Natural Science
Vol.4 No.4(2012), Article ID:18610,7 pages DOI:10.4236/ns.2012.44031
Hydroesterification of Nannochloropsis oculata microalga’s biomass to biodiesel on Al2O3 supported Nb2O5 catalyst
![]()
1GREETEC Laboratory, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil; *Corresponding Author: aalmaralesarceo@yahoo.es
2Ecology Department, University of Málaga, Málaga, Spain
3Materials Laboratory, West Zone State University Center (UEZO), Rio de Janeiro, Brazil
Received 30 January 2012; revised 29 February 2012; accepted 15 March 2012
Keywords: Biodiesel Microalgae; hydrolysis; esterification; Nannochloropsis oculata
ABSTRACT
Hydroesterification process has been presented biodiesel production from oil the green microalga Nannochloropsis oculata raw materials. Biodiesel studied in this work is the main product got from the hydroesterification of biomass the Nannochloropsis oculata and was obtained from esterification of fatty acid (product of a hydrolysis reaction) with methanol. It was used as catalyst the niobic acid pure and supported on δ-aluminum. The product was evaluated by gas chromatography and other analyses. The optimum conditions found in the conversion (%) for the hydrolysis reactions of the biomass (92.3%). Better results were observed in the algae concentration 20%, lead at 300˚C with 20% of catalyst. For esterification of fatty acids of Nannochloropsis oculata (92.24%), were observed the molar ratio methanol: fat acid 3, lead at 200°C with 20% of catalyst supported.
1. INTRODUCTION
In recent years, biodiesel is receiving widespread attention owing to non-toxic, biodegradable and renewable fuel. It also contributes no net carbon dioxide or sulfur to the atmosphere and emits less gaseous pollutants than the conventional diesel fuel [1]. Extensive studies have been conducted on using vegetable oils as diesel fuel. The focus has mainly been on oils like soybean, rapeseed, sunflower, and safflower [2] which are essentially edible in nature. Most recently, research effort has been aimed at identifying suitable biomass species, which can provide high energy outputs to replace the conventional fossil fuels [3]. Nevertheless, the cost of biodiesel production is still a major obstacle for large scale commercial exploitation; mainly due to the high feed cost of vegetable oils [2]. Microalgae are emerging as one of the most promising sources of biodiesel because of their high photosynthetic efficiency and faster growth as compared to any energy crop [4]. They reproduce quickly and can be harvested day after day [5]. However, the lipid content in the microalgae required to be high, otherwise the economic performance would be hard to achieve [6]. The production of biodiesel using microalgae requires that microalgae are sorted according to their growth rate, lipid content, and tolerance of high levels of CO2.
Biodiesel (fatty acid methyl esters; FAME) can be produced from oils/fats through transesterification of triglycerides (TG) with methanol. At present, most of the methods in transesterification use alkaline catalysts, even though this process needs sophisticated purification steps for removal of the catalyst and saponified products from free fatty acids. Our research group has, therefore, developed other catalyst processes: The process of hydroesterification. This process has been presented a promising alternative to the process of conventional production of biodiesel (transesterification). Since it favors the raw material conversion of any level of acidity and humidity. There reactions can be carried out with acid catalyst, that favors high conversions in a small range of time (30 minutes). The method involves, hydrolysis of oils/ fats to fatty acids (FA) in water and subsequent methyl esterification of FA to FAME.
Hydrolysis reaction of TG consists of three stepwise forward and backward reactions as shown in Figure 1. At each forward reaction step, one molecule of water is consumed producing one molecule of FA. For the backward reaction, on the other hand, G reacts with FA to return to MG. As in a similar manner, DG and MG also reverse to TG and DG, respectively, consuming one molecule of FA.
Methyl esterification of FA (Figure 2) is a major reaction to produce FAME whereas transesterification of TG is a major one in the conventional alkaliand acid-catalyzed methods. This esterification reaction is, therefore, an important step for high quality biodiesel fuel production.
This paper reports the results of study on the hydroesterification of Nannochloropsis oculata biomass and also, some properties for the obtained biodiesel.
2. METHODS
2.1. Materials
The algae Nannochloropsis oculata were harvested by continuous-flow centrifugation (separator, Alfa Laval) up to 10% dry matter in the slurry. The darkgreen algae slurry was spray-dried in a Niro Atomizer drier (input, 200˚C - 210˚C; output, 80˚C - 90˚C). The powder obtained (moisture content 7%) was preserved in plastic boxes for further use.
2.2. Experimental Procedure
Figure 3 shows the flow chart used in the process. This procedure was used as method of obtaining fatty acids for later conversion to biodiesel, so it was repeated countless times.
The final conversion of the hydrolysis process was calculated taking into account the acidity of initial value of algal oil and the final value of acidity of the fatty acid obtained. Acid value or neutralization number, is expressed in mg KOH required to neutralize 1 g of fatty acid methyl esters and is set to a maximum value of 0.8 mg KOH/g in the Brazilian norm (NBR 14448). This procedure was developed according to ASTM-D 664.
The esterification was carried out in the same reactor that hydrolysis, for 1 h, molar ratio methanol:fat acid 3, lead at 200˚C with 20% of catalyst (Nb2O5 pure and Nb2O5/Al2O3). The molar ratio the methanol/fatty acid was 3, by representing an excess of methanol. Temperature of 200˚C was taken keeping in mind that the acid
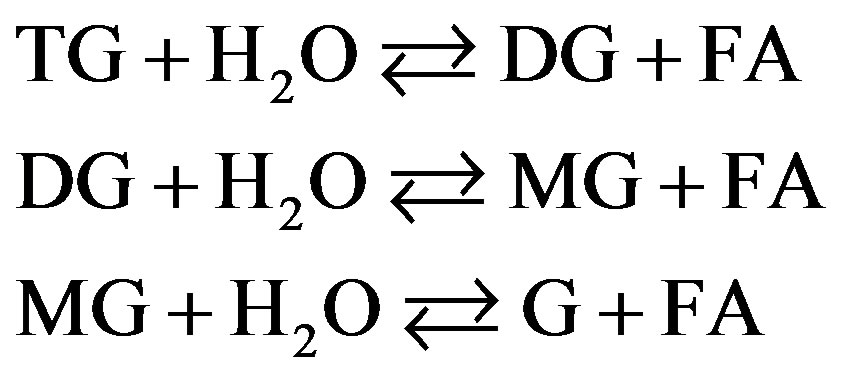
Figure 1. Three stepwise reactions for hydrolysis of triglycerides (TG) to fatty acids (FA).

