Advances in Bioscience and Biotechnology
Vol.4 No.7A1(2013), Article ID:34625,7 pages DOI:10.4236/abb.2013.47A1002
Cytokine profiles in sickle cell anemia: Pathways to be unraveled
![]()
1Centro de Pesquisas Gonçalo Moniz, Fundação Oswaldo Cruz, Salvador, Brasil
2Instituto de Ciência e Tecnologia do Sangue, Campinas, Brasil
3Universidade Estadual de Santa Cruz, Ilhéus, Brasil
4Faculdade de Farmacia da Universidade Federal da Bahia, Salvador, Brasil
Email: *mari@fiocruz.bahia.br
Copyright © 2013 Thassila Nogueira Pitanga et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 May 2013; revised 18 June 2013; accepted 25 June 2013
Keywords: Sickle cell anemia; Cytokines; Chemokine; Inflammation; Inflammasome
ABSTRACT
Sickle cell anemia (SCA) is a genetically inherited hemolytic disorder characterized by chronic inflammation. Cytokine expression affects the pivotal pathways that contribute to disease pathogenesis, but the mechanisms involved are not well understood. SCA is associated with a proinflammatory state, and an enhanced inflammatory response occurs during vasoocclusive crisis. The immune system thus plays an important role in this inflammatory condition, with several cell types secreting pro-inflammatory cytokines that contribute to the occurrence of common cyclical events in SCA patients, such as hemolysis, vascular occlusion and inflammation. Studies of these cytokines and chemokines in SCA patients have clarified the mechanisms that underlie this disease and highlighted the need for a better understanding of cytokine participation in SCA pathophysiology.
1. INTRODUCTION
Sickle cell anemia (SCA) is an inherited disorder characterized by homozygosis for the mutation that causes hemoglobin S (HbS) production. This point mutation (GAG > GTG) occurs in the sixth codon of the beta globin gene (HBB) and causes valine to replace glutamic acid in the sixth amino acid of the beta (β) globin chain of the hemoglobin molecule. SCA patients have a heterogeneous clinical outcome characterized by painful vaso-occlusive crises, stroke, priapism, pulmonary hypertension, acute chest syndrome (ACS) and chronic organ injuries. As a result of this mutation, deoxygenated hemoglobin molecules undergo a polymerization process that is considered the primary event leading to the pathogenesis of SCA [1].
Sickled red blood cells, as well as leukocytes, platelets and the vascular endothelium, are elements that obstruct vessels and trigger vaso-occlusive crises. The hemolysis that occurs in SCA can be both extravascular and intravascular. Intravascular hemolysis occurs when red blood cells (RBCs) rupture and release free hemoglobin into the plasma. Free hemoglobin has inflammatory and oxidant effects that lead to endothelium dysfunction. Other hemolysis products, including heme, reactive oxygen species (ROS) and reactive nitrogen species, are also released into the bloodstream, where they cause increased oxidative stress and decreased plasma levels of the vasodilator nitric oxide (NO) [2]. Increased ROS and RNS levels and decreased NO levels contribute to the activation of RBCs, leukocytes, platelets and endothelial cells. This activation leads to increased production of proinflammatory and anti-inflammatory cytokines, which gives SCA the characteristics of a chronic inflammatory disease [1,3] (Figure 1).
