Health
Vol. 4 No. 8 (2012) , Article ID: 21983 , 4 pages DOI:10.4236/health.2012.48071
Adjuvant effect of a synthetic Aluminium-Magnesium Silicate on chloroquine phosphate, against Plasmodium berghei
![]()
Department of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria; *Corresponding Author: maduikeezeibe@yahoo.com
Received 24 April 2012; revised 19 May 2012; accepted 12 June 2012
Keywords: Antiplasmodial resistance; chloroquine toxicity; Synthetic Aluminium-Magnesium Silicate
ABSTRACT
Effect a synthetic Aluminium-Magnesium Silicate (AMS) has on chloroquine was tested. Thirty, Plasmodium berghei-infected mice, in three experimental groups (7 mg/kg, 5 mg/kg and 3 mg/ kg) of 10 mice each, were treated. Two subgroups, in each experiment, were treated with chloroquine and with a chloroquine-AMS drug formulation, respectively. Five of the infected mice served as controls. Parasitaemia (%), Haemoglobin concentration (Hb), Red Blood Cells (RBC), rectal temperature and body weight were assessed. Parasitaemia of subgroups treated at 7 mg/kg were higher than that of the control. Also, at 7 mg/kg, there was mortality with chloroquine (20%) and with the chloroquineAMS drug (80%). At 5 mg/kg and 3 mg/kg, the AMS significantly (P < 0.05) improved ability of chloroquine to reduce plamodial parasitaemia, from 2.46 ± 0.21 to 1.57 ± 0.25 and from 3.82 ± 0.06 to 2.12 ± 0.08. It also significantly (P < 0.05) improved means of Hb and RBC from 12.25 ± 0.27 and 88.99 ± 5.72 to 12.68 ± 0.18 and 92.91 ± 4.01 and from 10.18 ± 3.00 and 63.39 ± 18.02 to 12.98 ± 0.47 and 95.23 ± 5.32. Body weight increased at 5 mg/kg, from 29.06 ± 1.95 to 32.66 ± 2.10 kg (P < 0.05) while at 3 mg/kg, rectal temperature reduced from 37.35 ± 0.32 to 36.84˚C ± 0.23˚C (P < 0.05). These results suggest, AMS worsened chloroquine toxicity at 7 mg/kg but potentiated its antiplasmodial activities at the lower doses.
1. INTRODUCTION
Protozoan parasites of the genus plasmodium are causative agents of malaria [1,2]. Malaria is a zoonotic disease. It affects man, zoo primates, avian species and rodents [3,4]. When species of plasmodium which naturaly infect animals were experimentaly passaged in human volunteers they produced clinical malaria in man [5].
Clinical signs of malaria in humanbeigns include fever, loss of weight and anaemia. Anaemia is diagnosed by determination of Red Blood Cell count (RBC), packed cell volume (PCV) and Haemoglobin concentration (Hb). It is a common consequence of malaria, especially in children [6]. Pathogenesis of anaemia in malaria are many. Haemolysis of infected red blood cells is the most important cause. The haemolysis depends on degree of parasitaemia, course of the disease and the number of febrile paroxysms that occur.
In humans, P. vivax predominantly invades about 2% of the immature red blood cells. P. malariae develops mostly in mature red blood cells and the parasitaemia is about 1%. P. falciparum affects red blood cells of all ages and its parasitaemia can be as high as 20% - 30%. This massive destruction of red blood cells accounts for severe anaemia associated with P. falciparum malaria. Even non parasitized RBCs could be removed from circulation by complement-mediated lysis and phagocytosis which result from immune complex deposition and complement activation [6].
Increased splenic clearance of parasitized and nonparasitized red blood cells, reduction in survival time of red blood cells, inhibition of erythropoeisis in the bone marrow, and drug induced haemolysis contribute to anaemia in malaria.
Malaria causes one to three million human deaths every year, in subsaharan Africa [7]. It is a serious health problem, especially among poor people and is itself, a cause of poverty [8]. Most countries in Africa spend upto 40% of their annual public health budgets in efforts to combat malaria [9].