Figure 2. Methyl esterification reaction of fatty acids (FA) to fatty acid methyl esters (FAME).
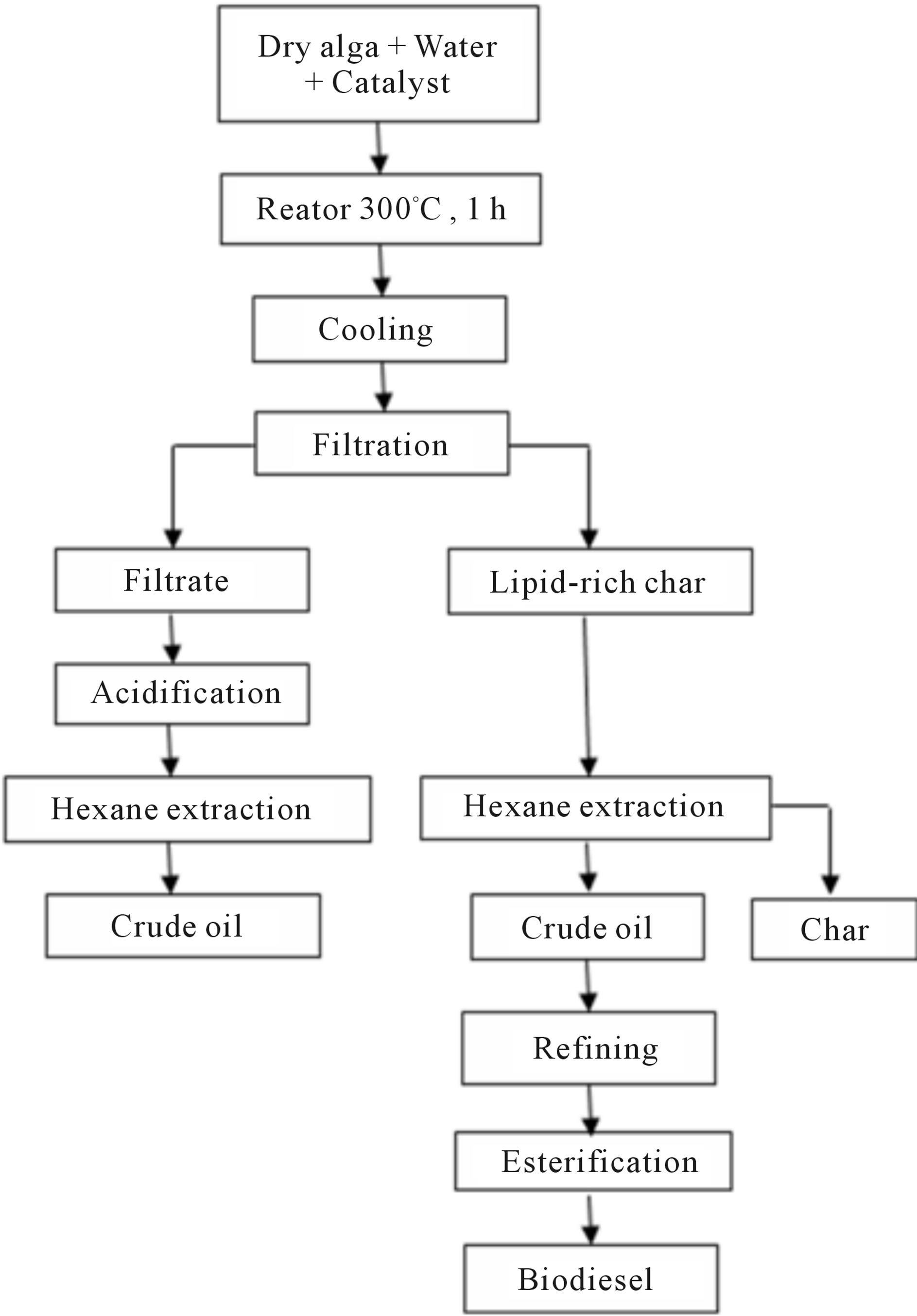
Figure 3. General scheme of the process.
esterification requires high temperatures to occur as previously studied by [7,8]. Aliquots were taken out by monitoring the esterification process, in 5, 10, 15, 20, 25, 30, 45 and 60 minutes, as measured by acidity. The final product of the reaction was three-time washed with deionised water, and subsequently was centrifuged to remove the aqueous layer compound by methanol and residual catalyst.
Proper application of the hydroesterification process depended heavily on available microalgae. A characteristic of microalgae commonly observed was wide lot-to-lot compositional variation due to nutritional, harvesting, and other subtle factors. Therefore, microalgae were highly desired that could be obtained in >100 g quantities. Additionally, the lipid content of the starting microalgae should be as high as possible and representative of strains that would eventually be utilized in the algal oil industry.
The supported catalyst (Nb2O5/Al2O3) was synthesized by wet impregnation. For example, to prepare 20 wt% Nb2O5 on Al2O3; first, necessary amounts of γ-aluminum (AIP) (98%, from Fluka Inc.), water and Nb2O5 were mixed. Finally, excess water was removed via rotary evaporation under vacuum at 60˚C - 80˚C during 20 minutes. The catalyst prepared was dried at 120˚C for 8 h, calcined at 300˚C for 2 h and stored in desiccators for further use.
2.3. Analytical Methods
The composition and quantity of methyl ester in biodiesel was determined according to biodiesel test method (EN 14103), using a HP 6890 series 2 gas chromatograph with a flame ionization detector. The capillary column was a DB-WAX column with a length of 30 m, a film thickness of 0.25 lm and an internal diameter of 0.32 mm. Helium was used as carrier gas and also as an auxiliary gas for the FID. One micro-litre of sample was injected using a 6890 Agilent Series Injector [9]. The fatty acid profile of the oil was measured following the International and European Standards ISO 5509 (to prepare the methyl esters) and EN 14103 (to measure the methyl esters profile), using the same chromatograph described previously.