Several cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), are associated with the activation of leukocytes, particularly monocytes and neutrophils, in SCA. Several other cytokines are also involved in the chronic inflammatory state that is present in SCA. The activation of cells and the release of cytokines stimulate the NF-κB transcription factor pathway, which regulates the production of interleukin-4 (IL-4), interleukin-6 (IL-6) and interleukin-8 (IL-8). IL-6 and
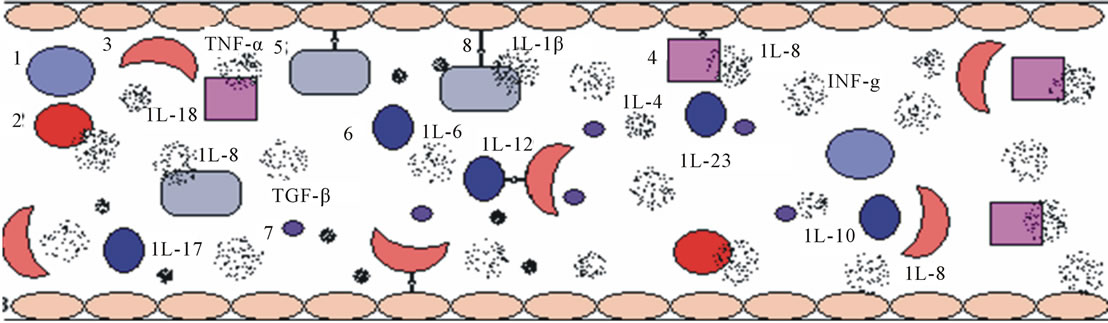
Figure 1. Representation of the vascular inflammatory environment, including the relevant cell types, corpuscles and cytokines. 1 = reticulocytes; 2 = red blood cells; 3 = sickled red blood cells; 4 = neutrophils; 5 = monocytes; 6 = lymphocytes; 7 = platelets and 8 = endothelial cells.
IL-8 production are also enhanced by the STAT3 intracellular pathway and proinflammatory activities [4]. Recently, the involvement of several other cytokines, such as IL-18, IL-17, IL-23, IL-12 and IL10, in inflammatory responses in SCA patients has been described. Because of the extensive participation of cytokines in the inflammatory processes involved in SCA pathology, this review of the cytokines and signaling pathways involved in SCA will contribute to an improved understanding of SCA pathology, especially the pathology of inflammatory vasoocclusive events.
2. INFLAMMASOME, IL-1β AND IL-18
The junction of many inflammatory molecules is the basis of the inflammasome [5]. This complex forms in response to danger signals [6], and the efficiency of the immune response depends not only on the presence of foreign antigens but also on the release of signaling molecules by stressed or damaged tissues [7]. In SCA, danger signals can be generated by hypoxia and metabolic acidosis, which lead to cell necrosis, hypokalemia and the formation of uric acid and ADP [8].
The imbalance or disruption of cellular integrity can trigger the formation of an inflammasome complex [5]. Inflammasomes activate a class of caspases known as inflammatory caspases [9], whose protease activity promotes the conversion of IL-1 beta (IL-1β) and IL-18 to their active forms [10]. Pro-inflammatory cytokines influence the clinical profile of SCA patients by increasing the adhesion of RBCs and leukocytes to the endothelium. These adhered cells trigger a cycle of increasing aggregation of sickled RBCs, platelets and neutrophils, which causes microvascular occlusion [11]. Qari et al. (2012) observed higher plasma concentrations of IL-1β in SCA patients during both the steady state and painful crises than in control subjects. However, higher levels were observed during the steady state than during painful crises. In 2011, Asare et al. demonstrated that plasma IL-1β levels are a good predictor of stroke outcome. In addition, high plasma IL-1β levels were associated with protection against stroke development in juvenile SCA patients with altered transcranial Doppler studies [12]. Other studies highlighted the need for an IL-1β-targeted therapeutic approach for the treatment of SCA in patients with a severe clinical profile [13].
Our team was the first to demonstrate that the inflammasome complex plays a role in SCA [14]. We observed increased serum IL-18 levels in SCA patients. Levels of this cytokine were positively correlated with classic danger signals, including uric acid and the hemolysis marker lactic dehydrogenase (LDH), which is indirectly related to oxidative stress. In SCA patients, intravascular hemolysis increases ROS production, which is a highly conserved signal involved in damage and stress sensing. The release of ROS promotes vascular occlusion by 2 mechanisms: classical endothelial activation and ROS-mediated activation of the inflammasome via membrane receptors like NALP3 [15]. In addition, SCA patients with the highest serum concentrations of uric acid displayed increased serum concentrations of IL-18, which strongly suggests that the inflammasome plays a role in SCA. These studies demonstrate that IL-1β and IL-18 are important players in vascular modulation and clinical pathology in SCA patients, but further analysis is needed to understand the interaction of these cytokines with other cytokines, oxidative molecules, classic prognosis markers and epigenetic factors.
