Engineering
Vol. 4 No. 3 (2012) , Article ID: 18330 , 7 pages DOI:10.4236/eng.2012.43018
Effect of Acidic Catalyst on Properties of Novel Conductive Copolymer Films Made of Pyrrole and Formyl Pyrrole
Department of Materials Science and Technology, Nagaoka University of Technology, Nagaoka, Japan
Email: takaomi@nagaokaut.ac.jp
Received December 27, 2011; revised January 12, 2012; accepted January 20, 2012
Keywords: Conductive Polymer; Copolymerization; Pyrrole; 2-Formyl Pyrrole; Trifluoroacetic Acid; Trichloroacetic Acid; Conductivity
ABSTRACT
Effect of acidic catalysis having carboxylic acid group was studied on properties of conductive copolymer films made of pyrrole (Py) and 2-formyl pyrrole (FPy). It was noted that trifluoroacetic acid (TFA) and trichloroacetic acid (TCA) were suitable for the preparation of copolymer films, which showed good properties in its strength and electrical conductivity of the copolymer films. When the concentration of TFA or TCA was increased in the monomer feed, the copolymerization yield became higher and the obtained films showed electrical conductivity in the range of 10–4 - 10–3 S/cm. FT-IR and UV-Vis spectra confirmed the formation of conjugate chemical structure in the copolymer film.
1. Introduction
Electrically conductive polymers with conjugated double bonds have been attracting in much attention of advanced materials. Especially, polypyrrole (PPy) is one of the most promising conductive polymers, because such polymer has higher conductivity and industrial stability in the conductive property relative to state rather than many other conductive polymers [1-8]. It was known that PPy could be easily prepared by chemical and electrochemical polymerization of pyrrole monomer in various organic solvents and in an aqueous media. In their preparation processes, polymerization condition and additives introduced into the reaction mixture influenced the properties of resultant conductive PPy [9-11]. For example, in electrochemical polymerization of PPy [12-14], there was difficulty to fabricate the resultant films because of the limitation of an electrode shape and size. Although chemical polymerization method could prepare a large quantity of PPy, it was known the resultant polymer was limited to powder shape [15-17]. In these cases, generally, acidic catalyst was used in such chemical polymerization of pyrrole to solve this problem. So far, Salmon and coworkers reported chemical polymerization of pyrrole for forming PPy films in the presence of sulfuric acid in ethanol [18]. They mentioned that the films were fragile and unstable in their polymer properties. Consequently, very few investigations were examined for single component PPy films prepared by chemical methods.
On the other hand, little was known about possible of copolymers of pyrrole and another monomer. For example, pyrrole and aniline [19-22], thiophene [23,24], furan [25], and N-substituted pyrrole [26,27] were prepared by chemical or electrochemical polymerization. Although some reports were described advantages of the obtained conductive polymers, their synthetic methods also included numerous steps and reactions facilitated by several instruments.
In contrast, there is possibility for preparation of novel conductive copolymer films by using pyrrole (Py) and 2-formyl pyrrole (FPy). This type of Py-FPy components could expect to construct conjugated structure between Py and Fpy showing methine group (Scheme 1). In a reaction involving Py and a compound containing formyl group for porphyrin, conjugated aromatic cyclic molecules, were normally obtained in the presence of carboxylic acid [28]. In the production of methine group of Py and FPy, it is interesting to form such copolymer backbone for conductive films. Therefore, effect of acid species used as catalyst was examined. Depending on properties of their copolymer films, the acidic catalyst would become important factor on the copolymerization of Py and FPy. In addition, there has been no report on such kind of copolymers made of Py and other monomers. In the present work, the role of acidic catalysts in the copolymerization was studied as a first report in detail by using a series of carboxylic acids in order to compare the properties of the resultant films.
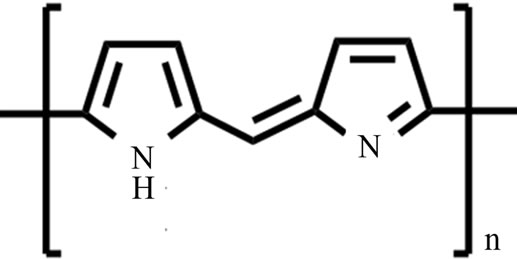
Scheme 1. Chemical structure of Py-FPy copolymer.
2. Experimental
2.1. Material
Pyrrole (Py) monomer was purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan) and distilled under reduced pressure after dehydration using calcium hydride for 24 hr. Pyrrole-2-carboxaldehyde (2-formyl pyrrole, FPy) was purchased from Tokyo Chemical Industry Co. Ltd. The carboxylic acid catalyst reagents including trifluoroacetic acid (TFA), difluoroacetic acid (DFA), formic acid (FA), acetic acid (AA), were obtained from Nacalai Tesque Inc. (Tokyo, Japan); trichloroacetic acid (TCA) were provided Tokyo Chemical Industry Co. Ltd. The chemical dopant, iodine (I2), was a product of Nacalai Tesque Inc. Water was distilled before use.