Methanol content was measured according to (EN 14110), using the same apparatus as that described for the methyl ester content. The capillary column was a DB-1 column with a length of 30 m, a film thickness of 3 lm and an internal diameter of 0.32 mm. Samples was injected automatically using a 7694E Agilent Headspace Sample. Trimethylsilylation of glycerol, monoand diglycerides, followed by gas chromatography using a 15 m capillary column coated with a 0.1 lm film of DB-5HT allows the determination of all analytes in a GC run [10]. For the quantitative determination of free glycerol, mono-, diand triglycerides, a calibration with the reference substances glycerol, mono-, diand triolein as well as two internal standards (1,2,4-butanetriol and tricaprin) was carried out. This procedure was developed according to ASTM-D 6584 and EN 14105.
The viscosity of biodiesel is about an order of magnitude lower than that of the parent oil and depends on the composition of alkyl esters. Kinematic viscosity was measured with a Canon-Fenske capillary viscosimeter immersed in a constant temperature (40˚C) bath (TAMSON TV 2000) following the Brazilian norm NBR 10441 and ASTM-D 445. The flash point method uses a closed-cup flash point tester Stanhope Seta series 3 (ISO 3679) and serves to restrict the amount of alcohol in the biodiesel fuel to a maximum of about 0.1% in the biodiesel fuel. The procedure was developed according to Brazilian norm NBR 14598.
Oxidative stability expresses the susceptibility to oxidation upon exposure to air of biodiesel [11]. Metrohm 743 Rancimat was used to determinate the induction period. This procedure was developed according to EN 14112.
Cold filter plugging point (CFPP) test calls for cooling a FAME sample at a specified rate and drawing it under vacuum through a wire mesh filter screen. CFPP is then defined as the lowest temperature at which 20 mL of sample safely passes through the filter within 60 s (European norm EN 116). An automatic tester ISL FPP 5Gs was used to carry out the determination of the CFPP [9, 12].
3. RESULTS AND DISCUSSION
The key processes involved in biodiesel production using microalgae are cultivation, harvest, lipid extraction (cell disruption), and the transesterification of the lipids [13]. Various reports from these topics has been publicated. However, studies of the hydrolysis process as a prelude to the production of biodiesel are extremely scarce. Its use could increase the number of raw material used actually for the biodiesel production, currently limited by high values of acidity and moisture. Furthermore, using hydrolysis can eliminate the process of drying and extraction of lipids with consequent economic benefits.
The fatty acid profile of the hydrolysis of biomass is summarized in Table 1. There are three main types of fatty acids that can be present in a triglyceride: saturated (Cn:0), monounsaturated (Cn:1) and polyunsaturated with two or three double bonds (Cn:2,3).
The high amount of saturated fatty acids found due to the fact that the cultivation of this alga was done in the open. Accordingly, the culture is exposed to high temperatures and high light intensities, two factors that directly influence on the accumulation of saturated fatty acids [14].
In gas chromatography, unidentified compounds were detected with the standards and according to available literature, can be attributed to the presence of even high
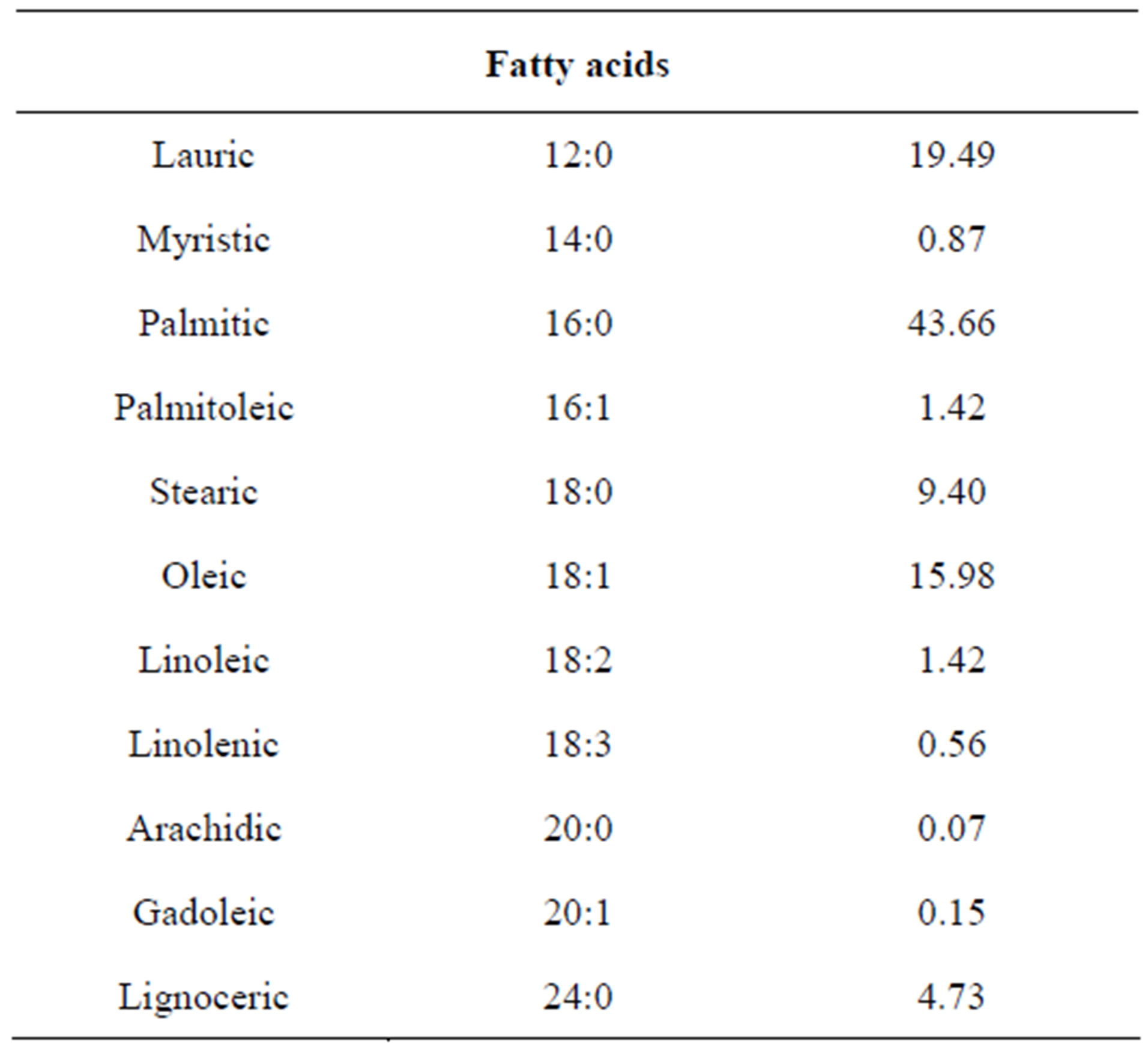
Table 1. Fatty acid compositions (wt%) of biomass from Nannochloropsis oculata.
