Natural Science
Vol. 5 No. 8A2 (2013) , Article ID: 35845 , 9 pages DOI:10.4236/ns.2013.58A2002
Use of ultrasound in food preservation
![]()
Department of Food Engineering, Faculty of Engineering, University of Gaziantep, Gaziantep, Turkey; *Corresponding Author: aykac@gantep.edu.tr
Copyright © 2013 Songül Şahin Ercan, Çiğdem Soysal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 25 June 2013; revised 25 July 2013; accepted 2 August 2013
Keywords: Ultrasound; Thermosonication; Manosonication; Food Preservation
ABSTRACT
Ultrasound is versatile and innovative technology due to its wide range of application and increase in knowledge and research studies. It is used in food industry for many purposes including analysis methods and food processings such as freezing, cutting, drying, tempering, homogenization, degassing, antifoaming, filtration and extraction. Ultrasound can be used as a promoter or alternative to food processing. There may be numereous advantages of using ultrasound for food processing such as effective mixing, increased mass transfer, reduced energy, reduced temperature and increased production rate. Due to the elimination of microorganisms and enzymes without destroying nutrients of foods, ultrasound can be used as an alternative method to thermal treatments in the food preservation. Additionally, low power ultrasound is thought to be an attractive nonthermal method due to overcome problems which occur during heat treatments such as physical and chemical changes, nutritional loss and change in organoleptic properties. This review summarizes mechanism, operation and latest potential applications of ultrasound in the food preservation.
1. INTRODUCTION
Foods are complex materials containing proteins, vitamins, carbohydrates, enzymes, fats, minerals, water and other organic ingredients with differing compositions. Processing and preservation of these foods require variety of different applications and cautions. Use of ultrasound in food processing includes extraction, drying, crystallization, filtration, defoaming, homogenization, meat tenderization and also use of ultrasound as preservation technique. Microbial and enzyme inactivation by use of ultrasound makes it possible to use in food preservation. Preservation techniques are applied to preserve foods for a long time and heat treatment is the most widely used method due to its high efficiency on microbial and enzyme inactivation. However many food ingredients are sensitive to heat and can be lost during thermal processing. Additionally, increasing consumer demand for minimally processed good quality and safe food products with natural flavor and taste, free from additives and preservatives, causes the need for the development of nonthermal methods for food preservation [1].
Ultrasound is one of the nonthermal methods that are used for foods in the last decades. It can be applied to solid, liquid and gas systems for different purposes. Its instrumentation can be fully automated and make rapid and precise measurements [2]. The principle aim of this technology is to reduce the processing time, save energy and improve the shelf life and quality of food products [3]. The advantages of ultrasound over the heat treatment include; minimization of flavor loss, greater homogenity and significant energy savings [4].
Although ultrasound has been extensively studied for food processing, its usage area increases and factors that affect the ultrasound efficiency remain to be determined for various systems. Use of ultrasound as food preservation technique is still in consideration and its efficiency needs to be evaluated for commercial application. This paper will mainly review the mechanism and application of ultrasound in food preservation especially for the inactivation of microorganisms and enzymes.
2. ULTRASOUND GENERATION
Ultrasonic wave producing system contains the generator, transducer and the application system. Generator produces electrical or mechanical energy and transducer converts this energy into the sound energy at ultrasonic frequencies. Three main types of transducers are reported as fluid-driven, magnetostrictive and piezoelectric transducers [5].
The fluid-driven transducer produces vibration at ultrasonic frequencies by forcing liquid to thin metal blade which can be used for mixing and homogenisation systems. The magnetostrictive transducer is made from a kind of ferromagnetic materials which change dimension upon the application of a magnetic field and these changes produce sought after mechanical vibrations. The efficieny of system is low somewhat 60% transfer to acoustic energy [6]. The piezoelectric transducers produce acoustic energy by changes in size produced by electrical signals in piezoceramic materials such as lead zirconate titanate, barium titanate and lead metaniobate. The piezoelectric transducers are most commonly used devices and are more efficient (80% - 95% transfer to acoustic energy) [5,6].
In application system a coupler device is used to transfer ultrasonic vibrations to the sample. This is generally obtained by ultrasonic bath and probe system. In ultrasonic baths, generally the transducers are fixed to the underside of the tank and most of the baths operated at around 40 kHz [6,7]. In probe systems the horns or probes are used to transmit or to amplify the ultrasonic signal. Their lengths must be half the wavelengths, or multiple, to maintain the resonant conditions of the system [5,7]. The horn shape defines the amplitude gain of ultrasonic signal. If the probe is the same diameter along its length then no gain in amplitude will occur but the acoustic energy will be simply transferred to the media [6].
3. CLASSIFICATION OF ULTRASOUND APPLICATION
Nowadays, ultrasound is an attractive subject in the food industry. Industries can use practically ultrasonic equipments and it is known as green novel technology due to its role in the environment sustainability. Methods of ultrasound applications can be divided into three: 1) Direct application to the product, 2) Coupling with the device, 3) Submergence in an ultrasonic bath [3]. Also, ultrasonic applications in the food industry are divided into two distinct categories according to the energy generated by sound field. These are low and high energy ultrasounds which are classified by their sound power (W), sound energy density (Ws/m3) and sound intensity (W/m2). Low energy (low power, low-intensity) ultrasound applications are performed at frequencies higher than 100 kHz and below 1 W/cm2 intensities. Small power level is used for low intensity ultrasound so that it is nondestructive and no change occurs in the physical or chemical properties of food. Low intensity ultrasound in the food industry is generally used for analytical applications to get information about the physicochemical properties of foods such as composition, structure and physical state [8-10].
High energy (high power, high-intensity) ultrasounic applications are performed generally at frequencies between 18 and 100 kHz and are intensities higher than 1 W/cm2 (typically in the range 10 - 1000 W/cm2) [10]. At this power, destruction can be observed due to the physical, mechanical or chemical effects of ultrasonic waves (e.g. physical disruption, acceleration of certain chemical reactions). High-intensity ultrasound has been used for many years to generate emulsions, disrupt cells and disperse aggregated materials. More recently it is used for many purposes such as modification and control of crystallization processes, degassing of liquid foods, enzyme inactivation, enhanced drying and filtration and the induction of oxidation reactions [9,11].
