Journal of Modern Physics
Vol. 2 No. 8 (2011) , Article ID: 6907 , 7 pages DOI:10.4236/jmp.2011.28091
Angular Distribution of Photoelectrons during Irradiation of Metal Surface by Electromagnetic Waves
Department of Physics, Samara State University, Samara, Russia
E-mail: volobuev@samaramail.ru
Received April 6, 2011; revised May 16, 2011; accepted June 3, 2011
Keywords: Photoeffect, Photoelectrons, Angular Distribution, Einstein’s Formula
ABSTRACT
Angular distribution of photoelectrons is investigated during the inner photoemissive effect for two variants: quantum of light basically reveals wave and basically corpuscular properties interacting with orbital electron. Distinction in angular distribution of photoelectrons for these variants is demonstrated. Angular distribution in the second variant is investigated for the nonrelativistic and relativistic cases.
1. Introduction
Interaction of quantums of electromagnetic radiation with substance can be investigated both from a wave position, and from a quantum position. From a wave position under action of an electromagnetic wave there are compelled fluctuations of an electronic orbit and nucleus of atoms. The energy of electromagnetic radiation going on oscillation of nucleus passes in heat. Energy of fluctuations of an electronic orbit causes repeated electromagnetic radiation with energy, smaller, than initial radiation.
From a quantum position character of interaction is more various. Interaction without absorption of quantums is possible: resonant absorption, coherent dispersion. The part of quantums is completely absorbed. Quantums can be absorbed without occurrence secondary electrons. Thus all energy of quantums is transferred fonons—to mechanical waves in a crystal lattice, and the impulse is transferred all crystal lattice of substance. At absorption of quantums can arise secondary electrons, for example, at an internal photoeffect [1]. Absorption of quantums with radiation of secondary quantums of smaller energy and frequency is possible, for example, at effect of Compton or at combinational dispersion. Secondary electrons at a photoeffect are used in photocells.
There is the problem of achieving of the maximum photoelectric flow during irradiation of the metal by flow of electromagnetic waves while designing of photocells. The depth of radiation penetration into metal during irradiation of its surface is defined by the Bouguer low [2]:
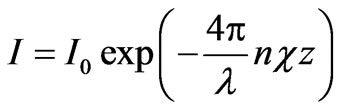 ,
,
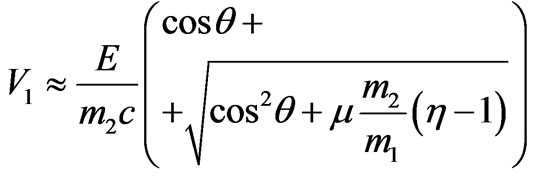
where I0—is the intensity of the incident wave, I—is the intensity on z-coordinate, directioned depthward the metal, l—is the wavelength of radiation, —is the product of refractive index by extinction coefficient.
—is the product of refractive index by extinction coefficient.
Let’s estimate the thickness of the metal at which intensity of light decreases in е = 2.718 times:
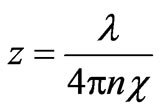
Average wavelength of a visible light for gold λ = 550 nm, 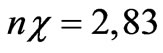 , therefore z = 15,5 nm. Considering [3] that lattice constant for gold а = 0,408 nm, it is possible to deduce that electromagnetic radiation penetrates into the metal on 40 atomic layers.
, therefore z = 15,5 nm. Considering [3] that lattice constant for gold а = 0,408 nm, it is possible to deduce that electromagnetic radiation penetrates into the metal on 40 atomic layers.
Therefore radiation interaction occurs basically of the top layers of atoms and angular distribution of electron escape from separate atoms, i.e. during the inner photoemissive effect, it will appreciably have an impact on distribution of electron escape from the metal surface.
As a result it is interesting to consider angular distribution of photoelectrons during the inner photoemissive effect.
2. Nonrelativistic Case
Although Einstein has explained the photoeffect nature in the early 20th century, various aspects of this phenomenon draw attention, till nowadays for example, the role of tunnel effect is investigated during the photoeffect [4].
In the description of angular distribution of the photoelectrons which are beaten out by photons from atoms, there are also considerable disagreements. For example it is possible to deduce that the departure of photoelectrons forward of movement of the photon and back in approach of the main order during the unitary photoeffect is absent, using the computational method of Feynman diagrams [5]. I is marked that photoelectrons don’t take off in the direction of distribution of quantum [6]. This conclusion is made on the basis of positions which in the simplified variant are represented by the following. The impulse of the taken off electron is defined basically by action produced by the electric vector of quantum of light on electron. If electron takes off in the direction of an electric vector of quantum it gets the impulse. On a plane set at an angle to a plane of polarization of quantum of light, (Figure 1) electron impulse value will be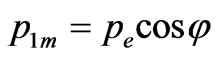 . Besides, if the electron impulse is set at an angle θ to the direction of quantum of light its value will be:
. Besides, if the electron impulse is set at an angle θ to the direction of quantum of light its value will be:
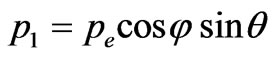 (1.1)
(1.1)
Therefore, photoelectron energy is equal to:
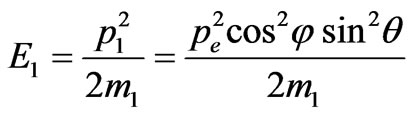 ,(1.2)
,(1.2)
where m1—is the electronic mass.