3. IL-6 AND IFN-γ
Sickle cell anemia is characterized by painful vasoocclusive crises (VOC), chronic inflammation and recurrent infections. In the early stages of an infection, CD4+ T cells differentiate into two main classes of effectors: TH1 cells induce cytotoxic CD8+ T cells and the inflammatory response, resulting in the production of cytokines, such as interleukin-12 (IL-12), interleukin-2 (IL-2), interferon gamma (IFN-γ) and TNF-γ; and TH2 cells produce cytokines such as IL-4, IL-5, IL-6 and IL-10, which are important in the production of antibodies. TH1 and TH2 cells play distinct roles in physiological and pathological conditions [16-18].
The interleukin-6 cytokine family promotes a variety of cellular functions, including differentiation, maturation, proliferation and survival. These cytokines are defined by their common usage of the widely expressed signal-transducing b subunit of the transmembrane receptor glycoprotein 130 (gp 130), which is a member of the class of type I cytokine receptors [19,20].
IFN-γ is a glycoprotein produced by CD4+ and CD8+ T cells after activation by natural killer (NK) cells. The immunoregulatory functions of IFN-γ are diverse and include the activation of mononuclear phagocytes, the upregulation of class I molecules of the major histocompatibility complex (MHC-I), the stimulation of NK cell cytolytic activity and the activation of neutrophils [21].
In a study performed in Oman, Pathare et al. (2004) demonstrated that the mean serum level of IFN-γ was higher in SCA patients than in control subjects. This difference was significant in patients during the steady state but not during crises [11]. In this study, the mean serum concentration of IL-6 was higher in SCA patients than in normal controls, and there was also a significant increase in IL-6 levels in crisis patients when compared to steady-state patients. Hibbert et al. (2005) showed that the IL-6 levels were significantly higher in the SCA group than in healthy subjects [22]. Veiga et al. (2013) used microarrays to investigate cytokine levels in SCA patients with periodontal inflammation and found that the SCA group displayed significantly higher levels of various cytokines (p < 0.05), including IFN-γ, than the control group. There was also elevated production of IL-6 in these patients than in control patients, but this difference did not reach statistical significance [23].
SCA patients with a history of chronic transfusion therapy were recruited from the hematology clinic at the Children’s Hospital and Research Center Oakland (CHRCO) at the time of a clinically indicated liver biopsy for the evaluation of iron overload. Inflammatory cytokine levels were higher in SCA patients than in control subjects. Although the observed differences in IFN-γ levels were not statistically significant, IL-6 levels were significantly higher in SCA patients than controls [24]. Plasma IL-6 levels were significantly elevated in patients during both painful crises and the steady state when compared to the age-matched control group. Surprisingly, IL-6 levels were significantly higher during the steady state than during painful crises. Plasma levels of IFN-γ showed a slight elevation during painful crises when compared to the steady state, but this difference was not statistically significant when compared to the healthy, age-matched control group [25].
The reported data concerning IL-6 and IFN-γ levels in SCA patients are inconsistent. Some investigators report elevated plasma levels of these proinflammatory cytokines, supporting a role for cytokine driven inflammation. Other investigators report normal or reduced levels of the same proinflammatory cytokines. These conflicting reports highlight the need for further studies regarding the role of these cytokines in SCA.