molecular weight hydrocarbons such as those found in other microalgae like Dunaliella tertiolecta and Botryococus braunii.
The initial acidity of the biomass (taken as algae oil acidity) was high (4.2%), totally impractical for traditional transesterification process. The final acidity value obtained was near 70%.
3.1. Influence of Temperature (T)
Figure 4 shows typical changes in the yield of FAME. A higher temperature resulted in a faster rate of FAME formation. In addition, the yield of FAME tended to increase quickly in early stage of the reaction. Then the rate of FAME formation became slow when the reaction proceeded. It seems apparent that the rate of FAME formation is nearly connected with concentration of FA in the reaction system.
The temperature of 200˚C (no catalyst) favored the rapid increase of the conversion, leading more rapid formation (about 30 minutes) of methyl ester. Figure 4 [15] in his studies of the transesterification of waste frying oils evaluated the influence of temperature on process hen the amount of catalyst fixed in 2%, the molar ratio of 6.128 and varied the temperature at 30˚C, 45˚C and 55˚C.These results led them to conclude that the temperature has a significant positive influence on the conversion of these fatty acid esters.
3.2. Influence of the Supported Catalyst (C)
The catalysts were calcined before being uses in the esterification process. The calcination favored the increase in acidic character of the catalyst, which triggered an increase in the conversion of fatty acids, in just 25 minutes of reaction.
In this conditions of reaction, no such pronounced differences were observed between the reaction without catalyst and in the presence of pure or supported catalyst (figure 5), conforming the overlap of the action of temperature on the action the catalysts.
According to [8] good results in the conversion process can be obtained when the catalyst have very acidic sites, as the niobium oxide.
This catalyst has a molecular structure that favors its thermal stability and the occurrence of minor problems related to diffusion. After 30 minutes of reaction was observed, the formation of plateau. This effect is described by [16] in reaction that reaching its maximum conversion have thermodynamic equilibrium.
In this work, we showed Al2O3 supported Nb2O5 catalysts were more active than pure in the production of biodiesel. The final conversion obtained was 92.24% and 87.43% by 20% Nb2O5/AL2O3 and 20% Nb2O5 respectively. Similar results had been obtained by [17] when
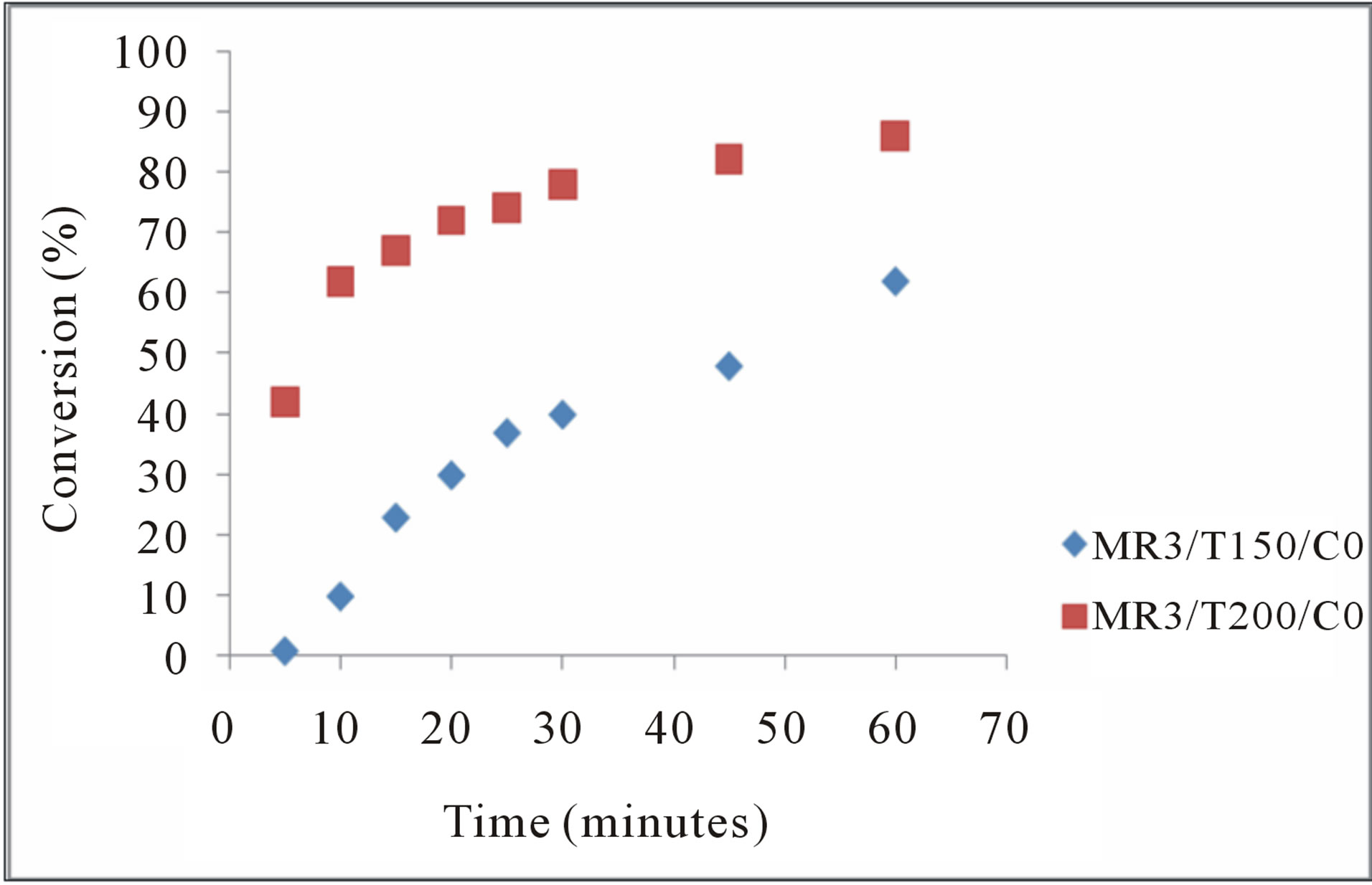
Figure 4. Influence of temperature on the conversion the FA to FAME. C0 = no catalyst.
