Journal of Cancer Therapy
Vol.3 No.4(2012), Article ID:21587,6 pages DOI:10.4236/jct.2012.34031
Pharmacological Isolation of Experimental Models of Drug-Resistant Hepatocellular Carcinoma Cell Line
![]()
Cellular Pathology and Molecular Genetics Group, Department of Life Sciences, Faculty of Science and Technology, Anglia Ruskin University, Cambridge, UK.
Email: benedict.odii@anglia.ac.uk
Received May 12th, 2012; revised June 16th, 2012; accepted June 30th, 2012
Keywords: Cancer; Cell Line; Drug-Resistant; Hepatocellular Carcinoma; Chemotherapy
ABSTRACT
Drug resistance is one of the major challenges facing the success of chemotherapy against human hepatocarcinoma (HCC) as well as other types of cancer. Studies with cell lines can serve as initial screening for agents that could modulate drug resistance. Development of a good experimental model of drug-resistant cells is a prerequisite for the success of such cellular studies; but could be laborious and generally time-consuming. Additionally, the high mortality rate associated with advanced HCC calls for a probe into the mechanism of resistance by developing experimental model that mimics clinical method of its treatment. Consequently, we have reported a simplified method of selection of drug-resistant hepatocarcinoma cells from human hepatocellular carcinoma (HEPG2) cell line using pharmacologic agents, cisplatin (CDDP) and 5-fluorouracil (5-FU). HEPG2 cell line was incubated for 24 hours with different concentrations of CDDP (0 - 20 µM) or 5-FU (0 - 100 µM). Cell viability was assayed by CCK-8 (Cell Counting Kit) analysis, and the inhibitory concentrations (IC50) for CDDP and 5-FU were established by dose-dependent cytotoxicity curves. The IC50(s) were confirmed by flow cytometric analysis of cell death due to CDDP or 5-FU. Clinical method of treatment was imitated by treating the parental HEPG2 cell line in pulse, at the optimal concentration of either CDDP or 5-FU for 4 to 6 hours. Induction was repeated 6 times, whilst allowing the cells to attain at least 70% confluence between intervals of induction. The resultant drug-resistant sub-lines, (HEPG2CR) and (HEPG2FR) were found to be stable after over 3 months of drug withdrawal and maintenance in drug-free medium. This was done with the views of establishing a simple, efficient and direct protocol for the development of good cellular models for the study of drug resistance in liver cancer, with possible application in other cancer types.
1. Introduction
Primary liver cancer is the fifth most common cancer worldwide and the third most deadly, accounting for approximately 600,000 deaths annually. Hepatocellular carcinoma (HCC), a primary malignancy of the hepatocyte, accounts for 85% to 90% of all primary liver cancer; out of which 80% of HCC cases occur in either sub-Saharan Africa or eastern Asia [1,2]. The continuous increase in HCC prevalence and high mortality rate as indicated by average survival rate of less than 12 months for patients diagnosed with advanced HCC, are reflectors of lack of effective therapy [3].
Cisplatin (CDDP) and 5-fluourouracil (5-FU) are two different and frequently used anticancer drugs with wide range of anti-tumour activities. CDDP has been used as a chemotherapeutic agent in many cancers, especially in testicular cancer and epidermal carcinomas of many organs, for which treatment is very successful [4]. 5-FU, a pyrimidine antimetabolite, is one of the first-line treatment options for gastrointestinal tumours and represents the most widely used chemotherapeutic agent in the management of hepatocarcinoma [5]. Treatment of human hepatocellular carcinoma with cisplatin has shown more effectiveness than any other anti-neoplastic agents; and when combined with 5-FU, cisplatin has been shown to induce additive and synergistic results [6,7]. For patients with advanced HCC, intra-arterial combination chemotherapy is one of the few successful options, and continuous use of CDDP and 5-FU has been shown to prolong the survival of such patients [8].