4. IL-8 AND TNF-α
Interleukin-8 is a pro-inflammatory member of the CXC chemokine family and is involved in both endothelial cell proliferation and angiogenesis [26]. This chemokine is produced by several types of cells, such as neutrophils, endothelial cells, macrophages and fibroblasts [27], and has a number of effects including re-arrangement of the cytoskeleton, changes in intracellular Ca++ levels, activation of integrins and promotion of protein-granule exocytosis and the respiratory burst [28]. The IL-8 receptors CXCR1 and CXCR2, which are expressed mainly by neutrophils, enhance neutrophil recruitment and promote defense against bacterial pathogens [26,29]. The proinflammatory cytokine TNF-α is produced mainly by monocytes/macrophages, but other cells, such as T-cells, smooth muscle cells, adipocytes and fibroblasts, can also produce this cytokine. TNF-α is named for its ability to stimulate tumor necrosis and regression in vivo [27]. Biological responses to TNF-α are mediated by two groups of receptors, TNFR55 and TNFR 75, which are present on the membrane of several types of cells, excluding RBCs [30].
IL-8 induces leukocyte chemotaxis, and TNF-α stimulates increased expression of adhesion molecules on endothelial cells, contributing to leukocyte adhesion. Both cytokines stimulate RBC adhesion to endothelial cells [11]. In addition, these cytokines induce neutrophil degranulation, capillary leak and vasoconstriction [11], and TNF-α inhibits cell proliferation and induces cell death [27].
IL-8 and TNF-α contribute to the vascular inflammatory state that is present in various inflammatory diseases, including SCA. As mentioned previously, these cytokines induce increased adhesion of RBC and leukocytes to the vascular endothelium, and this adhesion can cause vaso-occlusion and local hypoxia [11]. Several studies have shown that patients display higher levels of a number of cytokines, especially IL-8, during VOC than during the steady state [11,31-34]. In contrast to these findings, other studies showed that the levels of IL-8 were similar between patients in crises and patients in steady-state [35]. In addition, although SCA patients in VOC had higher levels of TNF-α than patients in the steady-state group, this difference was not statistically significant [36]. Hypoxia is a common consequence of vaso-occlusion. One study reported that hypoxia induces IL-8 expression [37], contributing to increased plasma IL-8 levels and vascular inflammation.
SCA patients who are treated with hydroxyurea (HU) display an altered profile of IL-8 and TNF-α levels. Treatment with HU is associated with increased serum levels of circulating IL-8 [31]. Other studies reported conflicting results, demonstrating that patients undergoing HU therapy displayed significantly lower plasma levels of IL-8 [34,38]. Other studies showed that HU did not significantly increase [36] or decrease [34] plasma TNF-α concentrations.
In addition, IL-8 levels are positively correlated with HbS and negatively correlated with F hemoglobin (HbF) [32]. Tavakkoli et al. (2004) showed that an increase in HbF concentration was not associated with a significant change in plasma TNF-α levels [36].
These results demonstrate that increased levels of circulating IL-8 and TNF-α are associated with increased hemolysis, vascular occlusion and inflammation. However, further studies are needed to understand the interrelationships of IL-8 and TNF-α with other proinflammatory cytokines in SCA patients.
5. IL-17 AND TGF-β
Ischemic events that result from the occlusion of major and minor vessels involve interactions between RBCs, leukocytes and the endothelium, and these interactions are regulated by cytokines secreted by leukocytes, adhesion molecules and, consequently, the immune response, which is involved in the initiation and development of crises in SCA [39]. The importance of cytokine IL-17 is well established [40], but recent data has demonstrated that IL-17 is produced by a subset of T cells, named Th17 cells, that is distinct from TH1 and TH2 cells [41]. IL-17 plays an important role in allergic responses and promotes inflammation by inducing the production of inflammatory cytokines and chemokines, the recruitment of neutrophils, the production of antibodies and the activation of T cells [42]. The differentiation of TH17 cells from naïve cells requires transforming growth factor-beta (TGF-β) and the subsequent expansion of the TH17 lineage requires IL-23 [43].