If 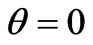 then photoelectron energy
then photoelectron energy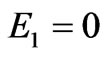 . Photoelectrons take off readies its maximum in the direction of a light vector or a polarization vector, i.e. an electric field vector of quantum of light. The same dependence is offered in the work [7]. The formula (1.2) has the simplified nature in comparison with [6,7], but convey correctly the basic dependent of distribution energy of a photoelectrons escape from the corners j and q.
. Photoelectrons take off readies its maximum in the direction of a light vector or a polarization vector, i.e. an electric field vector of quantum of light. The same dependence is offered in the work [7]. The formula (1.2) has the simplified nature in comparison with [6,7], but convey correctly the basic dependent of distribution energy of a photoelectrons escape from the corners j and q.
The lack of dependence (1.2) is that at its conclusion the law of conservation of impulse, wasn’t used and therefore there is no electron movement to the direction . Usage of the impulse conservation equation in [6,7] can’t be considered satisfactory since in the analysis made by the authors it has an auxiliary character. At the heart of the analysis [6,7] is the passage of electron from a discrete energy spectrum to a condition of a continuous spectrum under the influence of harmonious indignation, i.e., the matrix element of the perturbation operator is harmonious function of time. In other words, the emphasis is on the wave nature of the quantum cooperating with electron. Angular distribution of electron energy in the relative units, made according the formula (1.2) is shown on Figure 2, a curve 1.
. Usage of the impulse conservation equation in [6,7] can’t be considered satisfactory since in the analysis made by the authors it has an auxiliary character. At the heart of the analysis [6,7] is the passage of electron from a discrete energy spectrum to a condition of a continuous spectrum under the influence of harmonious indignation, i.e., the matrix element of the perturbation operator is harmonious function of time. In other words, the emphasis is on the wave nature of the quantum cooperating with electron. Angular distribution of electron energy in the relative units, made according the formula (1.2) is shown on Figure 2, a curve 1.
Let’s illustrate the correction to the formula (1.2) connected with presence of photon impulse, following [8]. Figure 3 demonstrates change of photoelectron impulse in the presence of a photon impulse. The conclusion made on the basis of the is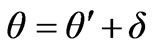 . Let’s find
. Let’s find
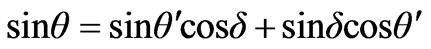 .
.
Considering that δ is too small we find
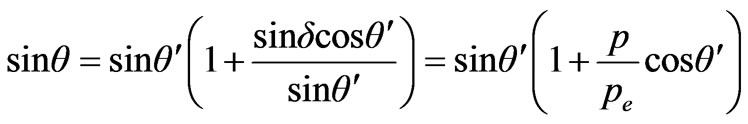 .
.
The law of sines for a triangle on Figure 3 is used. Further consideration
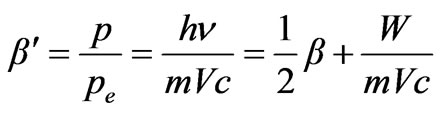 where
where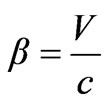 –is the relation of photoelectron speed to a speed of light in vacuum, W—is the work function of electrons from atom, we have
–is the relation of photoelectron speed to a speed of light in vacuum, W—is the work function of electrons from atom, we have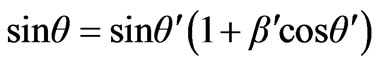 . Taking for granted that
. Taking for granted that  is small, we will transform (1.2) into
is small, we will transform (1.2) into
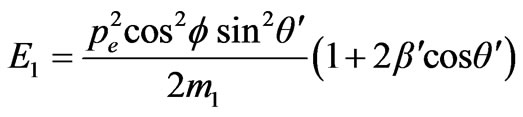
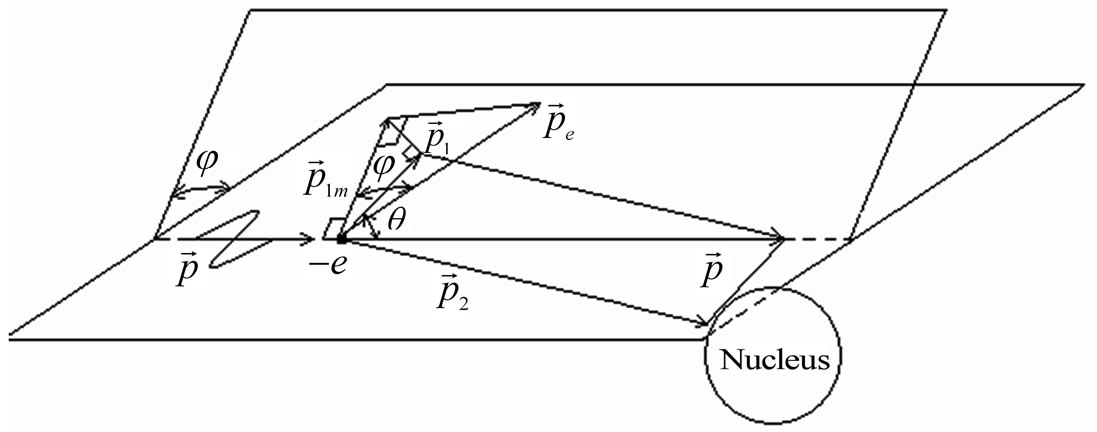
Figure 1. Direction of vectors of particle impulse at the inner photoemissive effect.