Many resistant tumour cells in humans are gradually acquired during chemotherapeutic administration. Drugresistant cell lines, selected by exposure to anticancer agents could serve as valuable tools for the illumination of factors underlying drug resistance [9]. The development of good experimental model of drug resistant cell line is a vital prerequisite for any study to understand the mechanisms of drug resistance. Until now, though, models for development of drug-resistant cell lines in various cell types have been reported. However, such models have either used the method of increasing continuous administration [10] or low-dosage intermittent incremental inducement [11]. These methods are associated with various and inconsistent dosages, without recourse to clinical method of chemotherapeutic administration from which cancer cells acquire resistance.
In the present study, we established CDDP-resistant and 5-FU-resistant cell lines from parental HEPG2 cell line by imitating the clinical pattern of chemotherapy, the pulse treatment. This was done following the establishment of the optimal concentrations of CDDP and 5-FU at which 50% cell death is achieved (the inhibitory concentration, IC50). In the end, highly stable, drug-resistant sub-lines of HEPG2 cell line were produced, and a simple protocol that adopts clinical method was established. This protocol would serve as a useful guide to researchers intending to produce drug-resistant cell line, especially for the study of drug resistance in HCC and other forms of liver cancer.
2. Materials and Methods
2.1. Pharmacologic Agents
5-fluorouracil (5-FU) and cisplatin (CDDP) (Sigma Aldrich, UK) were dissolved in double distilled water to yield working stocks at concentration of 20 mM and 2 mM respectively. The working stocks were stored at room temperature.
2.2. Cell Line and Culture Conditions
The parental human hepatocellular carcinoma (HEPG2) cell line was obtained from the European Collection of Animal Cell Cultures (ECACC). Cells were cryopreserved and then rapidly thawed and grown in RPMI 1640 medium (Invitrogen, UK), fully supplemented with 10% heatinactivated foetal bovine serum (FBS) (Invitrogen, UK), 1% glutamine (Invitrogen, UK) and 1% penicillin streptomycin (Invitrogen, UK). The physiological conditions of the cells were maintained at 5% CO2 and 37˚C in a humidified atmosphere. Logarithmically growing cells were at the second passage when they were cryopreserved with a freezing medium containing 10% dimethylsulphoxide (DMSO) (Sigma Aldrich, UK) and 90% supplemented RPMI 1640 medium (Invitrogen, UK). The freezing vials containing the cells were cryo-preserved in a cryovial containing isopropanol and maintained at –80˚C for 24 hours; and the vials containing the parental cells were finally maintained as working stock at –196˚C in liquid nitrogen.
2.3. Cell Viability Assay
Cell viabilities in response to chemotherapeutic induction, and the degrees of cytotoxicity of CDDP and 5-FU on the cells were measured using cell counting kit (CCK-8) (Sigma Aldrich, UK). The CCK-8 is made up of Dojindo’s highly water-soluble tetrazolium salt, WST-8[2- (2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt]; which produces a water-soluble, yellowish formazan dye upon reduction in an electron carrier [12]. The number of viable cells is directly proportional to the amount of the formazan dye generated in cells. Assays were done following manufacturer’s instruction. Briefly, cells were seeded at density of 104 cells per well in 96-well plates and preincubated overnight for adherence at physiological conditions of 5% CO2 and 37˚C, in a humidified atmosphere. The cells were then treated at concentrations of 0 µM to 20 µM for CDDP; and 0 µM to 100 µM for 5-FU. After 12 to 24 hours incubation, 10 µl of CCK-8 was added to each well and incubated at 37˚C for 2 hours; followed by the measurement of absorbance using an automated micro-plate reader ELx 800 (BioTek, UK) at 450 nm. Each experiment was done in triplicates and the results were the mean of at least three independent measurements and expressed as percentage of the absorbance of untreated control cells ± S.E.M.
