/mL) and 8.6 (hr), respectively. Thus, there were no significant differences in the pharmacokinetic parameters of SN-38 between day 1 and day 15. The mean Cmax, Tmax, AUC and MRT of CPT-11 determined on day 1 were 2.5 (ng/mL), 1.5 (hr), 11.0 (ng hr/mL) and 5.9 (hr), respectively. The mean Cmax, Tmax, AUC and MRT on day 15 were 2.0 (ng/mL), 1.5 (hr), 10.3 (ng hr/mL) and 6.3 (hr), respectively. Thus, there were no significant differences in the pharmacokinetic parameters of CPT-11 between day 1 and day 15.
Pharmacokinetics of 5-FU, uracil and FT. The mean Cmax of 5-FU, uracil and FT determined on day 3 were 250.8 (ng/mL), 7107.3 (ng/mL) and 8179.8 (ng/mL), respectively (Table 5). The mean AUC of 5-FU, uracil and FT were 592.1 (ng hr/mL), 10352.2 (ng hr/ml), and 44452.1 (ng hr/mL), respectively. The mean Cmax of 5-FU, uracil and FT on day 10 were 246.5 (ng/mL),
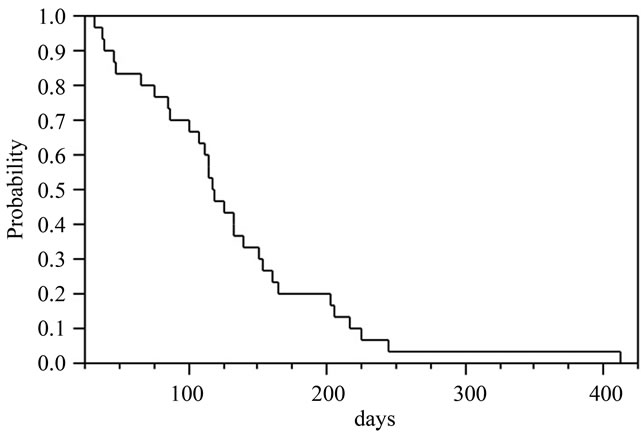
Figure 1. Kaplan-Meier plot of time to treatment discontinuation.
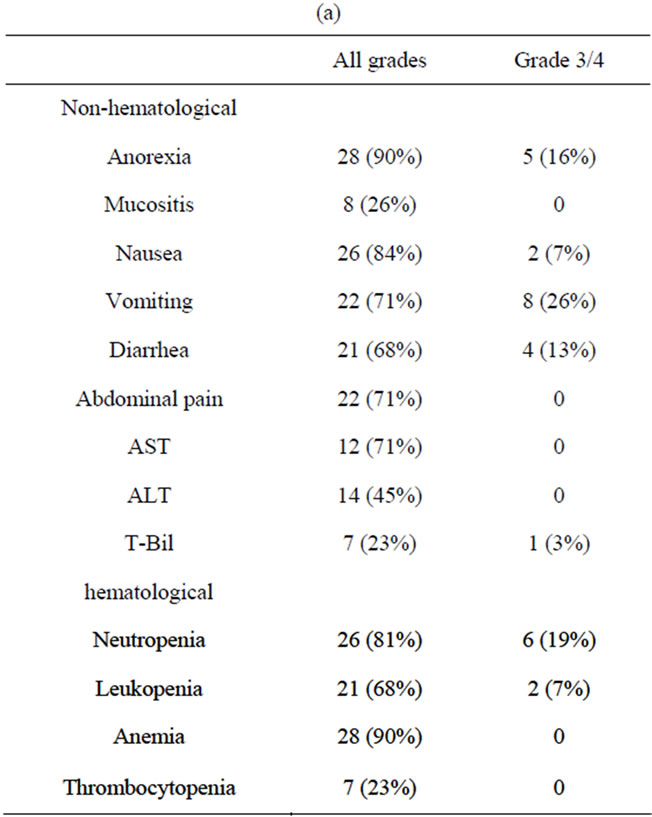
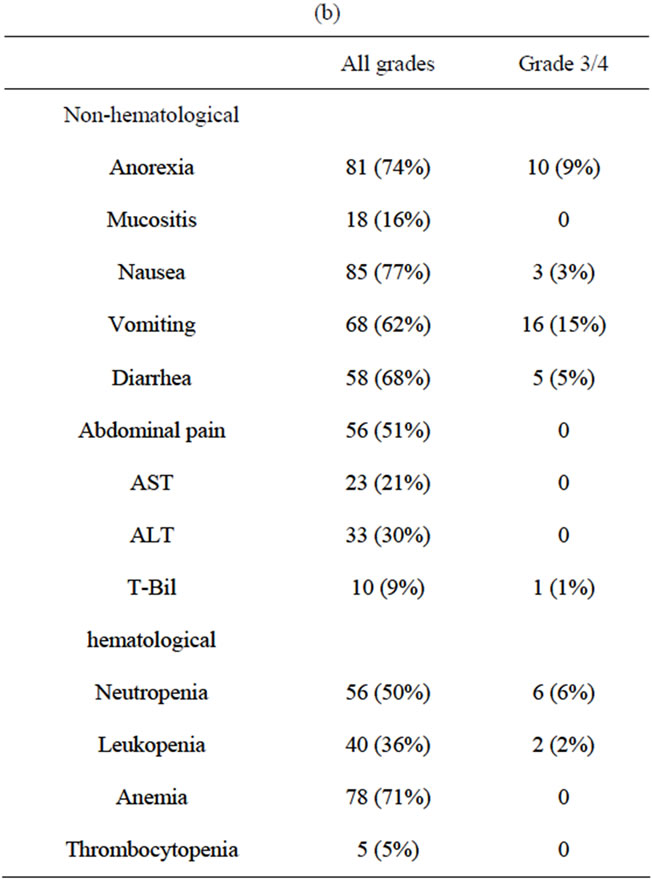
Table 3. (a) Toxicities according to NCI-CTC classification (per patient); (b) Toxicities according to NCI-CTC classification (per cycle).
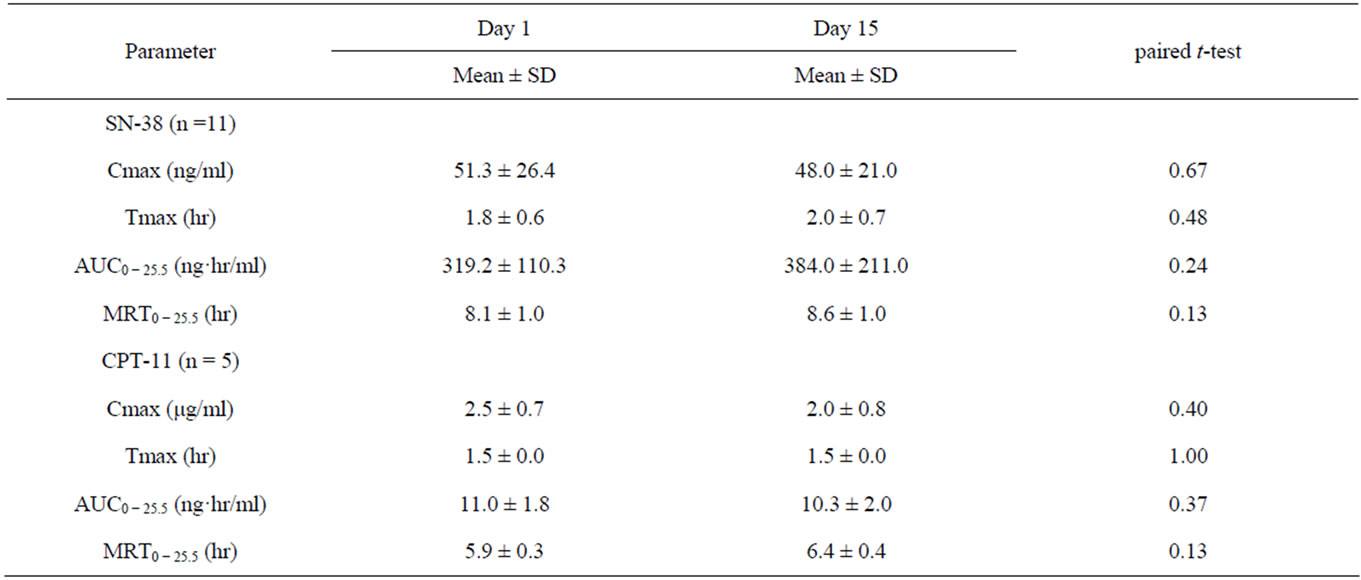
Table 4. Summary of SN-38 and CPT-11 pharmacokinetic parameters.