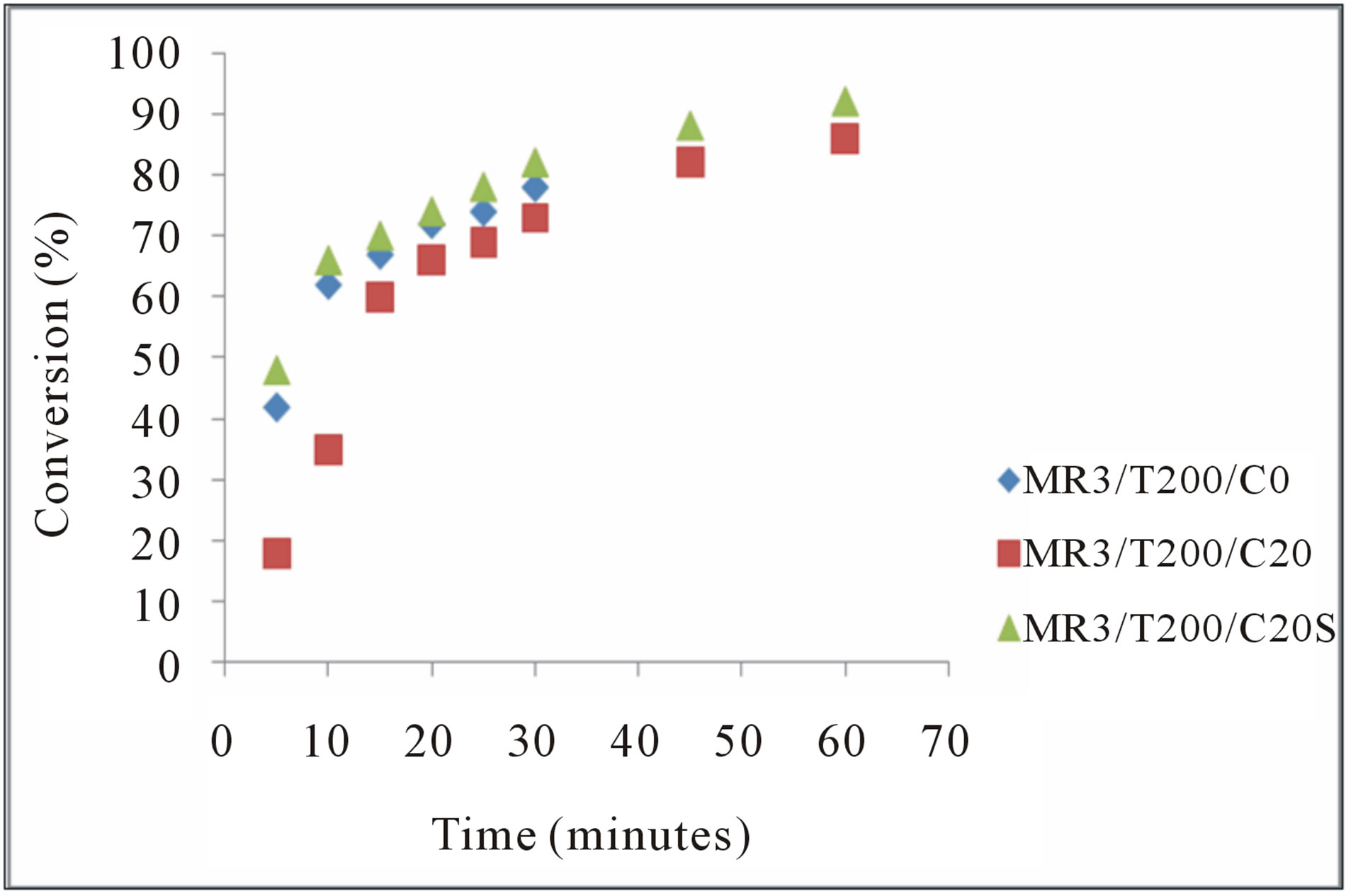
Figure 5. Influence of the supported catalyst on the conversion the FA to FAME. C20 = 20% pure catalyst and C20S = 20% supported catalyst.
study the transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts.
3.3. Influence of the Molar Ratio on the Conversion the FA to FAME (MR)
Figure 6 show the effect of molar ratio of methanol on methyl esterification reaction. Obviously found that a higher yield of FAME was achieved when a more than amount of methanol was added. This increase indicates that the forward esterification reaction rate was accelerated with the increase of methanol amount; similar to that observed with vegetable oil transesterification under excess methanol [18].