2.4. Determination of Inhibitory Concentrations
The inhibitory concentration otherwise known as IC50, is the optimal concentration at which the survival rate of HEPG2 cells reached the minimum following exposure to an appropriate anticancer drug [13]. Determination of this concentration (IC50) is primordial to the selection of cells for any drug resistance. Briefly, cells at the logarithmic growth phase (80% confluence) were harvested and seeded at a density of 104 cells per well in 96-well plates and incubated overnight for proper adherence. Subsequently, the drug-free medium was replaced with prewarmed fully supplemented fresh medium, containing 0 µM to 100 µM of 5-FU or 0 µM to 20 µM of CDDP. The cells were incubated for 24 hours, while maintaining the physiological conditions at 5% CO2 and 37˚C temperature, in a humidified atmosphere. After 24 hours incubation, cell viability assessment was conducted colorimetrically using cell counting kit-8 (CCK-8) (Sigma Aldrich, UK). The optical densities (OD) of cells treated at different drug concentrations were measured at 450 nm using a micro-plate reader ELx 800 (BioTek, UK); and cell viability was calculated as a measure of the optical density (OD) of treated cells relative to the optical density of the untreated controls, excluding the OD of blank controls. Dose-dependent response curve was plotted and the respective inhibitory concentrations (IC50) of CDDP and 5-FU were established.
2.5. Flow Cytometric Analysis of Cell Death
The inhibitory concentrations (IC50) were confirmed by the flow cytometric analysis of cell death using Annexin V-FITC kit (BD Biosciences, San Diego). Briefly, HEPG2 cells were grown to 80% confluence, and detached using 0.25% (w/v) trypsin in 5 mM EDTA (Invitrogen). The cells were seeded at the rate of 2 × 105 per ml of RPMI 1640 medium (Invitrogen), supplemented with 10% foetal bovine serum (Invitrogen), 1% Penicillin streptomycin (Invitrogen), and 1% L-glutamine (Invitrogen). After 24 hour incubation in appropriate concentrations of CDDP (0 - 16 µM) or 5-FU (0 - 90 µM), under physiological conditions of 5% CO2, 37˚C, in a humidified atmosphere; the cells were detached by trypsinization, washed and re-suspended in binding buffer. The cells were analysed for apoptosis following mixing with Annexin VFITC and propidium iodide (PI), and incubation in the dark for 5 minutes. Analysis was done using flow cytometer FACSCalibur (BD Biosciences, Europe).
2.6. Selection of Cells for Resistance
In selection of the cells for resistance, clinical method of treatment was adopted. The parental HEPG2 cell line was treated in pulse, at the IC50 of either CDDP or 5-FU for 4 to 6 hours. Induction was repeated 6 times, whilst allowing the cells to attain at least 70% confluence between induction intervals. After 6 complete cycles of induction, selected cells were maintained in drug-free RPMI 1640 medium containing appropriate supplements, and passaged at the attainment of 70% - 80% confluence. No further experiment was performed on the cells until after 4 weeks maintenance in drug-free medium, whilst testing the stability of the developed resistance.
2.7. Test for Resistance
The stabilities of the selected clones (HEPG2CR) and (HEPG2FR) were tested after 2 weeks, one month and 3 months of drug withdrawal. Briefly, the selected clones were harvested at exponential growth phase using 0.25% trypsin-EDTA. Cell counting was performed using heamatocytometer, and the cells were seeded in 96-well plate at the rate of 104 cells per well, in triplicates. Plates were maintained at 37˚C in a humidified atmosphere of 5% CO2. After overnight incubation for attachment, cells were incubated for 24 hours in RPMI 1640 medium containing appropriate concentration of CDDP (0 - 20 µM) or 5-FU (0 - 100 µM). Following the 24 hours incubation, the cells were further incubated for 2 hours in the presence of 10 µl of CCK-8 per well. The optical densities were measured and the new IC50 was obtained from a dose-dependent cytotoxicity curve for each of the drugs as previously described. The difference between the IC50 of the resistant clone and that of the parental cell line defines the degree of resistance.