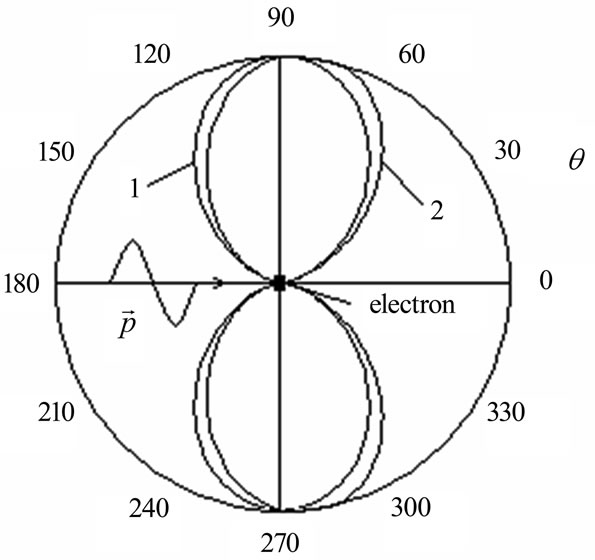
Figure 2. Angular distribution of photoelectrons during interaction of orbital electron with the electromagnetic wave.

Figure 3. The account of the impulse of quantum  using wave approach of electron interaction with the electromagnetic wave.
using wave approach of electron interaction with the electromagnetic wave.
Angular distribution of electron energy for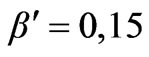 , made according to the (1.2), taking into account the correction is shown on Figure 2, a curve 2.
, made according to the (1.2), taking into account the correction is shown on Figure 2, a curve 2.
Thus scattering indicatrix of photoelectrons has received some slope forward, but to the direction of quantum impulse, i.e., at 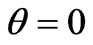 electrons don’t take off as before.
electrons don’t take off as before.
The formula (1.2) is accounted as a basis of the wave nature of light. For the proof of this position we will consider interaction of an electromagnetic wave with orbital electron. The description of orbital movement electron is done on the basis of Bohr semiclassical theory since interacting process of electron with an electromagnetic wave is investigated from the positions of classical physics, Figure 4. By the sine law from a triangle of speeds we find:
 ,(1.3)
,(1.3)
where Vt—is the speed of electron movement round the nucleus, V1—is the total speed of electron considering the influence on it of an electromagnetic wave.
By the law of cosines we have:
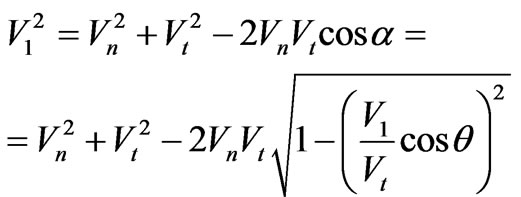 (1.4)
(1.4)

Figure 4. Attitude of components velocity of orbital electron during its interaction with the electromagnetic wave.
where Vn—is the component of the general speed of electron movement after its detachment from a nucleus which arises under the influence of electric field 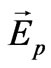 in the electromagnetic wave.
in the electromagnetic wave.
Solving (1.4) rather V1, we find:
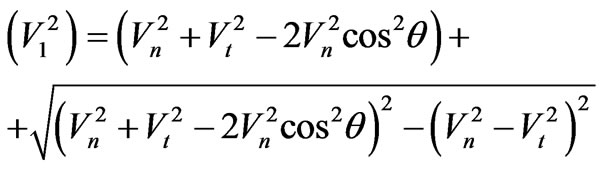 .(1.5)
.(1.5)
The condition of detachment electron from atom at any position of electron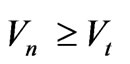 .