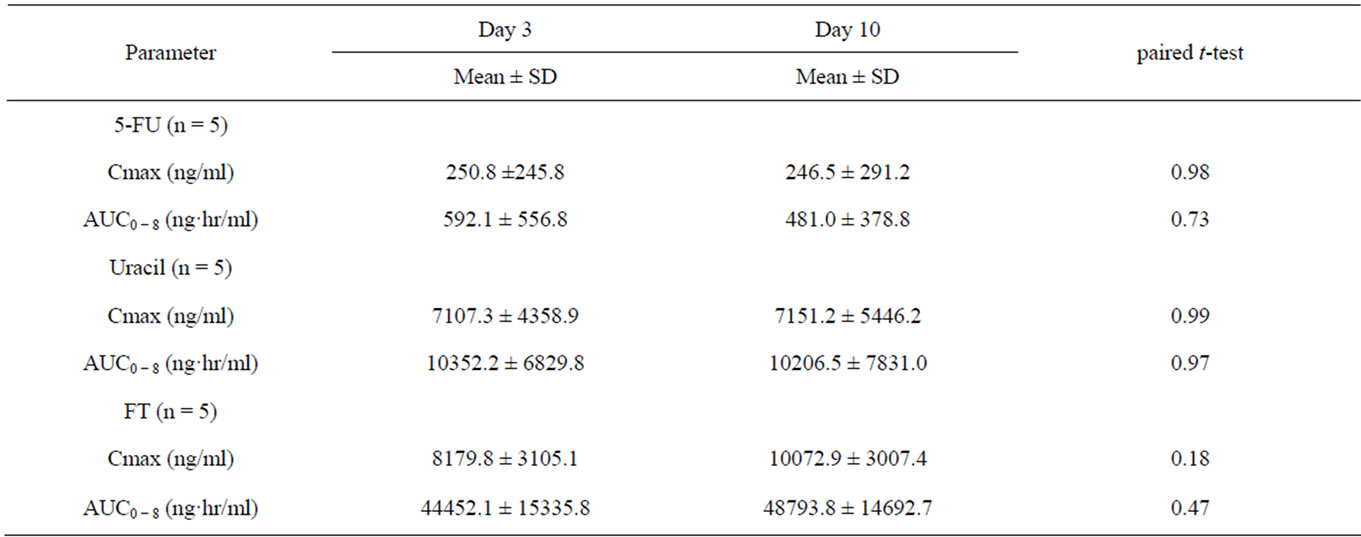
Table 5. Summary of 5FU, Uracil, and FT pharmacokinetic parameters.
7151.2 (ng/mL), and 10072.9 (ng/mL, respectively. The mean AUC of the three drugs on day 10 were 481.0 (ng hr/mL), 10206.5 (ng hr/mL), and 48793.8 (ng hr/mL), respectively. Thus, there were no significant differences in Cmax and AUC parameters of 5-FU, uracil and FT between day 3 and day 10.
4. Discussion
Our feasibility study shows that CPT-11 plus UFT combination chemotherapy is well tolerated with acceptable time to treatment discontinuation with no apparent increased toxicity or pharmacokinetic interactions. Hematological toxicity was comparatively mild, with grade 3/4 neutropenia being seen in 19% and leucopenia 7%. The incidence of grade 3/4 non-hematological toxicity was anorexia occurred in 16%, diarrhea 13%, vomiting 26%, Douillard et al. reported infusional 5-FU/LV plus CPT- 11 should be considered reference, and grade 3/4 toxicities of the resimen were neutropenia 29%, leucopenia 20%, diarrhea 44%, nausea 7% and vomiting 11% [10]. Our study showed that the regimen combining CPT-11 and UFT is considered to be feasible and safe for administration on an outpatient basis.