4. METHODS OF ULTRASOUND
Ultrasound can be used for food preservation in combination with other treatments by improving its inactivation efficacy. There have been many studies combining ultrasound with either pressure, temperature, or pressure and temperature.
1) Ultrasonication (US) is the application of ultrasound at low temperature. Therefore, it can be used for the heat sensible products. However, it requires long treatment time to inactivate stable enzymes and/or microorganisms which may cause high energy requirement. During ultrasound application there may be rise in temperature depending on the ultrasonic power and time of application and needs control to optimize the process [11].
2) Thermosonication (TS) is a combined method of ultrasound and heat. The product is subjected to ultrasound and moderate heat simultaneously. This method produces a greater effect on inactivation of microorganisms than heat alone (Table 1). When thermosonication is used for pasteurization or sterilization purpose, lower process temperatures and processing times are required to achieve the same lethality values as with conventional processes [12,13].
3) Manosonication (MS) is a combined method in which ultrasound and pressure are applied together. Manosonication provides to inactivate enzymes and/or microorganisms by combining ultrasound with moderate pressures at low temperatures. Its inactivation efficiency is higher than ultrasound alone at the same temperature.
4) Manothermosonication (MTS) is a combined method of heat, ultrasound and pressure. MTS treatments inactivate several enzymes at lower temperatures and/or in a shorter time than thermal treatments at the same temperatures [3]. Applied temperature and pressure
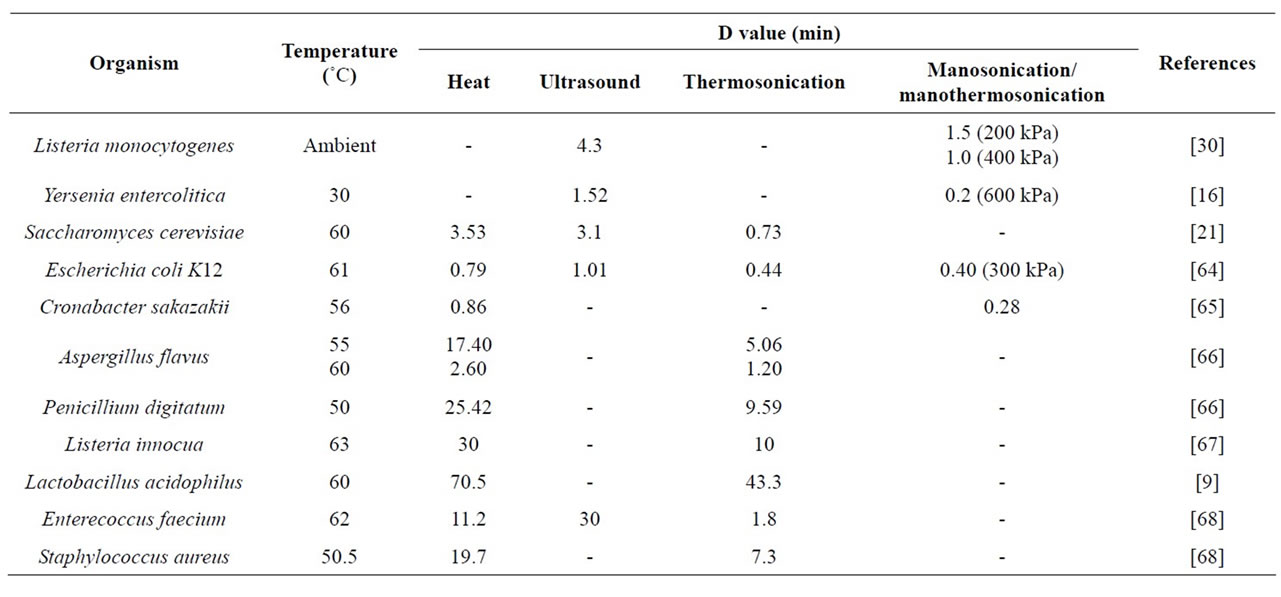
Table 1. Inactivation of microorganisms by using heat, ultrasound and pressure.
maximizes the cavitation or bubble implosion in the media which increase the level of inactivation. Microorganisms that have high thermotolerance can be inactivated by manothermosonication. Also some thermoresistant enzymes, such as lipoxygenase, peroxidase and polyphenoloxidase, and heat labile lipases and proteases from Pseudomonas can be inactivated by manothermosonication [14].
5. ULTRASOUND IN FOOD PRESERVATION
Consumer demand for fresher, higher quality and microbiologically safe and stable food has promoted research on nonthermal methods for the inactivation of microorganisms and enzymes. During nonthermal processing, the temperature of foods is held below the temperature normally used in thermal processing; therefore, a minimal degradation of food quality is expected [15]. Ultrasound is one of the nonthermal process which has been continuously suggested for food preservation. However, the high resistance of certain enzymes and bacterial spores to ultrasound treatment limit its application. To increase its lethality, ultrasound can be combined with pressure, with temperature or with both simultaneously.