2.2. Copolymerization of Py and FPy with Various Carboxylic Acids
Py (200 mg, 3 mmol) and FPy (286 mg, 3 mmol) were dissolved and then stirred in 2 mL of chloroform (CHCl3) in a sample tube (20 mL). Then, the solution containing each carboxylic acid (13 mmol) and CHCl3 (2 mL) was added to the monomer solution at room temperature, when the polymerization was started. Here, different carboxylic acid such as TFA, TCA, DFA, FA and AA were used for each copolymerization. For example, when the TFA was added to the monomer solution, the solvent color changed immediately from transparent brown to yellowish red. Then, the mixed solution was spin-coated onto the petri dish at 20 rpm using a spin coater (Opticoat MS-A100; Mikasa Co. Ltd., Japan). Each copolymerization was carried out for about 0.5 - 8 hrs at room temperature, and then a film was formed in the petri dish. It was confirmed that the black color film was insoluble in several solvents. After the copolymerization of Py and FPy, the resultant films were ground down and washed well with water and acetone. The precipitates were stirred with 3 wt% of NaOH in aqueous solution for 0.5 hr in order to remove the acid catalyst. Then, the solution was stirred with 2N HCl aqueous solution at 0.5 hr to convert them to acidity. The obtained black precipitates were dried in a vacuum oven at 60˚C for 12 hr. These samples were used for Fourier transfer infrared (FT-IR) spectrometry (Prestige-21; Shimadzu Corp., Japan). For the measurement of electrical conductivity, iodine was doped on the film. Each film was washed with water and acetone and then put in a closed vessel containing a small amount of iodine for chemical doping. Then, the electrical conductivity of the doped film was determined.
2.3. Measurements
Characteristics of the resultant Py-FPy copolymers including electrical conductivity, tensile strength, FT-IR and UV-Visible spectrophotometry were measured. The value of electrical conductivity of each film was obtained using a typical four-point method (Roresta-GP MCPT610; Mitsubishi Chemical Analytec Co. Ltd., Japan) at room temperature. Before measurement of the conductivity, the film was placed in I2 atmosphere for about 24 hr. Then the values of electrical conductivity of the doped film were determined with the four-point probe as attached to the surface of the film at five points. The tensile strength of the films was measured using a load cell (LTS-500N; Minebea Co. Ltd., Japan) for the sample specimen (2.5 cm × 7.5 cm). FT-IR spectra of the resultant films were also determined using a FT-IR spectrometer in the transmittance mode. Transmission spectra were obtained using KBr method. The spectral resolution was 4 cm–1 for each spectrum. The UV-Visible spectra of thin films having about 1 - 10 μm thickness on the quartz glass were measured using a UV-VIS-NIR spectrophotometer (V-570; Jasco Corp., Japan) in absorption mode.
3. Results and Discussion
3.1. Properties of Py-Fpy Copolymer Films Prepared in the Presence of Different Catalyst
In order to examine effect of catalyst on Py-FPy copolymer films, five species of carboxylic acids were used for the copolymerization. Table 1 shows several carboxylic acids used as catalyst for preparation of resultant copolymer films. It was obviously observed that the copolymerization time was varied when the chemical structure of the catalyst was changed. When the AA was used, surface tucking less time (STLT) observed on the film surface was about 8 hr. However, as the TFA catalyst was used, the value of STLT was about 0.5 hr, indicating the polymerization was quickly occurred. The resultant value of the yield also indicated that both TCA and TFA showed high yield of the copolymerization. In the cases of AA and FA, the yields became low relative to those of TFA, TCA and DFA. Furthermore, it was noted that the films obtained showed somewhat different view. As presented in Figure 1 for typical pictures of the resultant films copolymerized with TFA (a), TCA (b) and DFA (c), the view was like metallic black and different color grow. In contrast it was easily seen for FA (d) and AA (e) to lead fragile black films. As shown in Table 1,
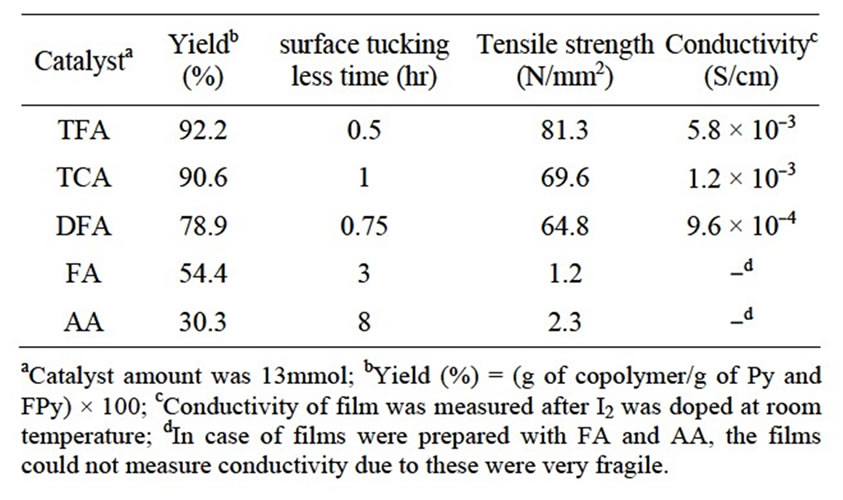
Table 1. Polymerization yield, surface tucking less time, tensile strength and conductivity of Py-FPy copolymer synthesized using various carboxylic catalysts.

Figure 1. Pictures of Py-FPy copolymer films prepared with TFA (a), TCA (b), DFA (c), FA (d) and AA (e).
the latter had very low tensile strength in the range of 1.2 - 2.3 N/mm2. However, the films produced with TFA and TCA showed good results in copolymer yield and film strength.