.
In case of equality of speeds  we have:
we have:
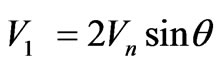 .(1.6)
.(1.6)
Distribution of speeds (1.6) corresponds to (1.2) and Figure 2, a curve 1. Thus, the parity (1.6) arises if to consider only the wave nature of the electromagnetic wave cooperating with orbital electron.
In [9] distribution of an angle of the electron escape is investigated only for a relativistic case. It is thus received that electrons are emanated mainly to a direction of photon distribution. However the done conclusion is also actually based on the formula (1.1). Therefore the drawback of the conclusion [9] is in absence in definitive formulas of angular distribution of electrons of nuclear mass m2. And after all the nuclear mass defines a share of the photon impulse which can incur a nuclear.
Let’s consider the phenomenon of the inner photoemissive effect from positions of corpuscular representation of quantum of light, Figure 1. The quantum of light by impulse 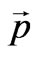 and energy Е beats out electron from atom, making A a getting out. Thus both laws of conservation of energy should be observed:
and energy Е beats out electron from atom, making A a getting out. Thus both laws of conservation of energy should be observed:
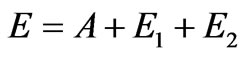 ,(1.7)
,(1.7)
where Е1—is the kinetic energy of taken off electron, Е2—is the kinetic energy of nucleus as well as the law of conservation of impulse:
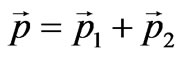 ,(1.8)
,(1.8)
where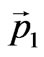 —is the impulse of taken off electron,
—is the impulse of taken off electron,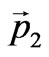 —is the impulse transferred to a nucleus.
—is the impulse transferred to a nucleus.
The formula (1.7) differs from Einstein’s standard formula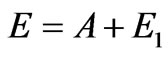 . The point is that Einstein’s formula means the absence of angular distribution of photoelectrons speed. Really, if energy of photon Е is set and work function A for the given chemical element is determined certain speed of the electron escape from atom is thereby set. It means that speeds of electrons, taking off to every possible directions are identical, and the problem of finding out their angular distribution is becoming incorrect.
. The point is that Einstein’s formula means the absence of angular distribution of photoelectrons speed. Really, if energy of photon Е is set and work function A for the given chemical element is determined certain speed of the electron escape from atom is thereby set. It means that speeds of electrons, taking off to every possible directions are identical, and the problem of finding out their angular distribution is becoming incorrect.
The value of the impulse transferred to a nucleus can be found using the formula, following (1.8):
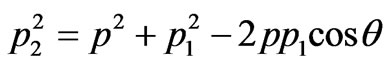 .(1.9)
.(1.9)
The system of Equations (1.7) and (1.9) to obtain a combined solution and the Equation (1.9) is convenient to express through energy. Taking into account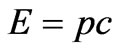 , where c—is the speed of light in vacuum,
, where c—is the speed of light in vacuum, 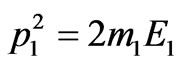 and
and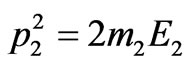 , we find:
, we find:
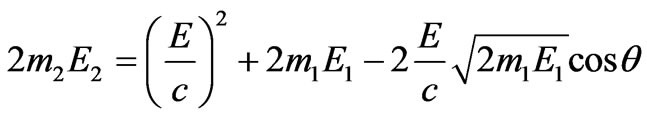 , (1.10)
, (1.10)
where m1—is the electronic mass, m2—is the nuclear mass.
Substituting in (1.10) kinetic energy of nuclear Е2 by (1.7), we have:
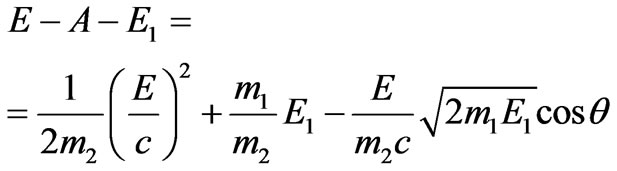 . (1.11)
. (1.11)
Let us introduce the following notation
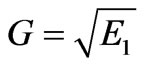 ,
, 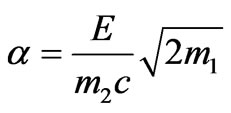 ,
, 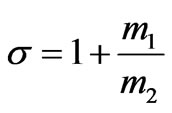 ,
, .
.