The quality of the biodiesels synthesized was tested according to the European Standard EN 14214. In Table 2, parameters for biodiesels synthesized from the biodiesel are given. Most of the parameters satisfied the limits imposed by the standard EN 14214. The degree of compliance of these parameters (ester content, methanol content, kinematic viscosity, acid value, triglycerides,
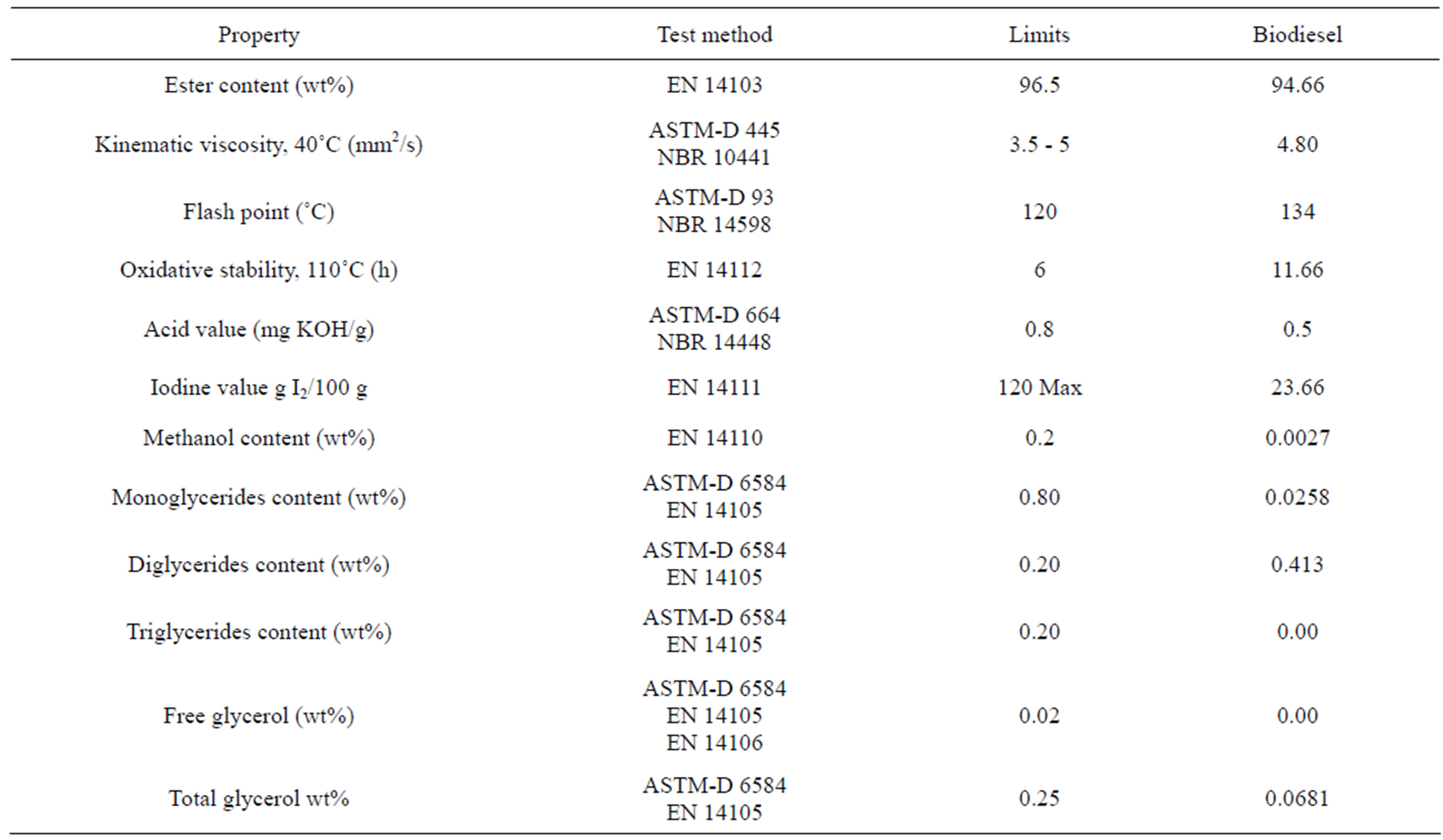
Table 2. Properties of biodiesel from Nannochloropsis oculata (UNE-EN 14214).
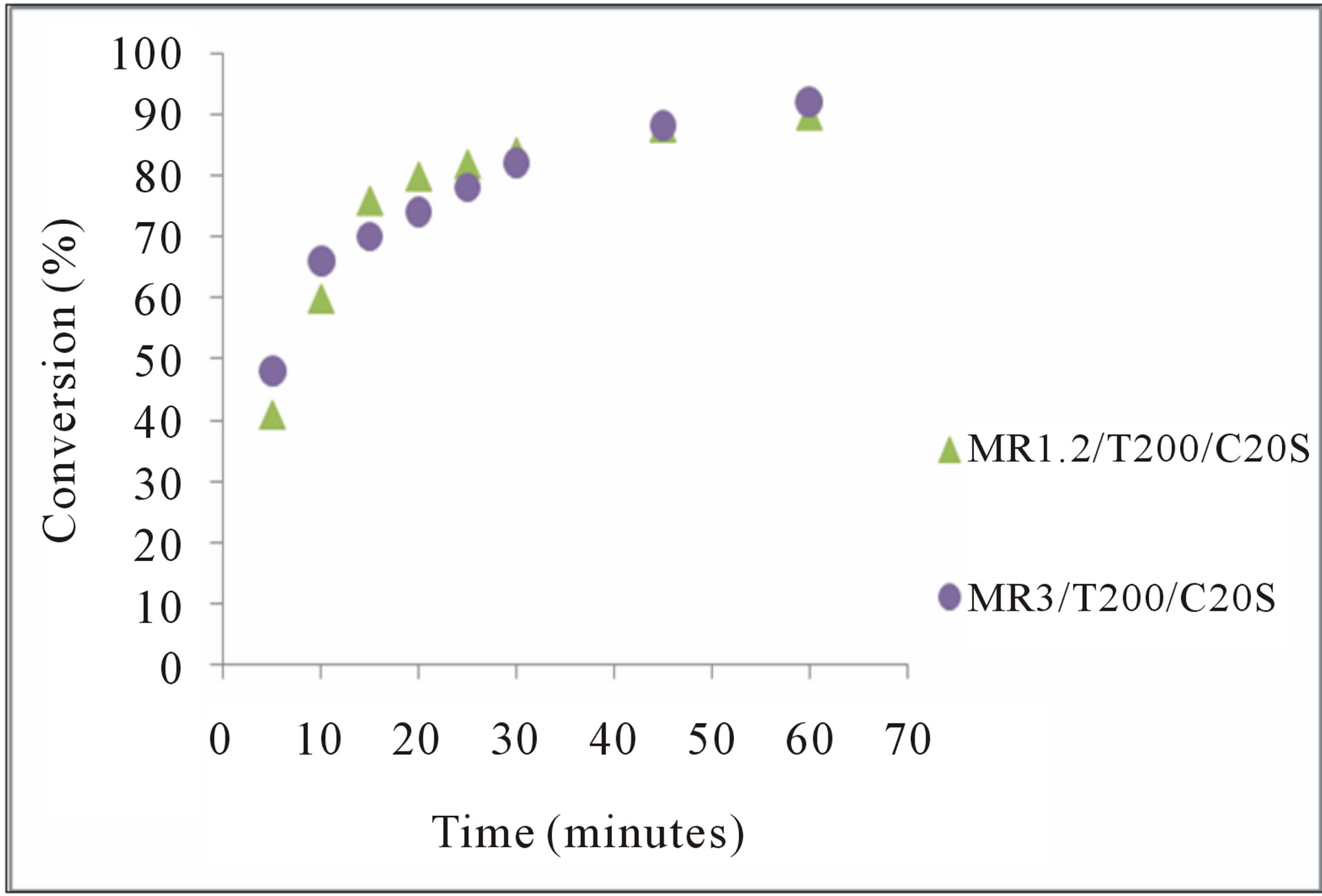
Figure 6. Influence of the molar ratio on the conversion the FA to FAME. C20S = 20% supported catalyst.