Figure 2 show FT-IR spectra of copolymer films prepared by each carboxylic acid before (a) and after (b) removal of the catalysts from the film. In spectra (a), each spectra had C=O stretching band at about 1680- 1640 cm–1 region. However, after washing the catalyst, the spectra (b) showed disappearance of the strong C=O stretching near 1680 - 1640 cm–1. When comparison was made in the maximum peak of TFA catalyst without and with the film, the values of wavenumber for the C=O band were 1780 and 1670 cm–1, respectively. Furthermore, the characteristic OH band of TFA could not appear in 3300 - 3400 cm–1 as observed in spectra (a). These results meant that the C=O group was interacted with the Py-FPy film and the acidic proton for protonating in the Py nitrogen of the film. In Figure 2(b), the FT-IR spectra of copolymer films for FA and AA showed still presence of the stretching band assigned with C=O, although washing operation was carried out. This meant that the brittle films were oxidized. It was also observed that appearance of the N-H stretching vibrations of two types (3380 and 3260 cm–1) and the C=C double bond stretching of the pyrrole ring of two types (1626 and 1538
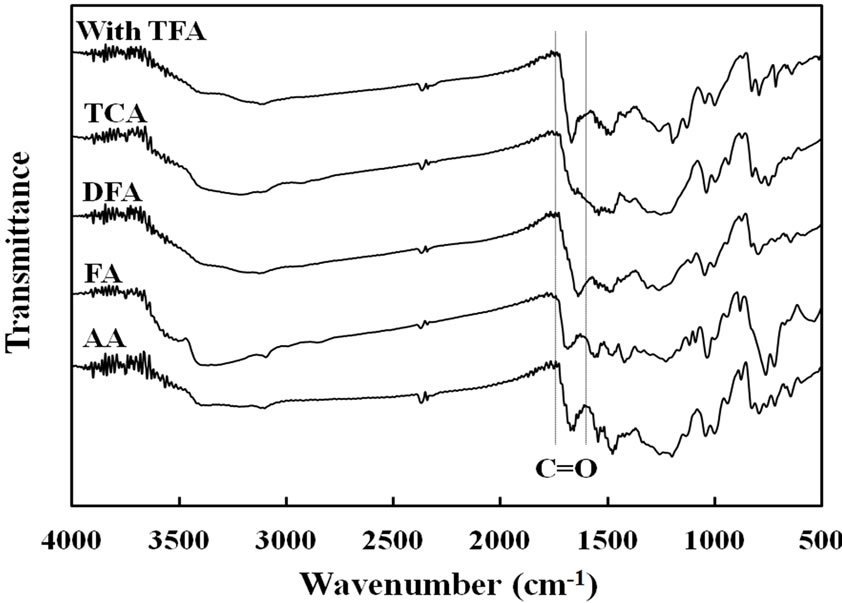 (a)
(a)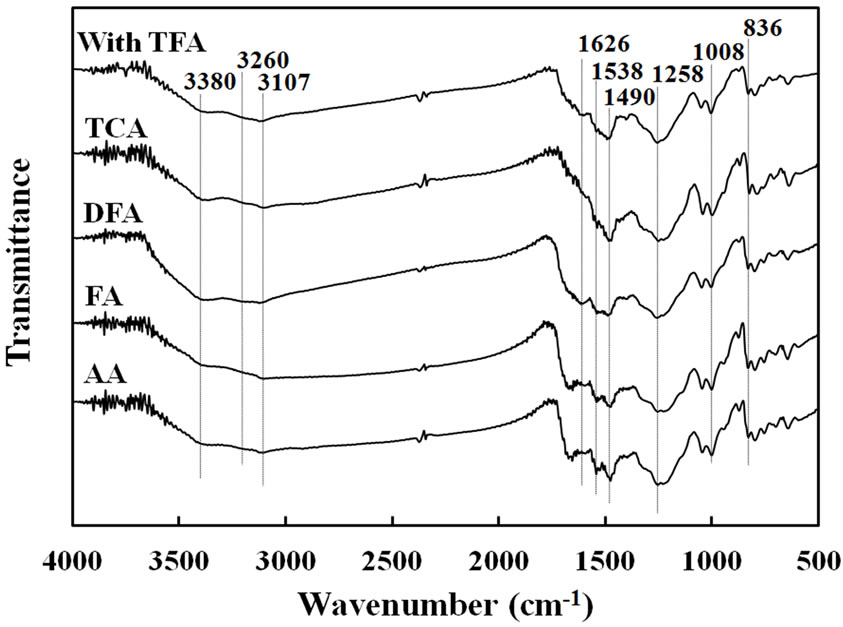 (b)
(b)
Figure 2. FT-IR spectra of Py-FPy copolymer films obtained by various carboxylic acids before removed catalysts (a) and after removed (b).
cm–1) indicated the presence of the Py and FPy segments in the each film. Also, the peak of aromatic C-H stretching at 3107 cm–1 and the peaks of C=N stretching at 1490 cm–1 was attributed to the formation of the conjugated structure in the film. The broad peak observed at 1258 cm–1 was assigned to -C=CHstretching from methine group of the copolymer. Additionally, the peak of C-H out-of-plane deformation vibration from methine group and that peak of the aromatic C-H out-of-plane deformation vibration appeared at 1008 and 836 cm–1, respecttively.