3. Results
3.1. Pharmacological Induction of Cell Death and Measurement of Cell Viability
To understand the effect of CDDP or 5-FU on HEPG2 cell viability and establish the degree of cytotoxicity due to these drugs, HEPG2 cell line was incubated for 24 hours with 0 µM to 20 µM of CDDP or 0 µM to 100 µM of 5-FU. Cell viabilities were measured by CCK-8 assay and HEPG2 cell line was found to be more susceptible to CDDP than 5-FU. Furthermore, with 5-FU, cytotoxicity was found to be directly proportional to dose and time. However, for CDDP, an approximately reciprocal degree of cytotoxicity was obtainable at both 12 hours treatment and 24 hours treatment with similar concentration (Figure 1).
3.2. Establishment and Confirmation of Inhibitory Concentrations (IC50)
To determine the concentration of CDDP or 5-FU beyond which HEPG2 cell survival was minimum, the cells were exposed to different concentrations of CDDP (0 µM to 20 µM) or 5-FU (0 µM to 100 µM) for 24 hours. The respective inhibitory concentration was calculated from a dose-dependent response curve, following analysis of cell viability by CCK-8 assay. For CDDP, cell survival was minimal beyond 4 µM (Figure 1), but in the case of 5-FU, cell survival was minimal beyond 50 µM (Figure 2). Hence, the IC50 of CDDP was found to be 4 µM while that of 5-FU was 50 µM. The inhibitory concentrations were confirmed by the flow cytometric analyses of cell deaths due to CDDP or 5-FU (data not shown).
3.3. Development of Drug Resistant Sublines and Test for Resistance
Following the establishment of the concentration of CDDP or 5-FU at which HEPG2 cell viability is reduced by 50%, the resistant sublines HEPG2CR and HEPG2FR were established by incubating the parental HEPG2 cell line at the IC50 of CDDP (4 µM) or 5-FU (50 µM, whilst imitating the clinical method of treatment. The cells were found to have developed resistance to either CDDP or 5-FU following six intervals of induction. A test for resistance was conducted after 2 weeks of growth in drugfree medium, and a comparison of the IC50 of the parental cells and those of the resistant cells revealed a remarkable increase. For CDDP, the IC50 of HEPG2CR was double that of the parental cell line, hence a 100% increase (Figure 3). However, for 5-FU, the IC50 of
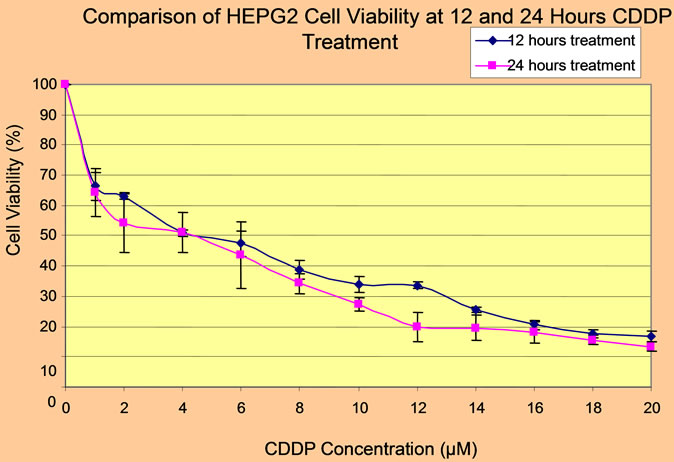
Figure 1. CCK-8 assay of viability of HEPG2 cell line in response to CDDP at different intervals.
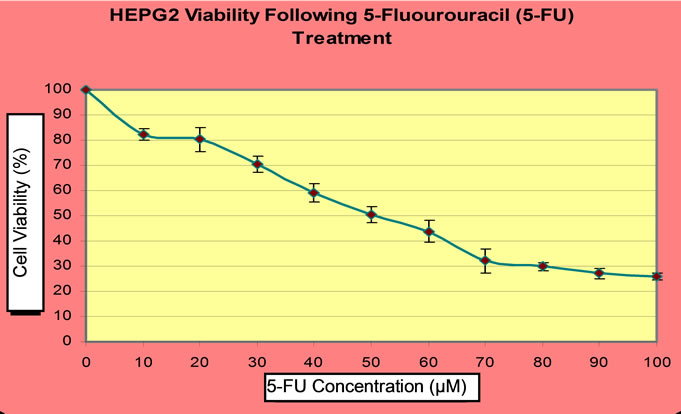
Figure 2. CCK-8 assay of HEPG2 cell viability following 5-FU treatment.