digplycerides, monoglycerides and glycerol content free and total and flash point) depends on the degree of oil refinement (previous pre-treatment step), the hydroesterification process (conversion) and the quality of phases purification step. The limit for each parameter is shown in Table 2.
3.4. Iodine Value
Iodine value is a measure of total un saturation within a mixture of fatty acid. It is expressed in grams of iodine which react with 100 g of the respective sample when formally adding iodine to the double bonds. The iodine value of a vegetable oil or animal fat is almost identical to that of the corresponding methyl esters [9].
Iodine value is limited to 120 g I2/100g in the European biodiesel standard (Table 2). The limit of 120 g I2/ 100g demanded by the European biodiesel standard excludes several promising oil sources such as soybean or sunflower seed oil, as well as grape seed oil, from serving as raw materials for biodiesel production [19]. The biodiesel of Nannochloropsis oculata satisfied the limit for the iodine value (23.66). This low value is due to the preponderance of saturated fatty acids as lauric, palmitic and stearic.
The limitation of unsaturated fatty acids is necessary due to the fact that heating higher unsaturated fatty acids results in polymerization of glycerides. This can lead to the formation of deposits or to deterioration of the lubricating [20].
The more unsaturation is present in the oil, the higher the iodine value [21-24]. Therefore, a higher degree of unsaturation than 137 make oils did not meet the European Standard for the iodine value. On the other hand, algae of N. oculata oil, rich in esters of saturated fatty acids such as palmitic (C16:0) and stearic (C18:0) acids, was the oil with a lower iodine value.
3.5. Oxidation Stability
Oxidation stability is one of the major issues affecting the use of biodiesel because of its content of polyunsaturated methyl esters [12]. A minimum Rancimat induction period of six hours is defined for biodiesel samples within UNE-EN 14214 (Table 2).This limit corresponds to the period of time passing before fatty acid methyl esters, aged at 110˚C under a constant air stream, are degraded to such an extent that the formation of volatile acids can be recorded through an conductivity increase. It is well known that it is very difficult to meet this limit for biodiesel fuels derived from many common raw materials, unless antioxidants are added to the biodiesel. All the biodiesels obtained achieve the minimum limit of six hours for oxidation stability (Table 2). Stability of fatty compounds is influenced by factors such as presence of air, heat, traces of metal, peroxides, light, or structural features of the compounds themselves, mainly the presence of double bonds [25]. The oxidation stability decreased with the increase of the contents of polyunsaturated methyl esters. Similar results were obtained by different authors [9,26,27]. Therefore, oils rich in palmitic and stearic acids generally show high stability. This is the explication for the high value obtained in our study (11.66 h).
3.6. Cold Filter Plugging Point
Distillate fuels typically develop operability problems such as wax settling and plugging of filters and fuel lines when overnight temperatures approach −10˚C and −15˚C [28]. The EN 14214 standard does not mention a lowtemperature parameter in its list of specifications; however, it discusses the use of a low-temperature filterability test, the cold filter plugging point (CFPP). Each country using can specify certain temperature limits for different times of year depending on climate conditions [12].
Table 2 shows the CFPP for the biodiesel synthesized. Microalgae biodiesel had poor low-temperature flow properties (16˚C of CFPP). If liquid biodiesel is cooled, the methyl esters of stearic and palmitic acid are the first amount to precipitate and therefore typically constitute a major share of material recovered from clogged biodiesel fuel filters [19].
3.7. Viscosity
Viscosity affects the atomization of a fuel upon injection into the combustion chamber and thereby ultimately the formation of engine deposits. The higher the viscosity, the greater the tendency of the fuel to cause such problems. High viscosity is the major fuel property why neat vegetable oils have been largely abandoned as alternative diesel fuel. Kinematic viscosity has been included in biodiesel standards (1.9 - 6.0 mm2/s in ASTM D6751 and 3.5 - 5.0 mm2/s in EN 14214). According to this the biodiesel obtained satisfied the limit for the viscosity value
(table 2). The viscosity of a transesterified oil, i.e., biodiesel, is about an order of magnitude lower than that of the parent oil [29,30]. The same behavior has the viscosity of hydroesterified oil of microalgae. Viscosity increases with chain length (number of carbon atoms) and with increasing degree of saturation. Value for kinematic viscosity fatty acid alkyl esters are included in Table 2.
3.8. Other Properties
The results of other parameters such as methanol content, monoglycerides content, diglycerides content, triglycerides content, free glycerol (wt%) and total glycerol, also behaved inside of norm. This may be explained by the own nature of the hydroesterification process and the thorough washing of the product before being analyzed.
4. CONCLUSIONS
The microalgae Nannochloropsis oculata is a good raw material to obtain a biodiesel of good quality. In this work, we showed Al2O3 supported Nb2O5 catalyst was more active than pure Nb2O5 in the production of biodiesel from the microalgae Nannochloropsis oculata. Among the mixed oxide catalysts, 20 wt% Nb2O5/Al2O3 was the most active. Excess methanol is necessary to achieve 94.66% biodiesel yield.