Figure 3 shows UV-Vis spectra of resultant films. It was noted that characteristic absorption band of the π-π* transition of polypyrrole at around 490 nm. Since Py and FPy had no absorption band at about 500 nm, this could be assigned with π-π* transition of the C=C double bond, which was formed by the copolymerization. When TFA, TCA, DFA, FA and AA were used, the π-π* transition band was appeared in 492, 490, 491, 457 and 455 nm, respectively. This fact indicated that the FPy group
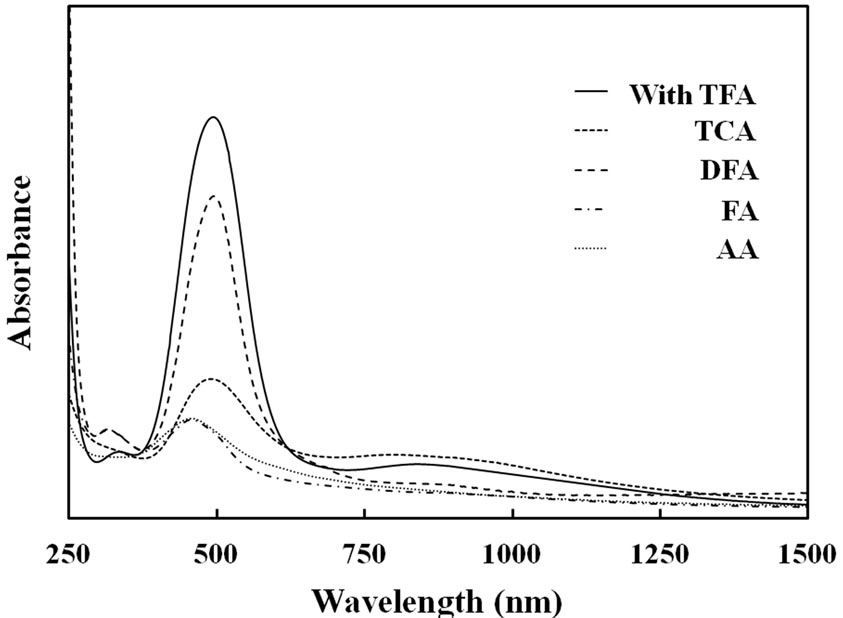
Figure 3. UV-Visible spectra of Py-FPy copolymer films obtained by various carboxylic acids.
was incorporated into the chemical structure of the conjugated polymer chains. In addition, when the TFA and TCA were used, weaker and broader band was appeared at about 900 nm. This strongly implied that the bipolaron state of polypyrrole was present in the films, when TFA and TCA were used as catalyst. This indicated that such strong acid strongly interacted with pyrrole segments to form bipolaron state.
3.2. Properties of Resultant Copolymer Films
Table 1 also shows the electrical conductivity of the resultant copolymer films. It was indicated that uses of the TFA and TCA catalyst could produce flexible films having higher electrical conductivity with 5.8 × 10–3 and 1.2 × 10–3 S/cm, respectively. In addition, the values of tensile strength strongly suggested that utilization of the TFA and TCA could obtain 81.3 and 69.6 N/mm2, respectively. This meant that the catalysts supported formation of the copolymer films showing stronger than cases of FA and AA. Therefore, TFA and TCA were selected for further investigations of catalyst feed for the resultant film strength.
Figure 4 shows copolymerization yields of Py and FPy in the presence of each catalyst amounts of 1, 3, 5, 7, 10, 13 and 15 mmol in the monomer feed. Here, monomer amounts of Py and FPy were each 3 mmol in the CHCl3 solution. As seen, the polymerization yields increased with increase of the catalyst amounts and became constant over 13 mmol. The high yield indicated that high concentration of the catalyst could consume both monomers to polymerize Py and FPy for the formation of the films. Figure 5 shows tensile strength of the copolymer films obtained in the presence of TFA and TCA with different feed amounts. As the amounts of the acid catalyst were increased, the tensile strength of the copolymer films became gradually high for the TFA and TCA. When the amounts were in the range of 10 - 15 mmol, the
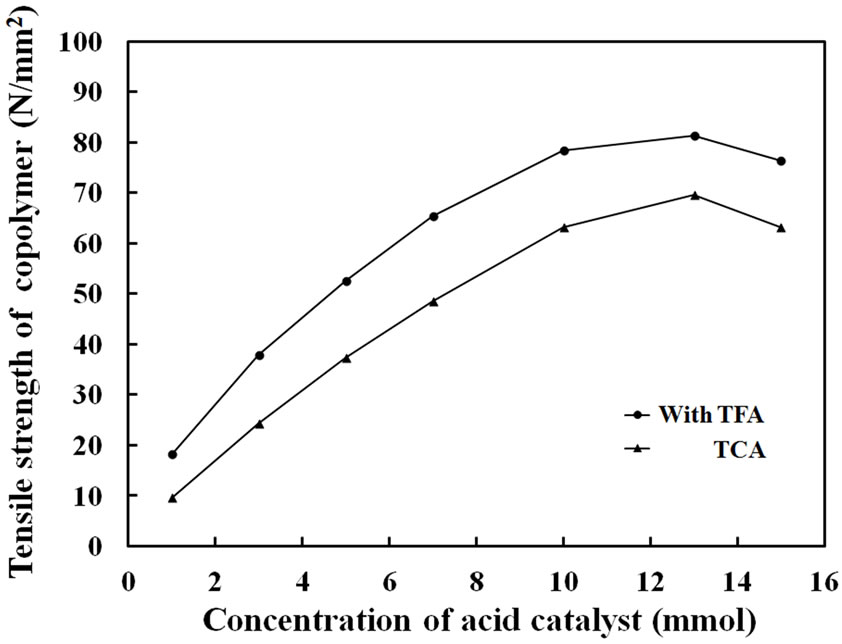
Figure 4. Polymerization yield of Py-FPy copolymer films obtained by each concentration of TFA and TCA.