Although 23 out of 31 patients had received prior treatment, favorable time to treatment discontinuation of 3.9 months was observed in the present study. It was comparable to the 2nd-line FOLFOX or FOLFIRI treatment. The median 2nd-line PFS was 4.2 months for FOLFOX therapy and 2.5 months for FOLFIRI therapy in the GRECOR study. We reported 16.1% of the overall response rate, moreover, the disease control ratio (CR +
PR + SD ratio) was 77.4%. In the GERCOR study, the 2nd-line disease control rate was 63% for FOLFOX chemotherapy and 35% for FOLFIRI chemotherapy [11]. It is considered that the high disease control ratio in the present therapy is correlated with 3.9 months of time to treatment discontinuation.
Oral fluoropyrimidines, such as UFT or capecitabine plays a role in recent developments of chemotherapy in MCRC. In general, oral anticancer drugs are convenient and easy in their administration, which enhanced patients’ compliances and improved quality of patients’ life. Orally administered UFT has clearly been shown to exert significant antitumor activity. The toxicity associated with UFT administration is slight, especially, a low incidence of hand-foot syndrome [12]. For these reasons, oral UFT has been widely used instead of parenteral 5-FU administration in Japan.
With regard to the combined use of CPT-11 and 5-FU, Falcone et al. reported that the AUC of SN-38 was attenuated by concomitant administration of CPT-11 with 5-FU, as a drug interaction [13]. Conversely, Saltz et al. reported that even when these drugs were administered simultaneously, 5-FU had no influence on the pharmacokinetics of CPT-11 or SN-38 [14]. Still, there are no definitive conclusions with the interaction between 5-FU and CPT-11. Our results revealed no significant differences in the Cmax, Tmax and AUC of either CPT-11 or SN-38, or in the Cmax and AUC of 5-FU, uracil or FT. One reason might be avoiding the same day administration of UFT and CPT-11. It is suggested that this treatment schedule does not produce any interaction between CPT-11 and UFT. However, the limitation of our study was not intended to assay the pharmacokinetic parameters in simultaneous administration. We measured FT pharmacokinetic parameters on day 3, which was 2 days after CPT-11 administration on day 1. The measurement of SN-38 pharmacokinetic parameters on day 15 was the next day of the last UFT administration (days 10 - 14). Whereas Falcone et al. and Salts et al. set out the SN-38 pharmacokinetic assessment of CPT-11 and 5-FU which was administered on the same day. Although the interval between the last UFT administration and the SN-38 sample collection on day 15 is less than 24 hours, it is difficult to discuss on the actual presence of drug interaction between CPT-11 and UFT without concomitant drug administration. Further assessments of pharmacokinetic study to reveal the presence of drug interaction between CPT-11 and UFT are necessary.
In conclusion, concomitant use of UFT plus CPT-11 treatment yielded relatively favorable time to treatment discontinuation and disease control rate, associated with mild adverse effects. It is applicable in cases when it is difficult to continue strong chemotherapy, such as elderly patients, 2nd-line treatment, or in case the patient unwilling portable infusion pump treatment. It is important to improve the therapeutic outcome through designing innovative administration methods, and to consider interactions between the constituent drugs by examining pharmacokinetics of oral fluoropyrimidines and CPT-11.
REFERENCES
- T. Andre, P. Colin, C. Louvet, et al., “Semimonthly versus Monthly Regimen of Fluorouracil and Leucovorin Administered for 24 or 36 Weeks as Adjuvant Therapy in Stage II and III Colon Cancer: Results of a Randomized Trial,” Journal of Clinical Oncology, Vol. 21, No. 15, 2003, pp. 2896-2903. doi:10.1200/JCO.2003.10.065
- A. de Gramont, J. F. Bosset, C. Milan, et al., “Randomized Trial Comparing Monthly Low-Dose Leucovorin and Fluorouracil Bolus with Bimonthly High-Dose Leucovorin and Fluorouracil Bolus plus Continuous Infusion for Advanced Colorectal Cancer: A French Intergroup Study,” Journal of Clinical Oncology, Vol. 15, No. 2, 1997, pp. 808-815.