5.1. Microbial Inactivation
Thermal treatment (i.e. pasteurization, ultra high temperature) is generally considered to be main method for the inactivation of bacteria but often result in some undesirable results such as formation of unwanted flavors and loss of nutrients. Nowadays, ultrasound is used for inactivation of microorganisms to overcome the undesirable results of thermal processing. Microbial inactivation mechanisms of ultrasound is simply explained by cavitation phenomena that caused by the changes in pressure [16,17]. Earnshaw, [18] explained that the extremely rapid creation and collapse of bubbles formed by ultrasonic waves in a medium creates the antimicrobial effect of ultrasound. During the cavitation process, localized changes in pressure and temperature cause breakdown of cell walls, disruption and thinning of cell membranes, and DNA damage via free radical production [4,19]. In fact, type of bacteria is an important criteria that changes the effectiveness of an ultrasound treatment [19]. Different kinds of microorganisms have different membrane structure. Such as, Gram-positive and Gram-negative bacteria do not show same behaviour against ultrasonic waves due to their different cell and membrane structures. Gram-positive bacteria have a thicker cell wall and lack of membrane and also Gramnegative bacteria have a thinner cell wall with an outer membrane [16]. Drakopoulou, et al. [20] examined the disinfection capability of ultrasound irradiation in the absence and presence of TiO2 particles on different bacteria groups, namely total coliforms (TC), faecal coliforms (FC), Pseudomonas spp. (PS), faecal streptococci (FS) and Clostridium perfringens species (CP), found in actual municipal wastewaters. They reported that Gramnegative bacteria are more readily susceptible to ultrasound inactivation than the gram-positive ones.
Effect of ultrasound on microbial inactivation also depends on intensity and frequency of ultrasound applied. Generally, frequency range of 200 - 600 kHz enhanced the effects of ultrasound on microorganisms. Wordon, et al. [21] suggested that high frequency of ultrasound was more effective in irradiation of microorganisms. Microbial inactivation using ultrasound has been investigated for application to a range of liquid foodstuffs. Levels of E. coli O157:H7 were reduced by 5 log cfu/mL with ultrasound in apple cider and the inactivation of E. coli K12 was enhanced using ultrasound at ambient temperatures. In the same study levels of Listeria monocytogenes in milk were reduced by 5 log cfu/mL when processed with ultrasound under mild heat conditions [22]. Kapturowska, et al. [23] investigated the use of sonication as an alternative method to inactivate yeast cells. Cells of Saccharomyces cerevisiae 2200 strain were sonicated in a 20 kHz horn-type sonicator. They found that the time, duty cycle, and power of ultrasounds significantly impacted the cell inactivation. After sonication, the count of live yeast cells decreased by 100 to 1000 times compared to their initial count expressed as cfu/cm3, this effect can be intensified by combing the activity of ultrasounds with a thermal factor.
Inactivations of microorganisms (especially spores) are resistant to environmental factors so that their inactivation is relatively difficult. Bacillus and Clostridium spores were found to be more resistant to heat and similarly resistant to ultrasound [24]. To inactivate resistant microorganisms generally ultrasound is applied with combination of pressure (manosonication), heat (thermosonication) or both pressure and heat treatments (manothermosonication) [16]. Effectiveness of microbial inactivation by these methods is dependent on the amplitude of the ultrasonic waves, exposure/contact time, volume of food being processed, the composition of the food and the treatment conditions [25]. When higher amplitudes were used, higher inactivation rate was observed and it could be due to an increase in the number of bubbles undergoing cavitation per unit of time [26] or to an increase in the volume of liquid in which cavitation is liable to occur [27].
D’Amico et al. [22] showed that ultrasound treatment combined with mild heat (57˚C) for 18 min. resulted in a 5-log reduction of L. monocytogenes in milk, a 5-log reduction in total aerobic bacteria in raw milk, and a 6-log reduction in E. coli O157:H7 in pasteurized apple cider. Juraga, et al. [28] work with high intensity ultrasound to investigate inactivation of Enterobacteriae in raw milk. For ultrasounds treatment, they used three parameters: temperature (20˚C, 40˚C and 60˚C), amplitude (120, 90 and 60 µm) and time (6, 9 and 12 min). They found that inactivation of microorganisms using ultrasound depends on the amplitude of the ultrasonic waves, the exposure/contact time with the microorganisms, and the temperature of treatment. The achieved results indicate significant inactivation of microorganisms under longer period of treatments with ultrasonic probe particularly in combination with higher temperature and amplitude.
Raso, et al. [29] investigated the inactivation of Bacillus subtilis spores by ultrasonic treatments under static pressure and a combined pressure and heat treatments. They showed that manosonication treatment at 500 kPa and 117 µm of amplitude for 12 min inactivated approximately 99% of the B. subtilis spore population. They reported that the ultrasound amplitude was also very effective on microbial inactivation. While manosonication treatment (20 kHz, 300 kPa, 70˚C, 12 min) at 90 µm inactivated 75% of the B. subtilis spore population, the same treatment at 150 µm inactivated 99.9% of this population. The manosonication treatments at temperatures higher than 70˚C (manothermosonication) led to more spore inactivation. In the range 70˚C - 90˚C, the combination of heat with a manosonication treatment (20 kHz, 300 kPa, 117 mm, 6 min) had a synergistic effect on spore inactivation.
Application of ultrasound (20 kHz, 117 μm) to Listeria monocytogenes under sublethal pressure (200 kPa) caused reduction of pH from 7.0 to 4.0. The acidic conditions had a much greater effect on the organisms resistance to heat than its sensitivity to ultrasonication [30].
5.2. Enzyme Inactivation
Enzymatic reactions produce undesirable changes in many foods during processing and storage periods. Heat treatment to eliminate enzymes is the commonly used method but it also destroys nutrients and may cause loss of food quality. For this reason, nonthermal technologies are being tested as an option for reducing the enzymatic activities in foods [31].
First enzyme inactivation by ultrasound was applied to pure pepsin almost 60 years ago and its inactivation mechanism was explained by cavitation. Since then, it has been proven that ultrasound is an effective method in the inactivation of enzymes when it is used alone or with temperature and pressure. There are many enzymes inactivated with ultrasound such as glucose oxidase [32], peroxidase [17,33], pectin methyl esterase [34], protease and lipase [35], watercress peroxidase [36] and polyphenoloxidase [15]. Table 2 summarizes the ultrasound application on enzymes.