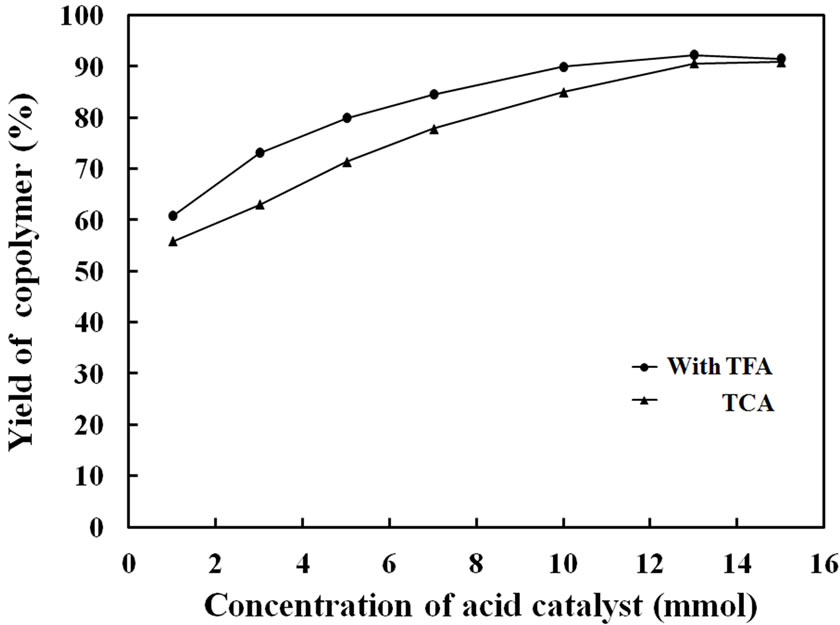
Figure 5. Tensile strength of Py-FPy copolymer films observed at different concentration of TFA and TCA.
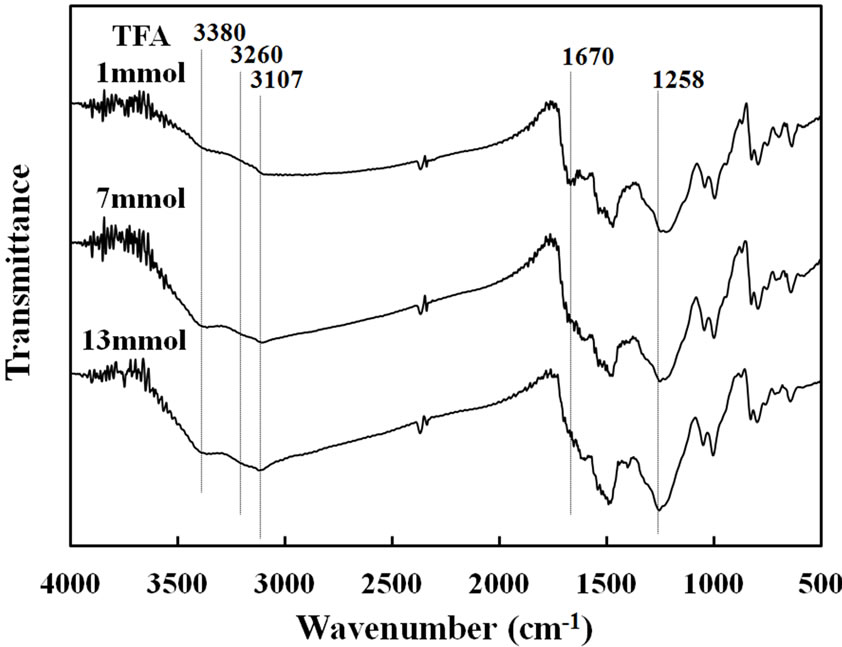
film strength was almost similar in about 80 and 70 N/mm2, for the TFA and TCA, respectively. As seen in Figure 6, FT-IR spectra of copolymer films prepared with each concentration of the catalysts had the two peaks of N-H stretching (3380 and 3260 cm–1). These peak intensities of the N-H stretching became strong with increase of the concentration of TFA. It was indicated that the acid made N-H form in the conjugated pyrrole ring by protonation of acidic catalyst. Also, the peak intensities of the aromatic C-H stretching were observed at 3107 cm–1 and -C=CHstretching at 1258 cm–1. These are due to the formation of conjugated structure in the copolymer. On the other hands, the C=O stretching at 1670 cm–1 became weaken, when the acid concentration was high. A similar tendency was seen in the case of TCA.