Then the Equation (1.11) will be transformed into:
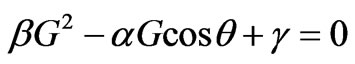
 . (1.12)
. (1.12)
Solving quadratic Equation (1.12) provided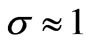 (electronic mass is much less that nuclear mass), we find:
(electronic mass is much less that nuclear mass), we find:
 . (1.13)
. (1.13)
Substituting in (1.13) accepted notation we have:
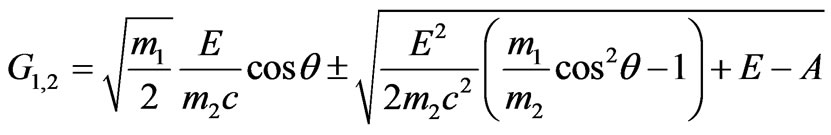 (1.14)
(1.14)
Considering that
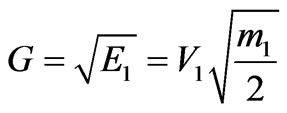
where V1—speed of photoelectrons provided
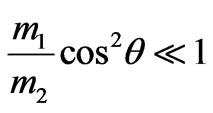 we find:
we find:
 .(1.15)
.(1.15)
Provided that nuclear mass is aiming to infinity  the formula (1.15) is transformed into Einstein's standard law for the photoeffect. Besides, this, as if it has been specified earlier, angular distribution of speed of photoelectrons disappears.
the formula (1.15) is transformed into Einstein's standard law for the photoeffect. Besides, this, as if it has been specified earlier, angular distribution of speed of photoelectrons disappears.
The condition 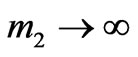 is fair in outer photoemissive effect when the photon impulse is transferred to the whole metal through single atoms. Therefore for an outer photoemissive effect, i.e. for interaction of the solid and the photon, Einstein’s formula
is fair in outer photoemissive effect when the photon impulse is transferred to the whole metal through single atoms. Therefore for an outer photoemissive effect, i.e. for interaction of the solid and the photon, Einstein’s formula 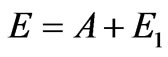 is applicable absolutely.
is applicable absolutely.
For the inner photoemissive effect in the formula (1.15) it is necessary to use effective nuclear mass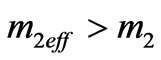 , considering attractive powers between atoms in substance.
, considering attractive powers between atoms in substance.
Transforming the formula (1.15), we get:
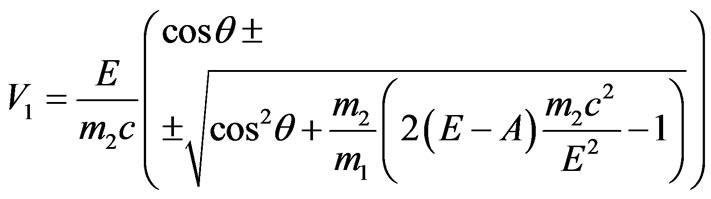 .(1.16)
.(1.16)
Let us nominate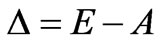 . Distribution of photoelectrons will arise at
. Distribution of photoelectrons will arise at
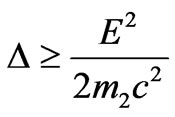 .
.
In the right part of the received inequality there is a very small value, therefore distribution of photoelectrons will arise practically at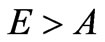 .
.
Let us nominate
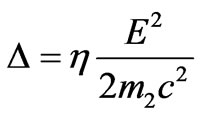 where
where 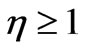 characterizes the value of exceedance of photon energy over work function in relative units. Thus the formula (1.16) takes the form:
characterizes the value of exceedance of photon energy over work function in relative units. Thus the formula (1.16) takes the form:
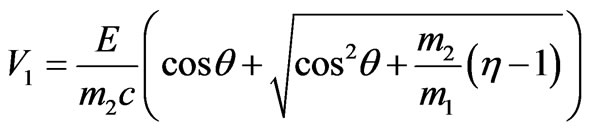 .(1.17)
.(1.17)
The analysis of the formula (1.17) shows that the root must to taking a plus since otherwise electron scattering basically goes aside, contrary to the direction of a falling photon. Angular distribution of the electron escape during the inner photoemissive effect in the relative units
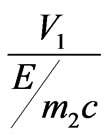
is shown in Figure 5, made according to the formula (1.17) with several values h for copper.
The Figure 5 makes it evident that speeds of photoelectrons become almost identical in all directions already at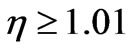 . Then Einstein’s formula
. Then Einstein’s formula 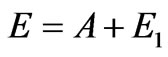 becomes fair and for the inner photoemissive effect. Considering that, for example, for copper the relation
becomes fair and for the inner photoemissive effect. Considering that, for example, for copper the relation
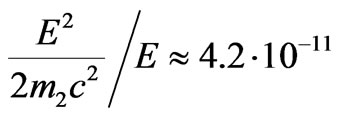
equivalent as far as the order of value is concerned
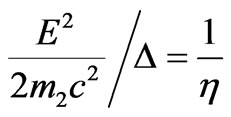
in the field of red photoelectric threshold (lr = 250 nm), it is possible to draw the conclusion that the evident difference of distribution of photoelectrons speeds from spherical, i.e. actually formula is violated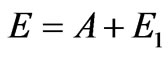 , can be observed only in very short wave part of spectrum g-radiations.