Ultrasound creates continuous vibration and produce stable cavitation bubbles which collapse due to the extreme local increase in pressure (1000 P) and temperature (5000 K) [37]. Also, because of shock waves strong shear and microstreaming the adjacent liquid is observed. All of these factors can cause modification of secondary and tertiary structure of protein due to the breakdown of hydrogen bonding or Van der Walls interaction in the polypeptide chains. These changes cause activity loss of many enzymes. The extreme pressure and temperature also lead to homolytic water molecule cleavage generating high energy intermediates such as hydroxyl and hydrogen free radicals. The free radical formed may re-
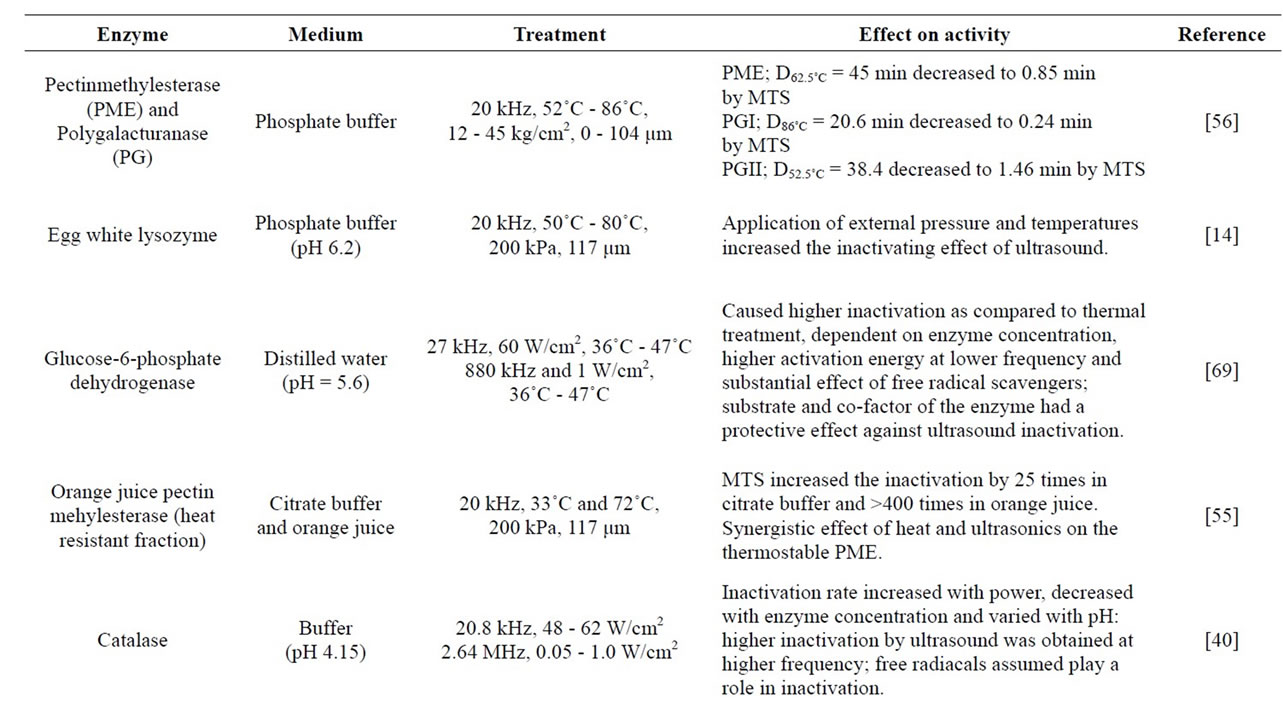
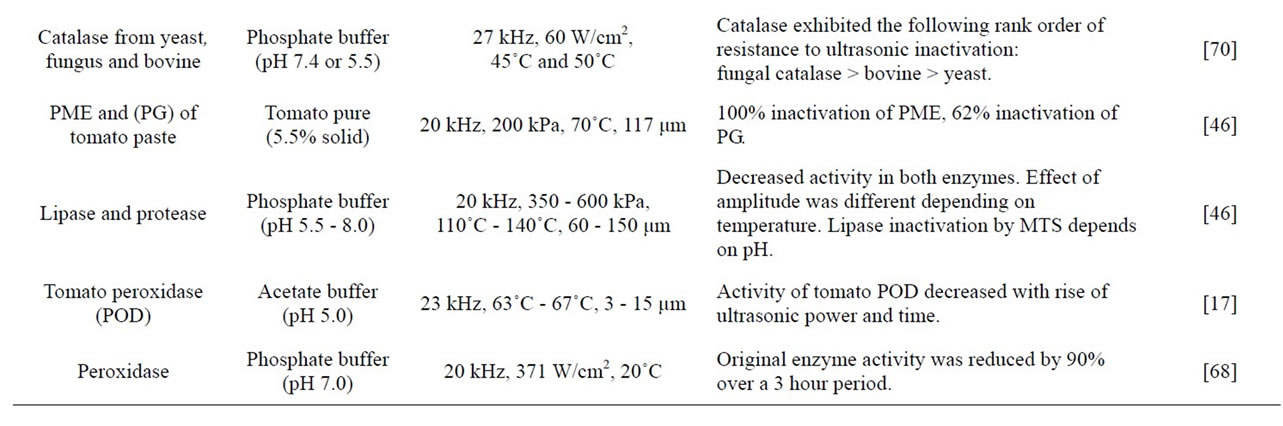
Table 2. Inactivation of enzymes by using heat, pressure and ultrasound treatments.
act with some amino acid residues that participate in enzyme stability, substrate binding or in the catalytic function with a consequent change in biological activity [31]. Such free radicals could recombine with amino acid residues of the enzymes. These residues are associated with structure stability, substrate binding and catalytic functions [36]. Distruption of tissue due to the ultrasonic application is an important criterion. As the amount of distruption tissue increases, surface area that contact with the enzymes and free radicals increases. For example, oxidases are usually inactivated by sonication while catalyses are affected at low concentrations. Reductases and amylases are highly resistant to sonication [7]. Production of free radicals during the ultrasonic application is crucially important in enzyme inactivation that is supported by several studies. Barteri, et al. [38] studied the inactivation of fumarase by ultrasound and explained that progressive oxidation of cystein by hydroxyl-free radicals and aggregation of the enzymes. They observed that disulfide linked aggregates formed during ultrasonic treatment causes inactivation of enzymes. The involvement of free radicals in the inactivation of trypsin has also been observed indirectly through the strong protective effect of mannitol against ultrasound inactivation. It is a free radical scavenger, as well as the presence of polypeptide fragments following sonications [39]. Also, some researches concluded that the role of free radicals on the ultrasound inactivation of enzymes has also been indirectly confirmed through the effect of free radical scavenging solutes in horseradish peroxidase, catalase and glucose-6-phosphatase dehydrogenase [40].