Figure 7 shows electrical conductivity of the copolymer films prepared with different catalyst concentration. In those cases the sample films were exposed to saturated I2 atmosphere at room temperature for 24 hr. It was particularly interesting that after I2 was doped, the values of
 (a)
(a)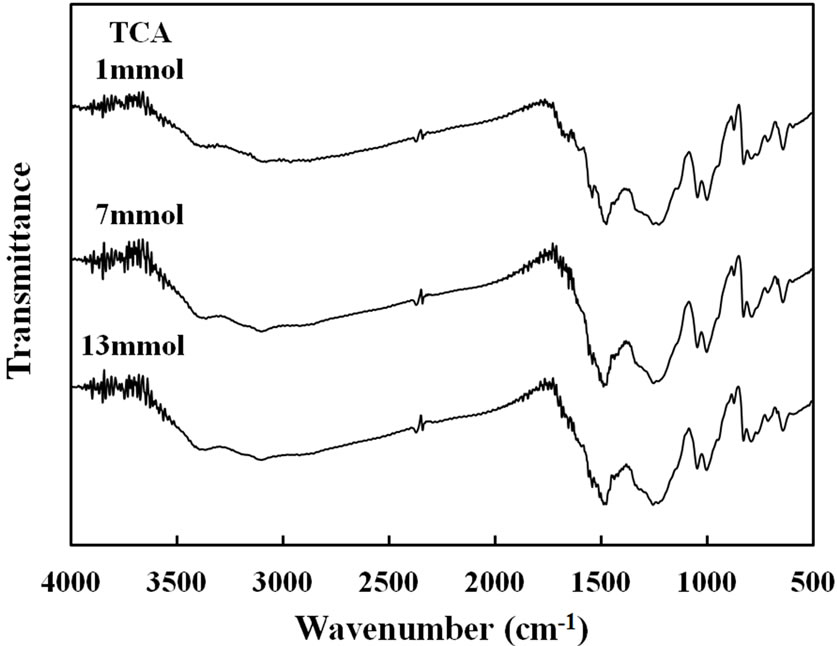 (b)
(b)
Figure 6. FT-IR spectra of Py-FPy copolymer films obtained at different concentration of TFA and TCA.
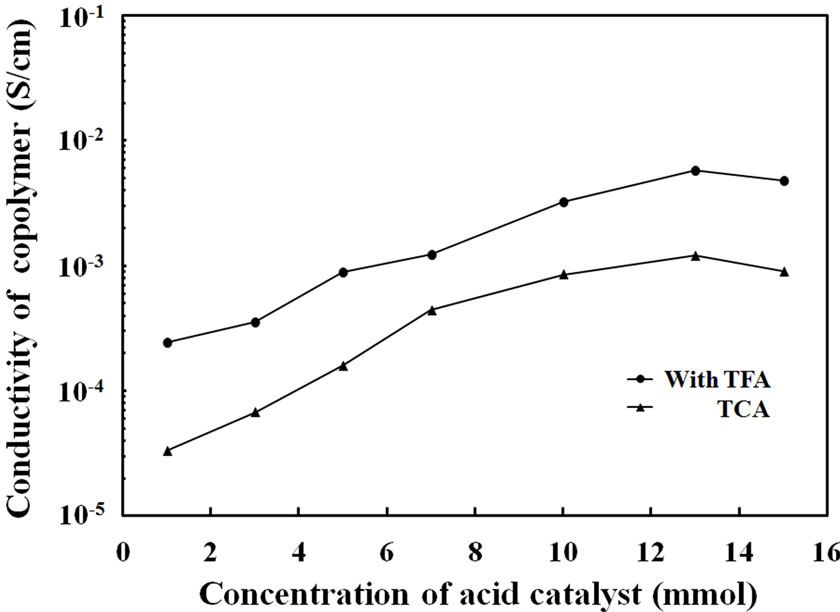
Figure 7. Electrically conductivity of Py-FPy copolymer films obtained by each concentration of TFA and TCA.
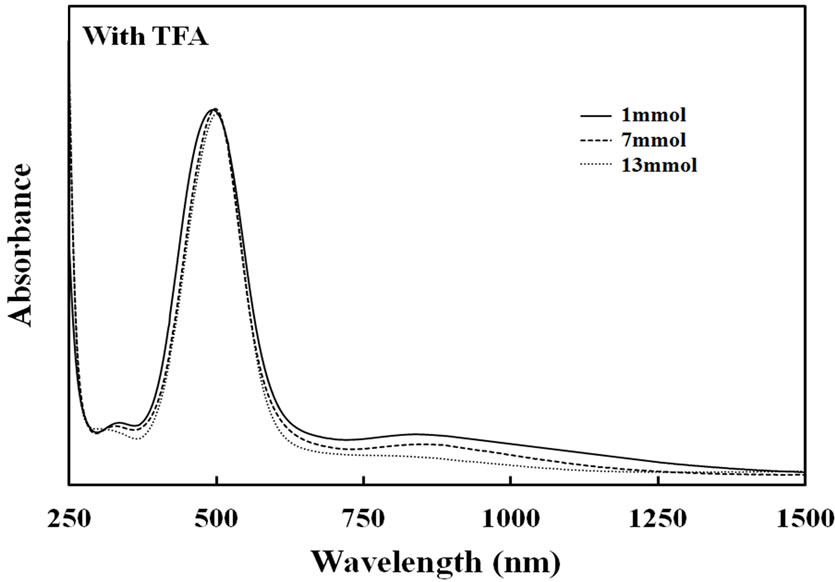
the conductivity was increased clearly with increased to show 10–3 to 10–4 S/cm and 10–3 to 10–5 S/cm for the TFA and TCA systems, respectively. Furthermore, to analyze the resultant films, UV-Vis spectra of thin films prepared by copolymerization of each catalyst concentration were measured. Figure 8 shows absorption spectra of the copolymer film prepared with TFA and TCA. The absorption band of the π-π* transition was observed at 492 nm for the copolymer films of the TFA system. It was obviously observed that when the amounts of the TFA catalyst were increased, the appearance of the broadening absorption of the bipolaron state between 700 - 1050 nm was observed. The tendency was similarly seen in the case of the TCA system. This indicated that higher acidic concentration could change the chemical structure of conjugated polymer chains. This caused contribution of higher conductivity of the films.