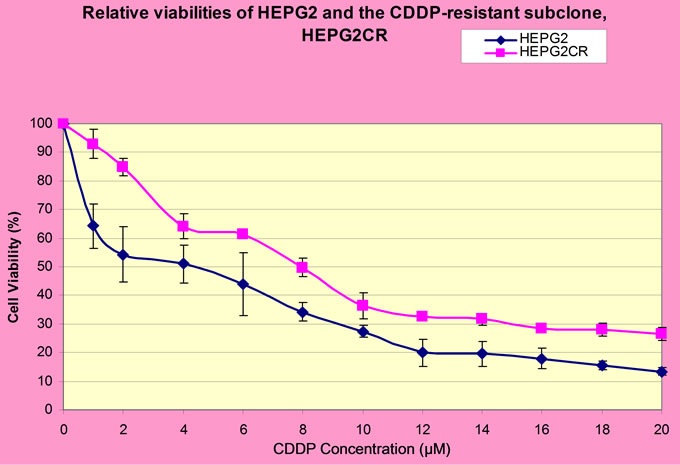
Figure 3. A comparison of the IC50 of parental HEPG2 cell line and that of the resistant subline (HEPG2CR).
HEPG2FR was found to be 65 µM as against the 50 µM of the parental cell line (Figure 4).
4. Discussion
Many resistant tumour cells in humans are gradually acquired during chemotherapy. Drug-resistant cell line models serve as useful paradigms for the clinical scenario of
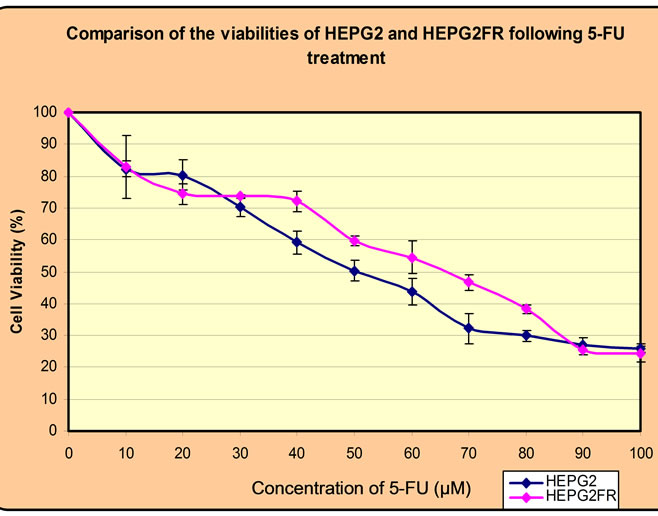
Figure 4. Relative IC50(s) of HEPG2 cell line and the 5- FU-resistant clone, HEPG2FR.
anticancer drug resistance. Cell line models with acquired resistance to a variety of anticancer agents have been variously developed in the quest to unravel the mechanisms underlying clinical drug resistance [13-15].
Primary liver cancers, especially HCC, are associated with very low survival rate and high mortality rate, occasioned by a high degree of resistance to chemotherapy. It is therefore, vitally important to carry out studies targeted to understanding the factors underlying the clinical drug resistance abilities of liver cancers. Such studies require good models of drug-resistant cell lines that are reflective of clinical drug resistance scenario for the cancer type under investigation. Consequently, we have reported herein, a simple, stepwise protocol for the establishment of drugresistant models of liver cancer, whilst imitating the clinical method of treatment. The human hepatocellular carcinoma (HEPG2) cell line was used as a model in the presence of anticancer agents, CDDP and 5-FU.