The roles of IL-17 and TGF-β in SCA are not well understood. Keikhaei et al. (2013) recently observed higher levels of IL-17 and TGF-β in steady-state patients than in healthy controls, but there was no difference between steady-state and crisis patients. The authors also demonstrated that HU-treated patients displayed lower levels of IL-17 than patients who did not receive this treatment [31]. Our team demonstrated a positive correlation between levels of free serum arginase and the levels of TGF-β in HbSS patients [44]. This finding raises the possibility that TGF-β induces upregulation of the arginase pathway and downregulation of the NO pathway. This switch in arginine metabolism could play a role in vascular activation and the increased serum arginase levels that lead to chronic hemolysis in HbSS individuals.
The role of IL-17 and TGF-β cytokines in HbSS pathophysiology remains unclear. Recent studies have demonstrated interesting changes in serum levels of these cytokines in SCA patients. These results warrant further investigation.
6. IL-12 AND IL-23
IL-12 and IL-23 are proinflammatory cytokines produced by macrophages and dendritic cells in response to microbial pathogens [45,46]. IL-12 regulates both innate and adaptive immunity. A major function of IL-12 is the induction of IFN-γ production by NK cells, T cells, B cells, and even antigen-presenting cells. Thus, IL-12 appears to be the main cytokine that regulates TH1 differentiation. In addition, IL-12 antagonizes TH 2 differentiation and the production of IL-4, IL-5 and IL-13 [45].
Like IL-12, IL-23 induces the production of IFN-γ by human T cells. In addition, IL-23 plays a key role in TH17 development by stabilizing both IL-17 expression and the TH17 phenotype. However, IL-23 is not a differentiation factor for TH17 cells and instead contributes to the induction of a pathogenic phenotype in TH17 cells [47]. IL-12 can also negatively affect the development, homeostasis and function of nTreg cells by limiting IL-2 expression [48].
There are few reports in the literature pertaining to the role of IL-12 in SCA. Taylor et al. (1999) [49] investigated the levels of several TH1 cytokines in both steady-state SCA patients and healthy control subjects, but detectable levels of IL-12 were not observed in either group. This result could be because the expression of significant amounts of IL-12 depends on microbial infection or altered immune responses, such as those observed in chronic immune-mediated diseases. Thus, it remains important to investigate IL-12 levels in SCA patients during crises. Hassan et al. (2009) performed a similar study of HbAS children who were infected with Plasmodium falciparum. Surprisingly, detectable levels of IL-12 were found in patients with mild malaria, but not in asymptomatic individuals. This finding could be related to the low levels of IL-10 that are typically associated with this infection, as IL-10 is a potent inhibitor of IL-12 [50].
With respect to the relationship between IL-23 and SCA, there is a report that investigated the possible association of IL-23 with arginase levels, an important molecule in the clinical outcome and in the vascular endothelium in inflammatory diseases. Hence, IL-23 was detected in steady-state patients, but it was not correlated to arginase, which was considered an interesting result [44].
7. ANTI-INFLAMMATORY CYTOKINES
IL-4 is an anti-inflammatory cytokine that participates in the regulation of the immune system at multiple levels. IL-4 promotes TH2 cell differentiation and inhibits TH1 cell differentiation. IL-4 is also a growth and survival factor that protects lymphocytes from apoptosis [51]. This cytokine plays an important role in the pathogenesis of allergic disease, particularly atopic asthma, and is essential for immune responses to parasitic infections [52]. In addition, IL-4 is affected by several alternative pathways of immune regulation, including alternative mRNA splicing. This process yields at least two functional isoforms of IL-4, full-length IL-4 and IL-4δ2. These variants have similar effects, but they can bind different types of receptors on the surface of target cells. Considering the varied functions of IL-4, abnormal regulation of this cytokine may cause immune disease [53].
The role of IL-4 in SCA is controversial. While some studies have reported that IL-4 levels increase during VOC, other studies have demonstrated that IL-4 levels are higher during the steady-state [39,54]. In 2000, an investigation of the TH2 cytokine levels in SCA patients revealed that plasma IL-4 levels were significantly higher among steady-state HbSS patients than HbAA and HbAS individuals. In addition, the ratios of plasma IL-2 to IL-4 and IFN-γ to IL-4 were significantly lower in HbSS patients than in the other groups, suggesting a possible mechanism for the predisposition of SCA patients to bacterial infections [54]. A recent study of SCA patients with asthma in Jamaica and London indicated that IL-4 levels may differ between children in developed countries and children in developing countries [55].