Inactivation of enzymes by ultrasonic treatment shows discrepancy amoung enzymes due to the different amino acid composition and the conformational structure of the enzyme [41]. Lopez and Burgos [42] explained that inactivation of peroxidase by manothermosonication is due to the splitting its prosthetic heme group, as for the mechanism of heat inactivation. However it was suggested that lipoxygenase inactivated by a free radical mediated mechanism [43] and possibly by denaturation of proteins [7]. Some enzymes, such as catalase, yeast invertase and pepsin are resistant to ultrasound [19].
The ultrasound stability of individual proteins varies between the enzymes [35,41,44,45] and also depends on ultrasound treatment conditions [34], the composition of treatment medium, treatment pH, and whether they are bound (e.g., membrane-bound proteins) or free (e.g., cytoplasmic proteins). Enzyme inactivation generally increases with increasing ultrasound power, ultrasound freqency, exposure time, amplitude level, cavitation intensity, processing temperature and processing pressure, but decreases as the volume being treated increases [34,35,41,46].
Ultrasound does not inactivate all enzymes at mild temperature conditions. Such as, Villamiel and de Jong [47] reported that ultrasound treatment (20 kHz, 120 μm) at temperature less than 55˚C did not inactivate alkaline phosphatase in milk, while only 22 and 14% inactivation was observed in γ-glutamyltranspeptidase and lactoperoxidase, respectively. Similar results were obtained for sonication (20 kHz, 7 - 40 W) of alkaline phosphatase in buffer [41].
Thermosonication is also a good alternative to the heat treatment for enzyme inactivation. Raviyan, et al. [34] indicate that when sonication is combined with heat, tomato pectin methylesterase is inactivated effectively compared to thermal treatment at the same temperature. Also, it was concluded that the thermosonication treatment is more effective in inactivation of a number of enzymes including the pectinmethylesterase, polygalacturonase and peroxidase than heat treatment [17,48,49].
The rate of inactivation of tomato pectinmethylesterase was greatly increased by combination of heat and ultrasound, with increasing cavitation intensity dramatically increasing the rate of inactivation [34]. Similar results were obtained for the inactivation of tomato peroxidase by heat and ultrasound [17]. De Gennaro, et al. [33] studied the effect of heat and thermosonication on the activity of horseradish peroxidase and they found that the decimal reduction time of peroxidase at 80˚C, reduces from 65 to 10 min when ultrasound is applied. Peroxidase was inactivated by combinations of heat and ultrasound at neutral [33] or low pH [50] and lipoxygenase has been shown to be inactivated at low sonication intensities [51].
Manothermosonication has reported to inactivate several enzymes at lower temperatures and/or in a shorter time than thermal treatments. Sensitivity of the enzymes to manothermosonication tratment is independent on the medium of treatment, the substrates, small co-solutes, and other proteins [35]. Manothermosonication has an increased effectiveness of enzyme inactivation compared with ultrasound alone [4]. Firstly in 1994, a research group headed by Burgos started the study of the application of manothermosonication to enzymes releavent to the food industry (peroxidase, lipoxygenase and polyphenoloxidase) in model buffer systems. Manothermosonication treatments proved to be much more efficient than heat treatment for inactivating these enzymes, especially those which are more thermally labile (lipoxygenase and polyphenoloxidase) [52]. Also, it was observed that manothermosonication is more effective than heat treatment alone (within the temperature range of 110˚C - 140˚C) for the inactivation of lipoxygenase, peroxidase and polyphenoloxidase, heat-resistant lipase from Pseudomonas fluorescens [53]. However, the effective improvement achieved using this combined treatment decreased as the treatment temperature increased. Manothermosonication could be useful to inactivate those enzymes within food materials that do not require such high temperatures for preservation [54].
Orange pectinmethylesterase was inactivated by heat (72˚C, D value of 500 min) and manothermosonication (72˚C, 20 kHz, 117 mm, 350 kPa) and manothermosonication gave a much lower D value (1.2 min) [55]. Pectic enzymes of tomatoes, pectic methylestearase and the two endopolygalacturonase isozymes are also inactivated by MTS treatments with much higher efficiency, both in model systems [56] and in tomato juice [46]. General trends arose from all these enzyme inactivation studies that thermolabile enzymes are more sensitive to ultrasound than those which are heat resistant [52].
Manothermosonication was reported to be effective for inactivation of protease and lipase from psychrotrophic Pseudomanas [53] and pectin methyl esterase from orange [55] and from tomatoes [56]. Vercet, et al. [35] reported that MTS is able to inactivate enzymes at a much higher rate than heat when these enzymes are not especially heat stable.
5.3. Effect of Ultrasound on Food Quality
Nowadays, food technology is aimed to reduce the nutrients loss during the processing and storage. Ascorbic acid is not stable against heat treatment and usually considered as an index of nutrient quality during processing and storage of foods [57]. It acts as a valid criterion for other sensorial or nutritional components, such as natural pigments and aromatic substances. Its concentration decreases during storage, depending on storage conditions such as temperature, oxygen content and light [58].
Tiwari, et al. [59] found that vitamin C retention of orange juice after ultrasonic treatment is higher when it is compared to thermal processing. Also, Cruz, et al. [49] found that ultrasonication was more effective in retention of vitamin C content of watercress then heat treatment. In addition, degradation of vitamin C in orange juice during storage at 20˚C was less after ultrasonic treatment than after temperature treatment only. The effects of ultrasound and temperature on vitamin C content of tomato extract were also studied. It was found that there is no significant effect of ultrasound whereas heat treatment significantly reduces vitamin C content of tomato extract [17].