, can be observed only in very short wave part of spectrum g-radiations.
The observed data of angular distribution of the photoelectrons which have been beaten out from a monolayer of atoms of copper by covering the nickel surface are
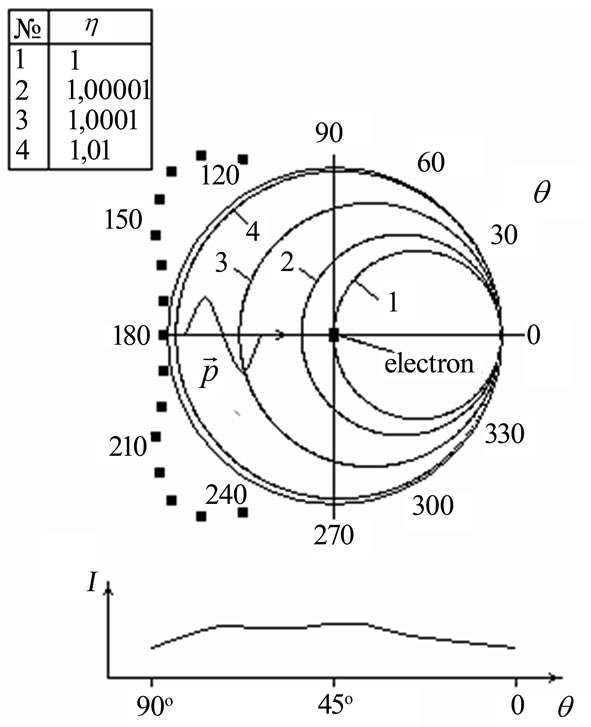
Figure 5. Angular distribution of photoelectrons during the inner photoemissive effect depending on parameter η during interaction of orbital electrons with light quantum. The results of experiments [10] are shown by black small squares. Below angular distribution of intensity I (in relative units) of photoelectrons is shown.
shown in Figure 5 by black small squares [10]. The wavelength of quanta allowed observing the photoeffect with 2р-atom shell of copper, but the photoeffect on nickel thus was absent. Experimental distribution of photoelectrons contradicts calculated distribution in Figure 2. Moreover, in distinction in Figure 2, small maxima of indicatrix of the distributions directed to an opposite direction of flight of light quanta at an angle of approximately 45˚ to the direction of light flux are observed. In [10] these maxima are explained by focusing properties of all population of atoms of the surface. The amplitude of maxima ascends with the increase of quantity of the monolayers of copper atoms on nickel.
Thus, angular distribution of photoelectrons will be absolutely various depending on whether what properties, wave or corpuscular are reveal by the light quantum in interaction with orbital electron. Only experiment can give the answer to the question what distribution it is true, Figure 2 or Figure 5. However existence of electron flux from an illuminated surface at normal light incidence [10], in the direction opposite to intensity of light, shows at the prevalence of corpuscular properties of light in its interaction with atoms.
3. Relativistic Case
Dealing with relativistic case of the inner photoemissive effect, the law of conservation of energy needs to be written down as:
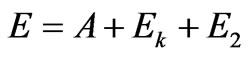 ,(2.1)
,(2.1)
where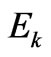 —is the kinetic energy of photoelectron.
—is the kinetic energy of photoelectron.
The law of conservation of impulse remains in the form (1.9). Using relativistic relation between the energy and the impulse for electron:
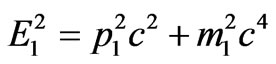 , (2.2)
, (2.2)
where Е1—is the total energy of electron, m1—is the electron rest mass, we will express the impulse of electron from (2.2) and we will substitute in (1.9). For convenience of the further transformations we will write down (2.2) into:
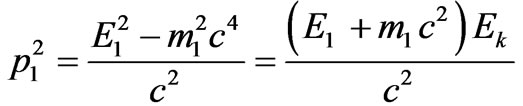 .(2.3)
.(2.3)
Formulating (2.3) the relation has been used:
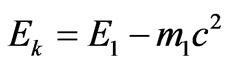 .(2.4)
.(2.4)
The Equation (1.9) will be transformed into:
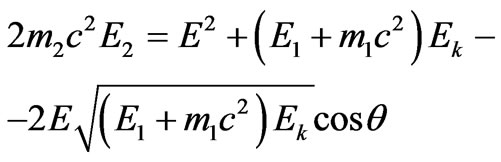 .(2.5)
.(2.5)
Because of that the nucleus that has a big mass and a relatively low speed after interaction with the photon, expression for relation of the impulse of the nucleus with its kinetic energy Е2 is used in the nonrelativistic form.