Chloroquine is one of the drugs of choice for prevention and treatment of malaria in the developing countries, because, it is cheap and effective [10]. However, in recent times cases of resistance to chloroquine by malaria parasites have been reported [11]. Need therefore exists to search for drugs to combine with chloroquine to improve its efficacy and still retain its low cost.
Aluminium-Magnesium Silicate (AMS) is cheap. It is also safe to combine with drugs for treatment of humanbeings and animals [12]. When in solution, its molecules form three dimentional colloidal structures round molecules of any drug it is combined with. By that mechanism, AMS stabilizes other drugs. To stabilize drugs means to protect the drugs from destruction. AMS may thus protect chloroquine from being rapidly degraded by metabolic processes. This will retain high concentration of chloroquine in blood of treated animals for a long time. When high concentrations of drugs are retained in blood for a long time, actions of such drugs improve [13]. By incorporating Ampicilline, piperazine and sulphadimidin in a synthetic AMS, their activities against Salmonella gallinarum, Helignosomoides bakeri and against Eimeria spp respectively, were improved [14-16]. A formulation of 20% chloroquine phosphate in the synthetic AluminiumMagnesium Silicate was therefore made, to test if it would improve antiplasmodial activity of chloroquine.
2. MATERIALS AND METHODS
Thirty five mice were infected by innoculating each mouse with 1 ml of blood which contained 2 × 105 parasitized Red Blood Cells. Ten days Post Infection (PI), they were divided into three experimental groups of 10 mice each. A group of five of the P. berghei-infected mice was left untreated to serve as control. Each experimental group was subdivided into two, each of five mice. The experiments were done with chloroquine phosphate at 7 mg/kg, 5 mg/kg and 3 mg/kg respectively. The two subgroups in each experiment were treated with chloroquine phosphate alone and with the drug formulation of 20% chloroquine phosphate in the synthetic AMS, respectively. The treatment was administered per os, daily for five days in each experiment.
A day post treatment (PT), body weight, percentage parasitaemia, rectal temperature, total Red Blood Cell (RBC) count and Heamoglobin concentration (Hb) were determined for each mouse in the six treated groups and in the control. This was repeated at one week, two weeks and three weeks PT. Mean values of the parameters for each group were recorded as the group’s weekly values.
3. RESULTS
Four mice (80%) and one mouse (20%) of the groups treated with 7 mg/kg chloroquine in AMS and 7 mg/kg chloroquine alone respectively, died between 7 to 14 days PT. Incorporating chloroquine phosphate in the AMS significantly improved its ability to reduce P. berghei parasitaemia at 5 mg/kg and at 3 mg/kg dose levels, from 2.46 ± 0.21 to 1.57 ± 0.25 (P < 0.05) and from 3.83 ± 0.06 to 2.12 ± 0.08 (P < 0.05) respectively. Mean parasitaemia, 4.15 ± 0.26 of the group treated with 7 mg/kg chloroquine phosphate alone, was higher than 3.60 ± 0.22 of the control (P < 0.05).
Mean Hb, 12.95 ± 0.25, 12.25 ± 0.27 and 12.68 ± 0.18 of the groups treated with 7 mg/kg chloroquine alone, 5 mg/kg chloroquine alone and 5 mg/kg chloroquine in AMS respectively, were significantly higher (P < 0.05) than 10.43 ± 2.64 of the only surviving mouse of the group treated with 7 mg/kg chloroquine in AMS and the mean, 10.18 ± 3.00 got in the group treated with 3 mg/ kg chloroquine alone.
Red blood cell counts, 95.19 ± 2.81, 92.91 ± 4.01 and 95.23 ± 5.32 of the groups treated with 7 mg/kg chloroquine phosphate alone, 5 mg/kg chloroquine phosphate in AMS and 3 mg/kg chloroquine phosphate in AMS respectively, were also, significantly higher (P < 0.05) than 88.99 ± 5.72 of the group treated with 5 mg/kg chloroquine alone and 85.55 ± 7.83 of the mouse treated with 7 mg/kg chloroquine in AMS. Least Rbc count, 63.39 ± 18.02 was got in the group treated with 3 mg/kg chloroquine alone.