Ultrasound is reported to have a minimal effect on the quality of fruit juices, such as orange juice [59], guava juice [60], blackberry juice [61] and strawberry juice [62]. Fruit juice colour is a primary factor considered by the consumer in assessing juice quality and sensory acceptance [63]. Color measurements during storage of orange juice after heat or ultrasound treatment indicated similar overall changes of a and b values, but significantly lighter products (L-values) resulting from the ultrasound treatment.
6. CONCLUSION
Ultrasound is considered to be an emerging technology in the food industry. It has advantages of minimizing flavor loss, increasing homogeneity, saving energy, high productivity, enhanced quality, reduced chemical and physical hazards, and is environmentally friendly. When it is applied with pressure and/or temperature its efficiency increases but cautions needed to determine and control nutritional loss. Also, process parameters and applied material change the results. Consequently, ultrasound is a good alternative method for the food preservation and processing and also no adverse effect on human health has been proven. Although there are many studies relating ultrasonic application in laboratory scale, its application in the food industry is not sufficiently common. Future studies should be focused on scale-up and standardization of treatment processes.
![]()
![]()
REFERENCES
- Ulusoy, H.B. Colak, H. and Hampikyan, H. (2007) The use of ultrasonic waves in food technology. Research Journal of Biological Science, 2, 491-497.
- Dolatowski, J.Z., Stadnik, J. and Stasiak, D. (2007) Application of ultrasound in food technology. Acta Scientiarum Polonorum Technologia Alimentaria, 6, 89-99.
- Chemat, F., Huma, Z. and Khan, M.K. (2011) Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry, 18, 813-835. doi:10.1016/j.ultsonch.2010.11.023
- Earnshaw, R.G., Appleyard, J. and Hurst, R.M. (1995) Understanding physical inactivation processes: Combined preservation opportunities using heat, ultrasound and pressure. International Journal of Food Microbiology, 28, 197-219. doi:10.1016/0168-1605(95)00057-7
- Mulet, A., Carcel, J., Benedito, C., Rossello, C. and Simal, S. (2003) Ultrasonic mass transfer enhancement in food processing. In: J. Welti-Chanes, F. Vélez-Ruiz and Barbosa-Cánovas, G.V., Eds., Transport Phenomena of Food Processing, Chapter 18, Boca Raton.
- Leadley, C.E. and Williams, A. (2006) Pulsed electric field processing, power ultrasound and other emerging technologies. In: Brennan, J.G., Ed., Food Processing Handbook, Wiley-VCH, Weinheim, 214-218. doi:10.1002/3527607579.ch7
- Mason, T.J. (1998) Power ultrasound in food processing—The way forward. In: Povey, M.J.W. and Mason, T.J. Eds., Ultrasound in Food Processing, Blackie Academic and Professional, London, 105-126.
- Jayasooriya, S.D., Bhandari, B.R., Torley, P. and Darcy, B.R. (2004) Effect of high power ultrasound waves on properties of meat: A review. International Journal of Food Properties, 2, 301-319. doi:10.1081/JFP-120030039
- Knorr, D., Zenker, M., Heinz, V. and Lee, D.U. (2004) Applications and potential of ultrasonics in food processing. Trends in Food Science and Technology, 15, 261- 266. doi:10.1016/j.tifs.2003.12.001
- McClements, D.J. (1995) Advances in the application of ultrasound in food analysis and processing. Trends in Food Science and Technology, 6, 293-299. doi:10.1016/S0924-2244(00)89139-6
- Zheng, L. and Sun, D.W. (2006) Innovative applications of power ultrasound during food freezing processes—A review. Trends in Food Science and Technology, 17, 16-23. doi:10.1016/j.tifs.2005.08.010
- Mason, T.J., Paniwnyk, L. and Lorimer, J.P. (1996) The uses of ultrasound in food technology. Ultrasonics Sonochemistry, 3, 253-260. doi:10.1016/S1350-4177(96)00034-X
- Villamiel, M., Hamersveld, V. and De Jong, P. (1999) Review: Effect of ultrasound processing on the quality of dairy products. Milchwissenschaft, 54, 69-73.
- Manas, P., Munoz, B., Sanz, D. and Condon, S. (2006) Inactivation of lysozyme by ultrasonic waves under pressure at different temperatures. Enzyme and Microbial Technology, 39, 1177-1182. doi:10.1016/j.enzmictec.2005.11.053
- Raso, J. and Barbosa-Canovas, G.V. (2003) Nonthermal preservation of foods using combined processing techniques. Critical Reviews in Food Science and Nutrition, 43, 265-285. doi:10.1080/10408690390826527
- Piyasena, P., Mohareb, E. and Mckellar, R.C. (2003) Inactivation of microbes using ultrasound: A review. International Journal of Food Microbiology, 87, 207-216. doi:10.1016/S0168-1605(03)00075-8
- Şahin Ercan, S. and Soysal Ç. (2011) Effect of ultrasound and temperature on tomato peroxidase. Ultrasonics Sonochemistry, 18, 689-695. doi:10.1016/j.ultsonch.2010.09.014
- Earnshaw, R.G. (1998) Ultrasound: A new opportunity for food preservation, In: Povey, M.J.W and Mason, T.J., Eds., Ultrasound in Food Processing, Blackie Academic and Professional, London, 183-192.
- Sala, F.J., Burgos, J., Condon, S., Lopez, P. and Raso, J. (1995) Effect of heat and ultrasound on microorganisms and enzymes. In: Gould, G.W., Ed., New Methods of Food Preservation, Blackie Academic and Professional, London, 176-204.
- Drakopoulou, S., Terzakis, S., Fountoulakis, M.S., Mantzavinos, D. and Manios, T. (2009) Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrasonics Sonochemistry, 16, 629-634. doi:10.1016/j.ultsonch.2008.11.011
- Wordon, B.A., Mortimer, B. and McMast, L.D. (2011) Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Research International, 47, 134-139. doi:10.1016/j.foodres.2011.04.038
- D’Amico, D.J., Silk, T.M., Wu, J.R. and Guo, M.R. (2006) Inactivation of microorganisms in milk and apple cider treated with ultrasound. Journal of Food Protection, 69, 556-563.