4. Conclusion
New conductive films of Py and FPy were reported in the present work. The effect of acidic catalysis on the formation
 (a)
(a)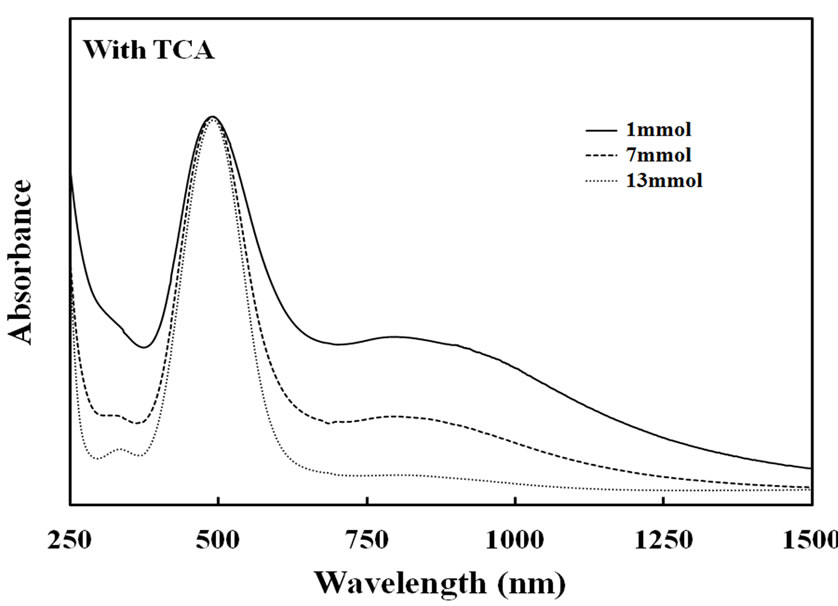 (b)
(b)
Figure 8. UV-Visible spectra of Py-FPy copolymer films obtained by each concentration of TFA and TCA.
of the copolymer films was studied for TFA, TCA, DFA, FA and AA. It was observed that the copolymer films prepared with TFA and TCA showed excellent tensile strength relative to that of AA and FA, indicating useful film formation. The electrical conductivity for the I2 doped was observed in the range of 10–4 to 10–3 S/cm and the increase of the acid concentration increased the conductivity. The results of FT-IR and UV-Visible spectra proved that formation of C=C conjugation including methine group from FPy in the copolymer films.
REFERENCES
- G. Tourillion and F. Garnier, “New Electrochemically Generated Organic Conducting Polymers,” Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, Vol. 135, No. 1, 1982, pp. 173-178. doi:10.1016/0022-0728(82)90015-8
- J. Joo, J. K. Lee, S. Y. Lee, K. S. Jang, E. J. Oh and A. J. Epstein, “Physical Characterization of Electrochemically and Chemically Synthesized Polypyrroles,” Macromolecules, Vol. 33. No. 14, 2000, pp. 5131-5136. doi:10.1021/ma991418o
- H. C. Kang and K. E. Geckeler, “Enhanced Electrical Conductivity of Polypyrrole Prepared by Chemical Oxidative Polymerization: Effect of the Preparation Technique and Polymer Additive,” Polymer, Vol. 41. No. 18, 2000, pp. 6931-6934. doi:10.1016/S0032-3861(00)00116-6
- L. X. Xia, X. G. Li and Y L. Yang, “Preparation, Properties and Applications of Polypyrroles,” Reactive and Functional Polymers, Vol. 47, No. 2, 2001, pp. 125-139. doi:10.1016/S1381-5148(00)00079-1
- A. J. F. Romero, J. J. L. Cascales and T. F. Otero, “Perchlorate Interchange during the Redox Process of PPy/ PVS Films in an Acetonitrile Medium. A Voltammetric and EDX Study,” The Journal of Physical Chemistry B, Vol. 109. No. 2, 2005, pp. 907-914. doi:10.1021/jp046601k
- P. M. Carrasco, H. J. Grande, M. Cortazar, J. M. Alberdi, J. Areizaga and J. A. Pomposa, “Structure-Conductivity Relationships in Chemical Polypyrroles of Low, Medium and High Conductivity,” Synthetic Metals, Vol. 156. No. 5-6, 2006, pp. 420-425. doi:10.1016/j.synthmet.2006.01.005
- C. Yang and P. Liu, “Water-Dispersed Conductive Polypyrroles Doped with Lignosulfonate and the Weak Temperature Dependence of Electrical Conductivity,” Industrial & Engineering Chemistry Research, Vol. 48. No. 21, 2009, pp. 9498-9503. doi:10.1021/ie900189j
- M. A. Chougule, S. G. Pawar, P. R. Godse, R. N. Mulik, Shashwati Sen and V. B. Pati, “Synthesis and Characterization of Polypyrrole (PPy) Thin Films,” Soft Nanoscience Letters, Vol. 1. No. 1, 2011, pp. 6-10. doi:10.4236/snl.2011.11002
- M. Omastová, M. Trchová, J. Kovářová and J. Stejskal, “Synthesis and Structural Study of Polypyrroles Prepared in the Presence of Surfactants,” Synthetic Metals, Vol. 138. No. 3, 2003, pp. 447-455. doi:10.1016/S0379-6779(02)00498-8
- K. S. Jang, H. Lee and B. Moon, “Synthesis and Characterization of Water Soluble Polypyrrole Doped with Functional Dopants,” Synthetic Metals, Vol. 143. No. 3, 2004, pp. 289-294. doi:10.1016/j.synthmet.2003.12.013
- H. D. Tran, K. Shin, w. G. Hong, J. M. D’Arcy, R. W. Kojima, B. H. Weiller and R. B. Kaner, “A TemplateFree Route to Polypyrrole Nanofibers,” Macromolecular Rapid Communications, Vol. 28. No. 24, 2007, pp. 2289- 2293. doi:10.1002/marc.200700581
- A. F. Diaz, K. K. Kanazawa and G. P. Gardini, “Electrochemical Polymerization of Pyrrole,” Journal of the Chemical Society, Chemical Communications, No. 14, 1979, pp. 635-636. doi:10.1039/C39790000635