Mean rectal temperature, 35.56˚C ± 0.82˚C and 35.73˚C ± 0.38˚C of the groups treated with 5 mg/kg chloroquine alone and 5 mg/kg chloroquine in AMS respectively, were significantly (P < 0.05) lower than 37.29˚C ± 0.38˚C of the mouse treated with 7 mg/kg chloroquine in AMS and 36.84˚C ± 0.32˚C, 36.84˚C ± 0.23˚C and 36.16˚C ± 0.35˚C got in the groups treated with 7 mg/kg chloroquine alone, 3 mg/kg chloroquine in AMS and in the control respectively.
Mean body weight, 32.66 ± 2.10 kg of the mice treated with 5 mg/kg chloroquine in AMS was significantly (P < 0.05) higher than 29.29 ± 0.51 kg, 29.17 kg and 29.06 ± 1.95 kg got in the groups treated with 7 mg/kg chloroquine alone, 7 mg/kg chloroquine in AMS and 5 mg/ kg chloroquine alone respectively. It was also more significantly (P < 0.05) higher than 26.65 ± 0.83 kg and 26.35 ± 0.61 kg of the groups treated with 3 mg/kg chloroquine alone and 3 mg/kg chloroquine in AMS respectively.
Effects of the synthetic AMS on ability of chloroquine phosphate to reduce P. berghei parasitaemia and on clinical signs of plasmodia infection in mice are as in tables 1-3.
4. DISCUSSION
Treatment with the chloroquine-AMS drug formulation reduced P. berghei parasitaemia more significantly than treatment with chloroquine alone. Vanderbilt [12] reported that AMS is a stabilizing agent. The three di-
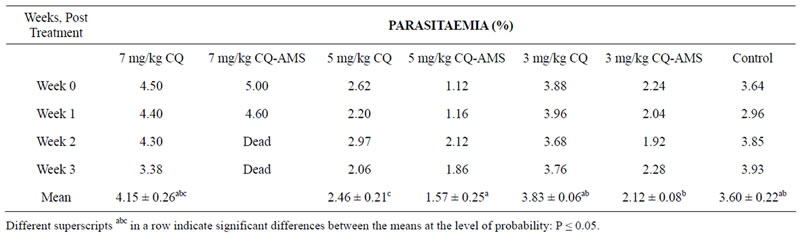
Table 1. Plasmodium berghei parasitaemia of mice treated with chloroquine phosphate in Aluminium Magnesium Silicate.
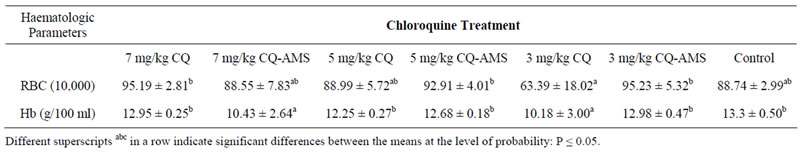
Table 2. Haemoglobin concentration and Red Blood Cell counts of P. berghei infected mice, treated with chloroquine phosphate in Aluminium Magnesium Silicate.
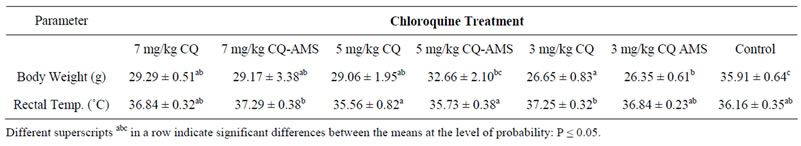
Table 3. Rectal temperature and body weight of P. berghei infected mice treated with chloroquine phosphate in Aluminium Magnesium Silicate.
mentional colloidal structures it forms round molecules of active drugs protect them from rapid degradation by metabolic processes. The synthetic AMS may have protected chloroquine from being rapidly degraded in blood of the treated mice. Retention of high concentrations of chloroquine in blood of the mice for longer time may have led to the significant reduction in parasitaemia.