Although IL-10 is mainly produced by activated CD8+ cells, it can also be produced by other types of cells, such as activated TH0, TH1 and TH2 cells, B lymphocytes, mast cells and lipopolysaccharide-activated monocytes. The synthesis of IL-10 is inhibited by IL-4 and by itself [56]. IL-10 is an anti-inflammatory cytokine whose main effect is inhibition of the synthesis of various cytokines, such as TNF-α, GM-CSF, IL-1, IL-6, IL-8 and IL-12, to promote the uptake and retention of iron within monocytes and the reticuloendothelial system. IL-10 also inhibits the proliferation of TH1 cells, decreasing cytolytic function and the secretion of TH1 cytokines and facilitating the development of a TH2 response [57].
There are conflicting reports on the role of IL-10 in SCA patients. One study demonstrated that patients undergoing HU therapy had high levels of IL-10, but the mechanism was not described [34]. In 2009, a Sudanese study of children with malarial infection demonstrated that asymptomatic children with sickle cell trait had significantly lower levels of IL-10 than HbAA children with severe malaria. However, these children had higher IL-10 levels than HbAA children with mild malaria. These results suggest that IL-10 has a protective effect against the occurrence of severe malaria in patients with sickle cell trait [50]. A study of inflammation and ironoverloading observed higher levels of IL-10 and lower levels of non-transferrin bound iron in SCA patients than in thalassemia patients, confirming the contribution of this cytokine to the regulation cellular iron status [24]. Thus, abnormal production of these anti-inflammatory cytokines can affect both cell-mediated and humoral immune responses and increase the risk of morbidity in sickle cell patients.
8. CONCLUSION
SCA is an inherited disorder characterized by homozygosis for HbS, and a number of cytokines have been implicated in disease severity. Understanding the roles of different cytokines in the pathology of SCA is essential for the development of effective therapies for this disease. While some cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, have been extensively studied in the context of SCA, the role of other cytokines in SCA pathology remains less clear. This review highlights several cytokines that are likely to be important in the pathophysiology of SCA.
![]()
![]()
REFERENCES
- Steinberg, M.H. and Rodgers, G.P. (2001) Pathophysiology of sickle cell disease: Role of cellular and genetic modifiers. Seminars in Hematology, 38, 299-306. doi:10.1016/S0037-1963(01)90023-X
- Rother, R.P., Bell, L., Hillmen, P. and Gladwin, M.T. (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA, 293, 1653-1662. doi:10.1001/jama.293.13.1653
- Frenette, P.S. (2002) Sickle cell vaso-occlusion: Multistep and multicellular paradigm. Current Opinion Hematology, 9, 101-106. doi:10.1097/00062752-200203000-00003
- Levy, D.E. and Darnell Jr., J.E. (2002) Stats: Transcriptional control and biological impact. Nature Reviews. Molecular Cell Biology, 3, 651-662. doi:10.1038/nrm909
- Martinon, F., Mayor, A. and Tschopp, J. (2009) The inflammasomes: Guardians of the body. Annual Review of Immunology, 27, 229-265. doi:10.1146/annurev.immunol.021908.132715
- Matzinger, P. (1994) Tolerance, danger, and the extended family. Annual Review of Immunology, 12, 991-1045. doi:10.1146/annurev.iy.12.040194.005015
- Kono, H. and Rock, K.L. (2008) How dying cells alert the immune system to danger. Nature Reviews Immunology, 8, 279-289. doi:10.1038/nri2215
- Shi, Y., Evans, J.E. and Rock, K.L. (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature, 425, 516-521. doi:10.1038/nature01991