The protocol primarily involves choice and establishment of parental cell line, assessment of sensitivity of parental cell line to drugs against which resistance is to be developed, establishment of the IC50 dose, selection of resistant cells, and test for resistance and stability.
The first step during the development of drug-resistant cell line is the choice of parental cell line. This is subject to a number of factors, such as tumour type and its relevance to the selecting agents under consideration [16]. The human hepatocellular carcinoma (HEPG2) cell line was chosen because it is the most prevalent [1,2] and deadliest [3] form of liver cancer. In relation to the selection agents, CDDP and 5-FU combination therapy is the most sensitive and preferred treatment option for patients with advanced HCC patients [8].
Following the choice of parental cell line, it is important to establish the degree of sensitivity of the cell line to the drugs in question. Such drug sensitivity assay is a prerequisite to the identification of the initial dose of treatment [16]. The sensitivity of parental HEPG2 cell line to CDDP or 5-FU was tested by measuring its viability using the cell counting kit-8. The results of the assays revealed that HEPG2 cell line is more sensitive to CDDP than 5-FU. The inhibitory concentration for CDDP or 5-FU was calculated as 4 µM or 50 µM respectively, from dose-dependent cytotoxicity curves.
On establishing the appropriate concentrations of CDDP and 5-FU at which the resistant sub-lines of HEPG2 cell line could be produced, the resistant cell lines were then established at these concentrations as described in the method section. The selection process mimicked the clinical method of treatment, as the parental cell line was treated in pulse, whilst allowing the cells to recover between treatment intervals. The resistant cell lines, HEPG2CR and HEPG2FR, were maintained in drug-free medium after complete selection. After the sixth induction, an increase in the IC50 for both drugs was an indication of resistance development. The IC50 of CDDP-resistant HEPG2 cell line doubled that of the parental cell line, whereas, that of 5-FU-resistnat cell line increased by a shorter margin relative to that of the parental cell line. This shows that HEPG2 cell line has stronger cytotoxicity memory for 5-FU; hence, the faster development of resistance to 5-FU relative to CDDP. The stability of the resistant cell lines was tested and they were found to be very stable in drug-free medium, as indicated by constant IC50 after months of maintenance in drug-free medium. This is against some reports that drug-resistant cell lines needed to grow in drug-containing medium in order to retain the stability of drug resistance [17]. Hence, our research provides the basis for the establishment of highly stable drug-resistant cell line, with minimal cost of production and maintenance.
In conclusion, development of good experimental models of drug-resistant cells is indispensable to the success of cellular and molecular studies of drug resistance mechanisms. The high mortality rate associated with advanced HCC calls for a probe into its mechanism of resistance to chemotherapy. Consequently, the protocol reported herein, could serve as a simplified method of selection of drugresistant hepatocellular carcinoma cells from human hepatocellular carcinoma (HEPG2) cell line using pharmacologic agents and assuming clinical treatment pattern. The stepwise method of selection as outlined, could serve as a firsthand guide for the selection of drug-resistant cell line needed for any liver cancer-related drug-resistance studies.
5. Acknowledgements
The project that resulted to this article was fully sponsored by the Government of Ebonyi State, Nigeria. Thanks to Martins, N. Elechi, the executive governor of Ebonyi State, for releasing the funds needed for this research.
REFERENCES
- M. Kumar, X. Zhao and X. W. Wang, “Molecular Carcinogenesis of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: One Step Closer to Personalized Medicine?” Cell & Bioscience, Vol. 1, No. 1, 2011, p. 5.