- D. A. Kaplin and S. Qutubuddin, “Electrochemically Synthesized Polypyrrole Films: Effects of Polymerization Potential and Electrolyte Type,” Polymer, Vol. 36. No. 6, 1995, pp. 1275-1286. doi:10.1016/0032-3861(95)93931-B
- S. M. Sayyah, S. S. Abd El-Rehim and M. M. El-Deeb, “Electropolymerization of Pyrrole and Characterization of the Obtained Polymer Films,” Journal of Applied Polymer Science, Vol. 90. No. 7, 2003, pp. 1783-1792. doi:10.1002/app.12793
- R. E. Myers, “Chemical Oxidative Polymerization as a Synthetic Route to Electrically Conducting Polypyrroles,” Journal of Electronic Materials, Vol. 15. No. 2, 1986, pp. 61-69. doi:10.1007/BF02649904
- K. Ishizu, H. Tanaka and R. Saito, “Microsphere Synthesis of Polypyrrole by Oxidation Polymerization,” Polymer, Vol. 37. No. 5, 1996, pp. 863-867. doi:10.1016/0032-3861(96)87266-1
- Y. Kudoh, “Properties of Polypyrrole Prepared by Chemical Polymerization Using Aqueous Solution Containing Fe2(SO4)3 and Anionic Surfactant,” Synthetic Metals, Vol. 79. No. 1, 1996, pp. 17-22. doi:10.1016/0379-6779(96)80124-X
- M. Salmon, K. K. Kanazawa, A. F. Diaz and M. Krounbi, “A Chemical Route to Pyrrole Polymer Films,” Journal of Polymer Science: Polymer Letters Edition, Vol. 20. No. 3, 1982, pp. 187-193. doi:10.1002/pol.1982.130200308
- B. Sari and M. Talu, “Electrochemical Copolymerization of Pyrrole and Aniline,” Synthetic Metals, Vol. 94. No. 2, 1998, pp. 221-227. doi:10.1016/S0379-6779(98)00010-1
- F. Fusalba and D. Bélanger, “Electropolymerization of Polypyrrole and Polyaniline-Polypyrrole from Organic Acidic Medium,” The Journal of Physical Chemistry B, Vol. 103. No. 42, 1999, pp. 9044-9054. doi:10.1021/jp9916790
- A. Prasannan, N. Somanathan, P. D. Hong and W. T. Chuang, “Studies on Polyaniline-Polypyrrole Copolymer Micro Emulsions,” Materials Chemistry and Physics, Vol. 116, No. 2-3, 2009, pp. 406-414. doi:10.1016/j.matchemphys.2009.04.014
- M. J. Antony and M. Jayakannan, “Self-Assembled Nionic Micellar Template for Polypyrrole, Polyaniline, and Their Random Copolymer Nanomaterials,” Journal of Polymer Science Part B: Polymer Physics, Vol. 47, No. 8, 2009, pp. 830-846.doi:10.1002/polb.21689
- S. Kuwabata, S. Ito and H. Yoneyama, “Copolymerization of Pyrrole and Thiophene by Electrochemical Oxidation and Electrochemical Behavior of the Resulting Copolymers,” Journal of the Electrochemical Society, Vol. 135, No. 7, 1988, pp. 1691-1695. doi:org/10.1149/1.2096098
- X. Li, M. Lu and H. Li, “Electrochemical Copolymerization of Pyrrole and Thiphene Nanofibrils Using TemplateSynthesis Method,” Journal of Applied Polymer Science, Vol. 86, No. 10, 2002, pp. 2403-2407. doi:10.1002/app.10893
- X. B. Wan, W. Zhang, S. Jin, G. Xue, Q. D. You and B. Che, “The Electrochemical Copolymerization of Pyrrole and Furan in a Novel Binary Solvent System,” Journal of Electroanalytical Chemistry, Vol. 470, No. 1, 1999, pp. 23-30. doi:10.1016/S0022-0728(99)00205-3
- M. Nishizawa, T. Sawagushi, T. Matsue and I. Uchida, “In Situ Characterization of Copolymers of Pyrrole and N-Methylpyrrole at Microarray Electrodes,” Synthetic Metals, Vol. 45. No. 2, 1991, pp. 241-248. doi:10.1016/0379-6779(91)91809-O
- Y. W. Chen-Yang, J. L. Li, T. L. Wu, W. S. Wang and T. F. Hon, “Electropolymerization and Electrochemical Properties of (N-hydroxyalkyl)Pyrrole/Pyrrole Copolymers,” Electrochimica Acta, Vol. 49, No. 12, 2004, pp. 2031- 2040. doi:10.1016/j.electacta.2003.12.033
- A. D. Adler, F. R. Longo, J. D. Finarelli, J. Goldmacher, J. Assour and L. Korsakoff, “A Simplified Synthesis for MesoTetraphenylporphine,” The Journal of Organic Chemistry, Vol. 32, No. 3, 1967, p. 476. doi:10.1021/jo01288a053