- Halle, A., Hornung, V., Petzold, G.C., Stewart, C.R., Monks, B.G., Reinheckel, T., Fitzgerald, K.A., Latz, E., Moore, K.J. and Golenbock, D.T. (2008) The Nalp3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology, 9, 857-865. doi:10.1038/ni.1636
- Martinon, F., Burns, K. and Tschopp, J. (2002) The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Molecular Cell, 10, 417-426. doi:10.1016/S1097-2765(02)00599-3
- Pathare, A., Al Kindi, S., Alnaqdy, A.A., Daar, S., KnoxMacaulay, H. and Dennison, D. (2004) Cytokine profile of sickle cell disease in Oman. American Journal of Hematology, 77, 323-328. doi:10.1002/ajh.20196
- Asare, K., Gee, B.E., Stiles, J.K., Wilson, N.O., Driss, A., Quarshie, A., Adams, R.J., Kutlar, A. and Hibbert, J.M. (2010) Plasma interleukin-1beta concentration is associated with stroke in sickle cell disease. Cytokine, 49, 39-44. doi:10.1016/j.cyto.2009.10.002
- Wanderer, A.A. (2009) Rationale for Il-1beta-targeted therapy to minimize hypoxic-ischemic encephalopathy. Journal of Perinatology, 29, 785-787. doi:10.1038/jp.2009.114
- Cerqueira, B.A., Boas, W.V., Zanette, A.D., Reis, M.G. and Goncalves, M.S. (2011) Increased concentrations of il-18 and uric acid in sickle cell anemia: Contribution of hemolysis, endothelial activation and the inflammasome. Cytokine, 56, 471-476. doi:10.1016/j.cyto.2011.08.013
- Dostert, C., Petrilli, V., Van Bruggen, R., Steele, C., Mossman, B.T. and Tschopp, J. (2008) Innate immune activation through nalp3 inflammasome sensing of asbestos and silica. Science, 320, 674-677. doi:10.1126/science.1156995
- Machado, P.R.L., Araújo, M.A.S., Carvalho L. and Carvalho, E. M. (2004) Mecanismos de resposta imune às infecções. Anais Brasileiro de Dermatologia, 79, 647-662. doi:10.1590/S0365-05962004000600002
- Frenette P.S. (2004) Sickle cell vaso-occlusion: Heterotypic, multicellular aggregations driven by leukocyte adhesion. Microcirculation, 11, 167-177.
- Janeway, C.A., Travers, P., Walport, M. and Shlomchik, M. (2001) Immunobiology. 5th Edition, Garland Publishing, New York, 90-100.
- Heinrich, P.C., Behrmann, I., Haan, S., Hermanns. H.M., Müller-Newen, G. and Schaper, F. (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochemistry Journal, 374, 1-20. doi:10.1042/BJ20030407
- Mansell, A. and Jenkins, B.J. (2013) Dangerous liaisons between interleukin-6 cytokine and toll-like receptor families: A potent combination in inflammation and cancer. Cytokine & Growth Factor Reviews. doi:10.1016/j.cytogfr.2013.03.007
- Abbas, A.K., Lichtman, A.H. and Pober, J.S. (2000) Section III—Effector mechanisms of the immune response. Celular and Molecular Immunology.
- Hibbert, J.M., Hsu, L.L., Bhathena, S.J., Irune, I., Sarfo, B., Creary, M.S., Gee, B.E., Mohamed, A.I., Buchanan, I.D., Al-Mahmoud, A. and Stiles, J.K. (2005) Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Experimental Biology and Medicine, 230, 68-74.
- Veiga, P.C., Schroth, R.J., Guedes, R., Freire, S.M. and Nogueira-Filho, G. (2013) Serum cytokine profile among Brazilian children of African descent with periodontal inflammation and sickle cell anaemia. Archives Oral Biology, 58, 505-510. doi:10.1016/j.archoralbio.2012.11.006
- Walter, P.B., Fung, E.B., Killilea, D.W., Jiang, Q., Hudes, M., Madden, J., Porter, J., Evans, P., Vichinsky, E. and Harmatz, P. (2006) Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. British Journal of Haematology, 135, 254- 263. doi:10.1111/j.1365-2141.2006.06277.x
- Qari, M.H., Dier, U. and Mousa, S.A. (2012) Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clinical and Applied Thrombosis Hemostasis, 18, 195-200. doi:10.1177/1076029611420992
- Li, A., Varney, M.L., Valasek, J., Godfrey, M., Dave, B.J. and Singh, R.K. (2005) Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis, 8, 63-71. doi:10.1007/s10456-005-5208-4
- Ikram, N., Hassan, K. and Tufail, S. (2004) Cytokines. International Journal of Pathology, 2, 47-58.