Substituting value Е2 in (2.5) from the Equation (2.1), we get:
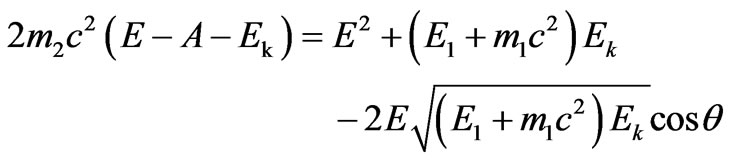 . (2.6)
. (2.6)
Let us nominate:
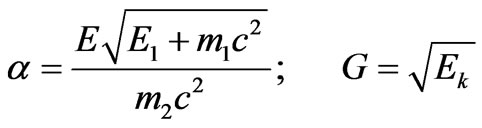 . (2.7)
. (2.7)
As a result (2.6) will be transformed into:
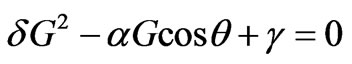
 . (2.8)
. (2.8)
The notation
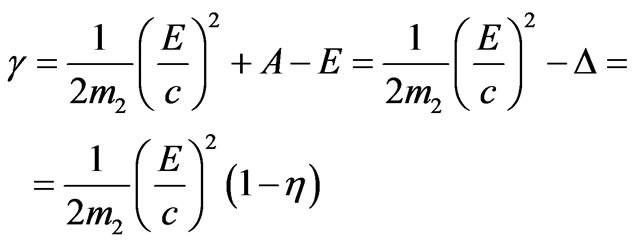
corresponds to item 1 section.
The value
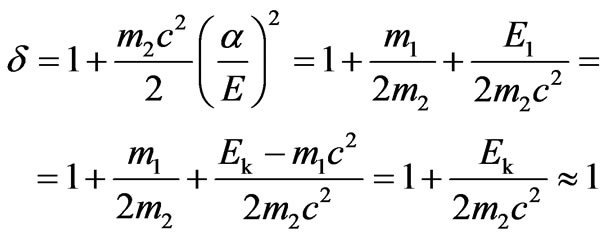 .
.
It is thus accounted for that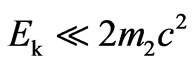 .
.
Solving the Equation (2.8), we get:
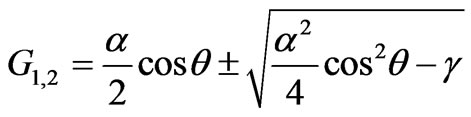 .(2.9)
.(2.9)
Substituting notations, we find:
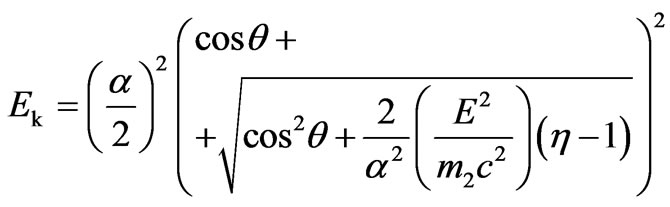 .(2.10)
.(2.10)
In contrast to the nonrelativistic case, the formula (1.17), formula (2.10) possesses in its right part value
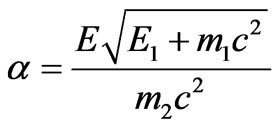
which depends on the total energy of electron Е1 the structure of which includes also kinetic energy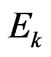 . But dependence of value a on
. But dependence of value a on 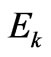 not strong as the total energy structure includes rather big rest energy of electron
not strong as the total energy structure includes rather big rest energy of electron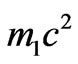 .
.
Considering that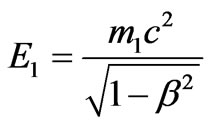 , where
, where 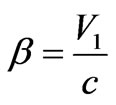 is the relative speed of the photoelectron, we find:
is the relative speed of the photoelectron, we find:
 .(2.11)
.(2.11)
Substituting the Equation (2.11) in the Equation (2.10) and considering that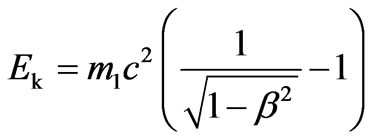 , we get:
, we get:
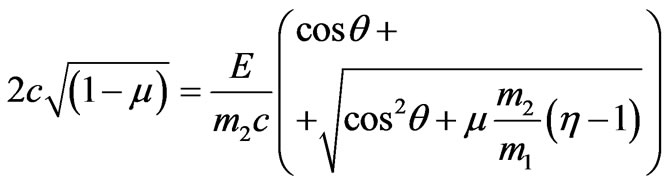 , (2.12)
, (2.12)
where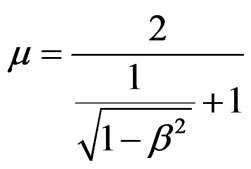 .
.