The oxidation stability and the cold filter plugging point increase with the increase of the content of saturated methyl esters, such as palmitic and stearic.
REFERENCES
- Vicente, G., Martinez, M. and Aracil, J. (2004) Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresource Technology, 92, 297- 305. doi:10.1016/j.biortech.2003.08.014
- Lang, X., Dalai, A.K., Bakhshi, N.N., Reany, M.J. and Hertz, P.B. (2001) Preparation and characterization of biodiesel from various bioils. Bioresource Technology, 80, 53-62. doi:10.1016/S0960-8524(01)00051-7
- Miao, X. and Wu, Q. (2004) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. Journal of Biotechnology, 110, 85- 93. doi:10.1016/j.jbiotec.2004.01.013
- Minowa, T., Yokoyama, S.-Y., Kishimoto, M. and Okakurat, T. (1995) Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel, 74, 1735-1738. doi:10.1016/0016-2361(95)80001-X
- Haag, A.L. (2007) Algae bloom again. Nature, 447, 520- 521. doi:10.1038/447520a
- Xu, H., Miao, X. and Wu, Q. (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. Journal of Biotechnology, 126, 499-507. doi:10.1016/j.jbiotec.2006.05.002
- Furuta, S., Matsuhashi, H. and Arata, K. (2004) Biodiesel fuel production with solid superacid catalysis fixed bed reactor under atmospheric pressure. Catalysis Communications, 5, 721-723. doi:10.1016/j.catcom.2004.09.001
- Carvalho, L., Britto, P., Matovanelli, R., Camacho, L., Antunes, O.A. and Aranda, D.G.A. (2005) Esterification of the fatty acid of palm by heterogeneous catalysis. Proceedings of the 13th Brazilian Congress of Catalysis and 3rd Mercosur Congress on Catalysis, Vol. 4, Uberlândia, 1-4.
- Knothe, G. (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Processing Technology, 86, 1059-1070. doi:10.1016/j.fuproc.2004.11.002
- Plank, C. and Lorbeer, E. (1995) Simultaneous determination of glycerol, and mono-, diand triglycerides in vegetable oil methyl esters by capillary gas chromatography. Journal of Chromatography A, 697, 461-468. doi:10.1016/0021-9673(94)00867-9
- Dunn, R.O. (2005) Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process Technology, 86, 1071-1085. doi:10.1016/j.fuproc.2004.11.003
- Knothe, G. (2006) Analyzing biodiesel: Standards and other methods. Journal of the American Oil Chemist’s Society, 83, 823-833. doi:10.1007/s11746-006-5033-y
- Lee, J.Y. (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresource Technology, 101, S75-S77.
- Garibay, A.H., Vázquez-Duhalt, M.P., Serrano, L. and Martínez, A.J. (2009) Biodiesel a partir de microalgas. BioTecnología, 13, 38-60.
- Marchetti, J.M., Miguel, V.U. and Errazy, A. F. (2006) Heterogeneous esterifications of oil with high amount of free fatty acids. Fuel, 86, 906-910. doi:10.1016/j.fuel.2006.09.006
- João, R.R., Santos, R.T.P., Marcio, N. and Arandra D.A.G. (2006) Simpósio Ibero-Americano de Catálise. Rio Grande do Sul, 17-22 September 2006.
- Umdu, M.T. and Erol, S. (2009) Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresource Technology, 100, 2828-2831. doi:10.1016/j.biortech.2008.12.027
- Umdu, E.S. (2008) Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. M.S. Thesis, Izmir Institute of Technology, Izmir, 2828-2831.
- Mittelbach, M. and Remschmidt, C. (2004) Biodiesel: The comprehensive handbook. Martin Mittelbach, Vienna.
- Mittelbach, M. (1996) Diesel fuel derived from vegetable oils, VI: Specifications and quality control of biodiesel. Bioresource Technology, 56, 7-11. doi:10.1016/0960-8524(95)00172-7
- Knothe, G. (2002) Structure indices in FA chemistry. How relevant is the iodine value? Journal of the American Oil Chemist’s Society, 9, 847-853. doi:10.1007/s11746-002-0569-4
- Knothe, G., Dunn, R.O. and Bagby, M.O. (1997) Biodiesel: The use of vegetable oils and their derivatives as alternative diesel fuels. In: Saha, B.C. and Woodward, J. Eds., Fuels and Chemicals from Biomass, American Chemical Society, Washington DC. doi:10.1021/bk-1997-0666.ch010
- Kyriakidis, N.B. and Katsiloulis, T. (2000) Calculation of iodine value from measurements of fatty acid methyl esters of some oils: comparison with the relevant American oil chemists society method. Journal of the American Oil Chemist’s Society, 77, 1235-1238. doi:10.1007/s11746-000-0193-3
- Lin, C.-Y., Lin, H.-A. and Hung, L.-B. (2006) Fuel structure and properties of biodiesel produced by the peroxidation process. Fuel, 85, 1743-1749. doi:10.1016/j.fuel.2006.03.010
- Bajpai, D. and Tyagi, V.K. (2006) Biodiesel: Source, production, composition, properties and its benefits. Journal of Oleo Science, 55, 487-502. doi:10.5650/jos.55.487
- McCormick, R.L., Ratcliff, M., Moens, L. and Lawrence, R. (2007) Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Processing Technology, 88, 651-657. doi:10.1016/j.fuproc.2007.01.006
- Park, J.-Y., Kim, D.-K., Lee, J.-P., Park, S.-C., Kim, Y.-J., Lee, J.-S. (2008) Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresource Technology, 99, 1196-1203. doi:10.1016/j.biortech.2007.02.017
- Dunn, R.O., Bagby, M.O. (1996) Low-temperature filterability properties of alternative diesel fuels from vegetable oils. Liquid fuel and industrial product from renewable resources. Proceedings of Third Liquid Fuel Conference, Nashville, 15-17 September 1996, 95-103.
- Dunn, R.O. and Knothe, G. (2001) Alternative diesel fuels from vegetable oils and animal fats. Journal of Oleo Science, 50, 415-426. doi:10.5650/jos.50.415
- Knothe, G. and Dunn, R.O. (2001) Oleochemical manufacture and applications. Sheffield Academic Press, Sheffield.