Dose of chloroquine usually employed in treatment of malaria in humans, is 7 mg/kg but in this experiment, mice treated at that dose level had higher parasitaemia than the untreated group. This may have been due to chloroquine toxicity. Chloroquine inhibits uptake of iron and destroys plasmodium-infected Rbcs [17,18]. Chloroquine toxicity also, results in immunosupression [19] and it has no effect on parasites that migrate to tissues of infected animals. Plasmodia parasites in tissues return to blood once concentration of antiplasmodial drugs in blood falls [18]. In immunosupressed animals, parasites that return to blood could have rapid multiplication. So, the high parasitaemia in the group treated with 7 mg/kg chloroquine alone, may be due to chloroquine toxicity. There was no mortality in the group of untreated mice. So, deaths recorded in the two subgroups treated at chloroquine dose of 7 mg/kg may also be due to chloroquine toxicity. Mortality of 20% in the group treated with chloroquine alone was significantly lower than 80% mortality in the group treated with the chloroquine-AMS drug formulation. This suggests that the AMS potentiated the chloroquine toxicity.
Wellem [19] reported that it is necessary to include supportive drugs, such as iron and vitamines in treatment of malaria to achieve effective cure. Noninclusion of these surportive chemotherapeutics in the experiments may be responsible for the high parasitaemia and mortalities recorded when chloroquine was used at dose of 7 mg/kg.
RBC counts and Hb values of the groups of mice treated with 7 mg/kg chloroquine alone, 5 mg/kg chloroquine in AMS and 3 mg/kg chloroquine in AMS were high, indicating that those mice had no anaemia but the group treated with 5 mg/kg chloroquine alone and the control had slight anaemia. Anaemia was more pronounced in the group treated with 3 mg/kg chloroquine alone and in the only survivor of the mice treated with 7 mg/kg chloroquine in AMS. Both chloroquine toxicity and malaria cause anaemia [17,19]. Anaemia of the groups treated with 5 mg/kg chloroquine alone and 3 mg/ kg chloroquine alone, may be a result of ineffective treatment of the plasmodial infections while that of the mouse treated with 7 mg/kg chloroquine in AMS may be due to chloroquine toxicity.
High mean rectal temperature and low mean body weight got in the group treated with 3 mg/kg chloriquine alone and in the mouse treated with 7 mg/kg chloroquine in AMS may also be due to same reasons. In the experiment with 5 mg/kg, the very low parasitaemia achieved by the treatments and the lower dose of chloroquine used may be responsible for absence of both anaemia and fever in mice of these subgroups. Highest mean body weight among the treated groups was also got in the subgroup of mice treated at 5 mg/kg with the Chloroquine phosphate-AMS drug formulation.
The control group had the highest mean body weight, had only slight reduction in RBC count and in Hb. Fever was also mild in this group. These findings agree with report of Carter and Diggs [20] that plasmodium infection in rats and mice do not produce serious clinical disease.
It has therefore been concluded that use of chloroquine at dose of 5 mg/kg in a chloroquine-AMS drug formulation may achieve better antiplasmodia actions than the 7 mg/kg chloroquine alone currently being used. Use of that lower dose will also reduce chloroqune toxicity that occured with chloroquine dose of 7 mg/kg. Supporting chloroquine treatment with iron and vitamines may further improve its antiplasmodial effect and reduce clinical signs in treated animals.