Considering that
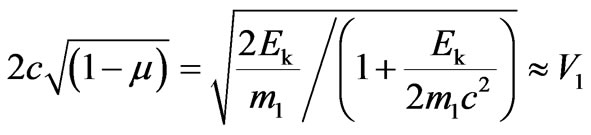 , at
, at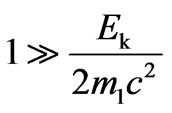 we find:
we find:
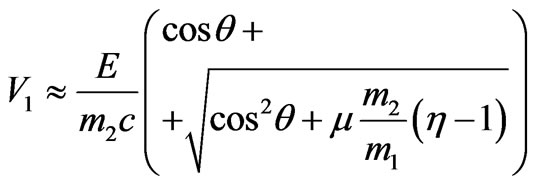 .(2.13)
.(2.13)
The formula (2.13) allows to consider relativistic effects at the photoeffect, in case of rather big speeds of photoelectrons. Thus, in contrast to (1.17), relativistic coefficient μ is introduced under the root.
The calculation of dependence m(b) shows on Figure 6, relativistic effects while calculating distribution of photoelectrons escape, can be neglected and (1.17) can
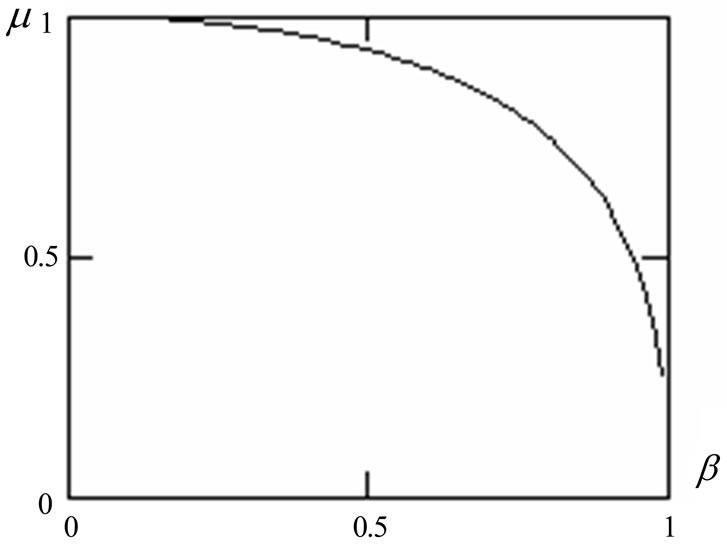
Figure 6. Dependence of relativistic coefficient b on relative speed of photoelectrons .
.
be used while the photoelectron speeds read approximately half the value of the light speed in the vacuum.
4. Conclusions
The analysis has shown that the start of electrons from atom at a photoeffect is almost spherical symmetric. It corresponds Einstein’s to formula. In Einstein’s formula there is no corner of a start of photoelectrons.The assumption, that in a direction of movement of a photon at a photoeffect of an electron does not take off unfairly. At designing photocells it is necessary to take into account presence of a stream electrons in a direction of electromagnetic radiation and in an opposite direction.
REFERENCES
- M. Ya. Amusia and V. K. Ivanov, “Intershell Interaction in Atoms,” Uzpehi Fizicheskih Nauk, Vol.152. No. 2. 1987, pp. 185-226. doi:10.3367/UFNr.0152.198706a.0185
- R. Ditchbern, “Physical Optics,” Science, Moscow, 1965, p. 413, 419, 497.
- N. Ashcroft, N. Mermin, “Solid-State Physics,” World, Moscow, 1979, Vol. 1. p. 82.
- E. L. Nolle, “Tunneling Mechanism of the Photoeffect in Metal Nanoparticles Activated by Caesium and Oxygen,” Uzpehi Fizicheskih Nauk, Vol. 177. No. 10, 2007, pp. 1133-1137.
- A. I. Mihajlov and I. A. Mihajlov, “A Double Nuclear Photoeffect in Relativistic Region, Angular and Energy Distributions of Photoelectrons,” Jounal of Experimental and Theoretical Physics, Vol. 114, No. 5, 1998, pp. 1537- 1554.
- V. G. Levich, “The Course of Theoretical Physics,” PhysMathGiz, Moscow, Vol. 2, 1962, p. 658.
- A. S. Davydov, “The Quantum Mechanics,” PhysMathGiz, Moscow, 1963, p. 366.
- M. A. Blohin, “Physics of Roentgen Rays,” State Publishing House, Moscow, 1957, p. 261.
- V. B. Berestetsky, E. M. Lifshits and L. P. Pitaevskij, “Quantum Electrodynamics,” Science, Moscow, Vol. 4, 1989, p. 249.
- D. A. Steigerwald and W. F. Jr. Egelhoff, “Finding of the Position of the Atoms in the Surface Layers of Solid,” Physical Review Letters, Vol. 60, No. 24, 1988, p. 2558. doi:10.1103/PhysRevLett.60.2558