- Kapturowska, A., Stolarzewicz, I. and Chmielewska, I. (2011) Ultrasounds—A tool to inactivate yeast and to extract intracellular protein. Zywnosc-Nauka Technologia Jakosc, 18, 160-171.
- Dehghani, M.H. (2005) Effectiveness of ultrasound on the destruction of E. coli. American Journal of Environmental Sciences, 1, 187-189. doi:10.3844/ajessp.2005.187.189
- USDA (2000) Kinetics of microbial inactivation for alternative food processing technologies: Ultrasound. US Food and Drug Administration Report. http://www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm103342.htm
- Suslick, K.S. (1990) Sonochemistry. American Association for the Advancement of Science, 247, 1439-1445.
- Suslick, K.S. (1988) Homogeneous sonochemistry. In: Ultrasound. Its Chemical, Physical, and Biological Effects. VCH Publishers, New York, 123-163.
- Juraga, E., Salamon, B.S. and Herceg, Z. (2011) Application of high intensity ultrasound treatment on Enterobacteriae count in milk. Mljekarstvo, 61, 125-134.
- Raso, J., Palop, A. and Condon, S. (1998) Inactivation of Bacillus subtilis spores by combining ultrasound waves under pressure and mild heat treatment. Journal of Applied Microbiology, 85, 849-854. doi:10.1046/j.1365-2672.1998.00593.x
- Pagan, R., Manas, P., Alvarez, I. and Condon, S. (1999) Resistance of Listeria monocytogenes to ultrasonic waves under presure at sublethal (manosonication) and lethal (manother-mosonication) temperatures. Food Microbiology, 16, 139-148. doi:10.1006/fmic.1998.0231
- Feng, H., Barbosa-Canovas, G.V. and Weiss, J. (2011) Ultrasound technologies for food and bioprocessing, Springer, New York. doi:10.1007/978-1-4419-7472-3
- Guiseppi-Elie, A., Choi, S-H. and Geckeler, K.E. (2009) Ultrasonic processing of enzymes: Effect on enzymatic activity of glucose oxidase. Journal of Molecular Catalysis, 58, 118-123. doi:10.1016/j.molcatb.2008.12.005
- De Gennaro, L., Cavella, S., Romano, R. and Masi, P. (1999) The use of ultrasound in food technology I: Inactivation of peroxidase by thermosonication. Journal of Food Engineering, 39, 401-407. doi:10.1016/S0260-8774(99)00028-X
- Raviyan, P., Zhang, Z. and Feng, H. (2005) Ultrasonication for tomato pectinmethylesterase inactivation: Effect of cavitation intensity and temperature on inactivation. Journal of Food Engineering, 70, 189-196. doi:10.1016/j.jfoodeng.2004.09.028
- Vercet, A., Burgos, J., Crelier, S. and Lopez-Buesa, P. (2001) Inactivation of protease and lipase by ultrasound. Innovation Food Science and Technologies, 2, 139-150. doi:10.1016/S1466-8564(00)00037-0
- Cruz, R.M.S., Vieira, M.C. and Silva, C.L.M. (2006) Effect of heat and thermosonication treatments on peroxidase inactivation kinetics in watercress (Nasturtium officinale). Journal of Food Engineering, 7, 8-15. doi:10.1016/j.jfoodeng.2004.11.007
- Suslick, K.S. (1994) The chemistry of ultrasound. From The Yearbook of Science and the Future. Encyclopaedia, Britannica, Chicago, 138-155.
- Barteri, M., Diociaiuti, M., Pala, A. and Rotella, S. (2004). Low frequency ultrasound induces aggregation of porcine fumarase by free radicals production. Biophysical Chemistry, 111, 35-42. doi:10.1016/j.bpc.2004.04.002
- Tian, Z.M., Wan, M.X., Wang, S.P. and Kang, J.Q. (2004) Effects of ultrasound and additives on the function and structure of trypsin. Ultrasonics Sonochemistry, 11, 399- 404.
- Potapovich, M.V., Eremin, A.N. and Metelitza, D.I. (2003) Kinetics of catalase inactivation induced by ultrasonic cavitation. Applied Biochemistry and Microbiology, 39, 140-146. doi:10.1023/A:1022577611056
- Özbek, B. and Ülgen, K.O. (2000) The stability of enzymes after sonication. Process Biochemistry, 35, 1037- 1043. doi:10.1016/S0032-9592(00)00141-2
- Lopez, P. and Burgos, J. (1995) Peroxidase stability and reactivation after heat treatment and monothermosonication. Journal of Food Science, 60, 451-455,482. doi:10.1111/j.1365-2621.1995.tb09801.x
- Lopez, P. and Burgos, J. (1995) Lipoxygenase inactivation by manothermosonication: Effects of sonication physical parameters, pH, KCl, sugars, glycerol, and enzyme concentration. Journal of Agricultural and Food Chemistry, 43, 620-625. doi:10.1021/jf00051a012
- Lopez, P., Sala, F.J., de la Fuente, J.L., Condon, S., Raso, J. and Burgos, J. (1994) Inactivation of peroxidase, lipoxygenase and polyphenol oxidase by manothermosonication. Journal of Agricultural and Food Chemistry, 42, 252-256. doi:10.1021/jf00038a005
- Vercet, A., Burgos, J. and Lopez-Buesa, P. (2002) Manathermosonication of heat resistant lipase and protease from Pseudomonas fluorescence: Effect of pH and sonication parameters. Journal of Dairy Research, 69, 243- 254. doi:10.1017/S0022029902005460
- Vercet, A., Sanchez, C., Burgos, J., Montanes, L. and Lopez-Buesa, P. (2002) The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. Journal of Food Engineering, 53, 273- 278. doi:10.1016/S0260-8774(01)00165-0
- Villamiel, M. and de Jong, P. (2000) Influence of high intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. Journal of Agricultural and Food Chemistry, 48, 472-478. doi:10.1021/jf990181s
- Wu, J., Gamage, T.V., Vilkhu, K.S., Simons, L.K. and Mawson, R. (2008) Effect of thermosonication on quality improvement of tomato juice. Innovative Food Science and Emerging Technologies, 9, 186-195. doi:10.1016/j.ifset.2007.07.007
- Cruz, R.M.S., Vieria, C.M. and Silva, C.L.M. (2008) Effect of heat and thermosonication treatments on watercress (Nasturtium officinale) vitamin C degredation kinetics. Innovative Food Science and Emerging Technologies, 9, 483-488. doi:10.1016/j.ifset.2007.10.005
- Yoon-Ku, J., Park, S.O. and Bong-Soo, N. (2000) Inactivation of peroxidase by hurdle technology. Food Science and Biotechnology, 9, 124-129.