REFERENCES
- Singh, B., Kim-Sung, L. and Matusop, A. (2004) A large focus of naturally acquired plasmodium knowlesi infection in humanbeings. Lancet, 363, 1017-1024. doi:10.1016/S0140-6736(04)15836-4
- Mueller, I., Zimmerman, P.A. and Reeder, J.C. (2007) Plasmodium malria and plasmodium ovale—“Bashful malaria parasite”. Trends in Parasitology, 23, 278-283. doi:10.1016/j.pt.2007.04.009
- Vincke, I.H. and Lips, M. (1948) Plasmodium berghei. Annales de la Societe Belge de Medecine Tropical, 28, 97- 104.
- Escalante, A. and Ayala, F. (1994) Phylogeny of the malarial genus, plasmodium, derived from Rna gene sequences. Proceedings of the National Academy of Sciences USA, 91, 11373-11377. doi:10.1073/pnas.91.24.11373
- Collins, W.E. and Aikawa, M. (1977) Plasmodia of nonhuman primates. In: Kreier, J.P., Ed., Parasitic Protozoa, Academic Press, New York.
- Chen, Q., Schlichtherle, M. and Wahlgren, M. (2000) Molecular aspects of severe malaria. Clinical Microbiology Review, 13, 439-450. doi:10.1128/CMR.13.3.439-450.2000
- Snow, R.W., Querra, C.A., Noor, A.M., Myint, H.Y. and Hay, S.I. (2005) The global distribution of clinical episodes of plasmodium falciparium malaria. Nature, 434, 214- 217. doi:10.1038/nature03342
- Etling, M., Mcfarland, D.A., Schultz, L.J. and Chitsulo, L. (1994) Economic impact of malaria in malawian households. Annals of Tropical Medecine and Parasitology, 45, 74-79.
- WHO (2009) Economic costs of malaria: Roll back malaria WHO partnership.
- Wards, B.P. and Fidock, D. (2004) Understanding how the malaria parasite resists chloroquine. Molecular Cell, 15, 867-877.
- Wellems, T.E. (2002) Plasmodium, chloroquine resistance and the search for a replacement antimalarial drug. Science, 298, 124-126. doi:10.1126/science.1078167
- Technical Literature-Veegum Inc. (1992) The versatile ingredient for pharmaceutical formulations. www.rtvanderbilt.com
- Brent, W., Gigi, H.R., Khalid, H.I. and John, C.R. (2001) What do we realy know about antibiotic pharmacdynamics? Pharmacotherapy, 21, 3028-3088.
- Ezeibe, M.C.O., Anosa, G.N., Okorie, O.K., Elendu-Eleke, N.P., Okoroafor, O.N., Ngene, A.A. and Chikelu, O.N. (2012) Aluminium-Magnesium Silicate enhances antibacterial activity of ampicilline trihydrate, against Salmonella gallinarum. http://precedings.nature.com/documents/6814/version/1/files/npre20126814-1.pdf
- Ezeibe, M.C.O. (2011) The synthetic Aluminium-Magnesium Silicate. Great AP Express Pub., Nsukka.
- Ezeibe, M.C.O., Ofafor, U.C., Okoroafor, O.N., Eze, J.I., Ngene, A.A., Animoke, P.C. and Mbuko, I.J. (2011) Effect of Aluminium-Magnesium Silicate on anticoccidial activity of sulphadimidin. Tropical Veterinary, 29, 41-44.
- Emerson, L.R., Martin, E.M., Rodger, K., Martin, D., Kyle, E., Vahey, M. and Wirth, D.F. (2001) Relationship between chloroquine toxicity and acquisition in Saccharomyces cerevisae. Antimicrobial Agents and Chemotherapy, 46, 114-117.
- Hempelmann, E. (2007) Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol Research, 100, 671- 676. doi:10.1007/s00436-006-0313-x
- Wildig, J., Michon, P., Siba, P. and Melombo, M. (2006) Parvovirus B 19 infection contrbutes to severe anaemia in young children in Papua New Guinea. Journal of Infectious Diswases, 194, 146-153.
- Carter, R. and Diggs, C.I. (1977) Plasmodia of rodents in: Parasitic protozoa. Kreier, J.P., Ed., Academic Press, New York.