- C. H. Kohne, J. Wils, M. Lorenz, et al., “Randomized Phase III Study of High-Dose Fluorouracil Given as a Weekly 24-Hour Infusion with or without Leucovorin Versus Bolus Fluorouracil plus Leucovorin in Advanced Colorectal Cancer: European Organization of Research and Treatment of Cancer Gastrointestinal Group Study 40952,” Journal of Clinical Oncology, Vol. 21, No. 20, 2003, pp. 3721-3728. doi:10.1200/JCO.2003.11.122
- G. Masci, M. Magagnoli, P. A. Zucali, et al., “Minidose Warfarin Prophylaxis for Catheter-Associated Thrombosis in Cancer Patients: Can It Be Safely Associated with Fluorouracil-Based Chemotherapy?” Journal of Clinical Oncology, Vol. 21, No. 4, 2003, pp. 736-739. doi:10.1200/JCO.2003.02.042
- Y. Maehara, Y. Kakeji, S. Ohno, et al., “Scientific Basis for the Combination of Tegafur with Uracil,” Oncology (Williston Park), Vol. 11, Suppl. 10, 1997, pp. 14-21.
- J. Y. Douillard, P. M. Hoff, J. R. Skillings, et al., “Multicenter Phase III Study of Uracil/Tegafur and Oral Leucovorin versus Fluorouracil and Leucovorin in Patients with Previously Untreated Metastatic Colorectal Cancer,” Journal of Clinical Oncology, Vol. 20, No. 17, 2002, pp. 3605-3616. doi:10.1200/JCO.2002.04.123
- D. H. Ho, R. Pazdur, W. Covington, et al., “Comparison of 5-Fluorouracil Pharmacokinetics in Patients Receiving Continuous 5-Fluorouracil Infusion and Oral Uracil plus N1-(2’-Tetrahydrofuryl)-5-Fluorouracil,” Clinical Cancer Research, Vol. 4, No. 9, 1998, pp. 2085-2088.
- P. Therasse, S. G. Arbuck, E. A. Eisenhauer, et al., “New Guidelines to Evaluate the Response to Treatment in Solid Tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada,” Journal of the National Cancer Institute, Vol. 92, No. 3, 2000, pp. 205-216. doi:10.1093/jnci/92.3.205
- E. Matsushima, K. Yoshida and R. Kitamura, “Determination of S-1 (Combined Drug of Tegafur, 5-Chloro-2,4- Dihydroxypyridine and Potassium Oxonate) and 5-Fluorouracil in Human Plasma and Urine Using High-Performance Liquid Chromatography and Gas Chromatography-Negative Ion Chemical Ionization Mass Spectrometry,” Journal of Chromatography B Biomedical Sciences and Applications, Vol. 691, No. 1, 1997, pp. 95- 104. doi:10.1016/S0378-4347(96)00429-X
- J. Y. Douillard, D. Cunningham, A. D. Roth, et al., “Irinotecan Combined with Fluorouracil Compared with Fluorouracil Alone as First-Line Treatment FOr Metastatic Colorectal Cancer: A Multicentre Randomized Trial,” Lancet, Vol. 355, No. 9209, 2000, pp. 1041-1047. doi:10.1016/S0140-6736(00)02034-1
- C. Tournigand, T. Andre, E. Achille, et al., “Folfiri Followed By FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study,” Journal of Clinical Oncology, Vol. 22, No. 2, 2004, pp. 229-237. doi:10.1200/JCO.2004.05.113
- M. Malet-Martino and R. Martino, “Clinical Studies of Three oral Prodrugs of 5-Fluorouracil (Capecitabine, UFT, S-1): A Review,” Oncologist, Vol. 7, No. 4, 2002, pp. 288-323. doi:10.1634/theoncologist.7-4-288
- A. Falcone, A. Di Paolo, G. Masi, et al., “Sequence Effect of Irinotecan and Fluorouracil Treatment on Pharmacokinetics and Toxicity in Chemotherapy-Naive Metastatic Colorectal Cancer Patients,” Journal of Clinical Oncology, Vol. 19, No. 15, 2001, pp. 3456-3462.
- L. B. Saltz, J. Kanowitz, N. E. Kemeny, et al., “Phase I Clinical and Pharmacokinetic Study of Irinotecan, Fluorouracil, and Leucovorin in Patients with Advanced Solid Tumors,” Journal of Clinical Oncology, Vol. 14, No. 11, 1996, pp. 2959-2967.
NOTES
*Corresponding author.