- Thakur, B.R. and Nelson, P.E. (1997) Inactivation of lipoxygenase in whole soy floor suspension by ultrasonic cavitation. Die Nahrung, 41, 299-301. doi:10.1002/food.19970410510
- Sun, D.W. (2005) Emerging technologies for food processing. Elsevier Academic Press, USA, 323-345.
- Vercet, A., Lopez, P. and Burgos, J. (1997) Inactivation of heat resistant lipase and protease from Pseudomonas fluorescens by manothermosonication. Dairy Science, 80, 29-36. doi:10.3168/jds.S0022-0302(97)75909-5
- Zeuthen, P. and Sorensen, B.L. (2003) Food Preservation Techniques. CRC Press, Washington, 303-337. doi:10.1533/9781855737143
- Vercet, A., Lopez, P. and Burgos, J. (1999) Inactivation of heat resistant pectinmethylesterase from orange by manothermosonication. Journal of Agricultural and Food Chemistry, 47, 432-437. doi:10.1021/jf980566v
- Lopez, P., Vercet, A. and Burgos, J. (1998) Inactivation of tomato pectic enzymes by manothermosonication. Zeitschrift für Lebensmittel-Untersuchung und Forschung A, 207, 249-252.
- Nicoleti, J.F., Silveira-Junior, V., Telis-Romero, J. and Telis, V.R.N. (2004) Ascorbic acid degradation during convective drying of persimmons with fixed temperature inside the fruit. Proceedings of the 14th International Drying Symposium, Sao Paulo, 1836-1843.
- Matei, N., Soceanu, A., Dobrinas, S. and Magearu, V. (2009) Kinetic study of ascorbic acid degradation from grapes. Ovidius University Annals of Chemistry, 20, 132- 136.
- Tiwari, B.K., Donnell, C.P.O., Muthukumarappan, K. and Cullen, P.J. (2009) Ascorbic acid degredation kinetics of sonicated orange juice during storage and comparison with thermally pasteurized juice. LWT Food Science and Technology, 42, 700-704. doi:10.1016/j.lwt.2008.10.009
- [61] Cheng, L.H., Soh, C.Y., Liew, S.C. and Teh, F.F. (2007) Effects of sonication and carbonation on guava juice quality. Food Chemistry, 104, 1396-1401. doi:10.1016/j.foodchem.2007.02.001
- [62] Tiwari, B.K., O’Donnell, C.P. and Cullen, P.J. (2009) Effect of sonication on retention of anthocyanins in blackberry juice. Journal of Food Engineering, 93, 166-171. doi:10.1016/j.jfoodeng.2009.01.027
- [63] Tiwari, B.K., O’Donnell, C.P., Patras, A. and Cullen, P.J. (2008) Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. Journal of Agriculture and Food Chemistry, 56, 10071-10077. doi:10.1021/jf801824v
- [64] Adequnte, A., Tiwari, B.K., Scannell, A., Cullen, P.J. and O’Donnell, C. (2010) Modelling of yeast inactivation in sonicated tomato juice. International Journal of Food Microbiology, 137, 116-120. doi:10.1016/j.ijfoodmicro.2009.10.006
- [65] Lee, H., Zhou, B., Liang, W., Feng, H. and Martin, S.E. (2009) Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: Microbial responses and kinetics modeling. Journal of Food Engineering, 93, 354-364. doi:10.1016/j.jfoodeng.2009.01.037
- [66] Arroyo, C., Cebrián, G., Pagán, R. and Condón, S. (2012) Synergistic combination of heat and ultrasonic waves under pressure for Cronobacter sakazakii inactivation in apple juice. Food Control, 25, 342-348. doi:10.1016/j.foodcont.2011.10.056
- [67] Lopez-Malo, A., Palou, E., Jimenez-Fernandez, M., Alzamora, S.M. and Guerrero, S. (2005) Multifactorial fungal inactivation combining thermosonication and antimicrobials. Journal of Food Engineering, 67, 87-93. doi:10.1016/j.jfoodeng.2004.05.072
- [68] Bermudez-Aguirre, D. and Barbosa-Canovas, G.V. (2008) Study of butter fat content in milk on the inactivation of Listeria innocua ATCC 51742 by thermo sonication. Innovative Food Science and Emerging Technologies, 9, 176-185.
- [69] Brennan, J.G. (2006) Food processing handbook. WileyVCH, Germany, 215-220.
- [70] Karaseva, E. and Metelitza, D.I. (2006) Stabilization of glucose-6-phosphate dehydrogenase by its substrate and cofactor in an ultrasonic field. Russian Journal of Bioorganic Chemistry, 32, 436-443. doi:10.1134/S1068162006050062
- [71] Potapovich, M.V., Eryomin, A.N. and Metelitza, D.I. (2005) Ultrasonic and thermal inactivation of catalases from bovine liver, the methylotrophic yeast Pichia pastoris, and the fungus Penicillium piceum. Applied Biochemistry and Microbiology, 41, 529-537. doi:10.1007/s10438-005-0096-3