- D. M. Parkin, F. Bray, J. Ferlay and P. Pisani, “Global Cancer Statistics,” CA: A Cancer Journal for Clinicians, Vol. 55, No. 1, 2005, pp. 74-108. doi:10.3322/canjclin.55.2.74
- A. K. Nowak, P. K Chow and M. Findlay, “Systemic Therapy for Advanced Hepatocellular Carcinoma: A Review,” European Journal of Cancer, Vol. 40, No. 10, 2004, pp. 1474-1484. doi:10.1016/j.ejca.2004.02.027
- D. Wang and S. J. Lippard, “Cellular Processing of Platinum Anticancer Drugs,” Natural Reviews. Drug Discovery, Vol. 4, No. 4, 2005, pp. 307-320. doi:10.1038/nrd1691
- Y. X. Li, Z. B. Lin and H. R. Tan, “Wild Type p53 Increased Chemosensitivity of Drug-resistant Human Hepatocellular Carcinoma Bel7402/5-FU Cells,” Acta Pharmacologica Sinica, Vol. 25, No. 1, 2004, pp. 76-82.
- M. Okamura, K. Hashimoto, J. Shimada and H. Sakagami, “Apoptosis-Inducing Activity of Cisplatin (CDDP) against Human Hepatoma and Oral Squamous Cell Carcinoma Cell Lines,” Anticancer Research, Vol. 24, No. 1, 2004, pp. 655-661.
- H. Tanioka, et al., “Combination Chemotherapy with Continuous 5-Fluorouracil and Low-Dose Cisplatin Infusion for Advanced Hepatocellular Carcinoma,” Anticancer Research, Vol. 23, No. 1, 2003, pp. 1891-1897.
- H. Nagai and Y. Smino, “Therapeutic Strategy for Advanced Hepatocellular Carcinoma by Using Combined Intra-Arterial Chemotherapy,” Recent Patents on Anticancer Drug Discovery, Vol. 3, No. 1, 2008, pp. 220-226. doi:10.2174/157489208786242296
- X. Yan, et al., “Biological Comparison of Ovarian Cancer Resistant Cell Lines to Cisplatin and Taxol by Two Different Administrations,” Oncology Reports, Vol. 17, No. 1, 2007, pp. 1163-1169.
- D. Vandier, et al., “Transactivation of the Metallothionein Promoter in Cisplatin-Resistant Cancer Cells: A Specific Gene Therapy Strategy,” Journal of the Natinonal Cancer Institute, Vol. 92, No. 1, 2000, pp. 642-647. doi:10.1093/jnci/92.8.642
- A. K. Godwin, et al., “High Resistance to Cisplatin in Human Ovarian Cancer Cell Lines Is Associated with Marked Increase of Glutathione Synthesis,” Proceedings of the National Academy of Science of the United States of the America, Vol. 89, No. 7, 1992, pp. 3070-3074. doi:10.1073/pnas.89.7.3070
- S. Shibata, et al., “Measuring Cell Viability and Proliferation,” The Journal of Immunology, Vol. 177, No. 1, 2006, p. 3564.
- L. Zhang, et al., “Fluorouracil Selectively Enriches Stemlike Leukemic Cells in a Leukemic Cell Line,” International Journal of Biological Sciences, Vol. 6, No. 5, 2010, pp. 419-427.
- G. Chen, et al., “Prevalence of Multidrug Resistance Related to Activation of the mdr1 Gene in Human Sarcoma Mutants Derived by Single Step Doxorubicin Selection,” Cancer Research, Vol. 54, No. 1, 1994, pp. 4980-4987.
- H. Takeshita, et al., “Experimental Models for the Study of Drug Resistance in Osteosarcoma: P-glycoprotein-Positive, Murine Osteosarcoma Cell Lines,” The Journal of Bone and Joint Surgery, Vol. 78, No. 1, 1996, pp. 366- 375.
- H. M. Coley, “Development of Drug-Resistant Models,” In: S. P. Langdon, Ed., Methods in Molecular Medicine, Vol. 88: Cancer Cell Culture: Methods and Protocols, Humana Press Inc., Totowa, 2004, pp. 267-274.
- P. R. Twentyman, N. E. Fox, K. A. Wright and N. M. Bleehen, “Derivation and Preliminary Characterisation of Adriamycin-Resistant Lines of Human Lung Cancer Cells,” British Journal of Cancer, Vol. 53, No. 1, 1986, pp. 529- 537. doi:10.1038/bjc.1986.83