- Graves, D.T. and Jiang, Y. (1995) Chemokines, a family of chemotactic cytokines. Critical Reviews in Oral Biology and Medicine, 6, 109-118. doi:10.1177/10454411950060020101
- Wolf, M., Delgado, M.B., Jones, S.A., Dewald, B., ClarkLewis, I. and Baggiolini, M. (1998) Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. European Journal of Immunology, 28, 164-170. doi:10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S
- Popa, C., Netea, M.G., van Riel, P.L., van der Meer, J.W. and Stalenhoef, A.F. (2007) The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. Journal of Lipid Research, 48, 751-762. doi:10.1194/jlr.R600021-JLR200
- Keikhaei, B., Mohseni, A.R., Norouzirad, R., Alinejadi, M., Ghanbari, S., Shiravi, F. and Solgi, G. (2013) Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. European Cytokine Network.
- Cajado, C., Cerqueira, B.A., Couto, F.D., Moura-Neto, J.P., Vilas-Boas, W., Dorea, M.J., Lyra, I.M., Barbosa, C.G., Reis, M.G. and Goncalves, M.S. (2011) TNF-alpha and IL-8: Serum levels and gene polymorphisms (-308G >A and -251A>T) are associated with classical biomarkers and medical history in children with sickle cell anemia. Cytokine, 56, 312-317. doi:10.1016/j.cyto.2011.07.002
- Gonçalves, M.S., Queiroz, I.L., Cardoso, S.A., Zanetti, A., Strapazoni, A.C., Adorno, E., Albuquerque, A., Sant’Ana, A., dos Reis, M.G., Barral, A. and Barral Netto, M. (2001) Interleukin 8 as a vaso-occlusive marker in Brazilian patients with sickle cell disease. Brazilian Journal of Medical and Biology Research, 34, 1309-1313.
- Lanaro, C., Franco-Penteado, C.F., Albuqueque, D.M., Saad, S.T., Conran, N. and Costa, F.F. (2009) Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. Journal of Leukocyte Biology, 85, 235-242. doi:10.1189/jlb.0708445
- Michaels, L.A., Ohene-Frempog, K., Zhao, H. and Douglas, S.D. (1998) Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood, 92, 3148-3151.
- Tavakkoli, F., Nahavandi, M., Wyche, M.Q. and Perlin, E. (2004) Plasma levels of TNF-alpha in sickle cell patients receiving hydroxyurea. Hematology, 9, 61-64. doi:10.1080/1024533032000158869
- Kim, K.S., Rajagopal, V., Gonsalves, C., Johnson, C. and Kalra, V.K. (2006) A novel role of hypoxia-inducible factor in cobalt chlorideand hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. Journal Immunology, 177, 7211-7224.
- Saleh, A.W., Hillen, H.F. and Duits, A.J. (1999) Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematologica, 102, 31-37. doi:10.1159/000040964
- Musa, B.O., Onyemelukwe, G.C., Hambolu, J.O., Mamman, A.I. and Isa, A.H. (2010) Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clinical and Vaccine Immunology, 17, 602-608.
- Kolls J.K. and Linden, A. (2004) Interleukin-17 family members and inflammation. Immunity, 21, 467-476. doi:10.1016/j.immuni.2004.08.018
- Harrington, L.E., Hatton, R.D., Mangan, P.R., Turner, H., Murphy, T.L., Murphy, K.M. and Weaver, C.T. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology, 6, 1123-1132. doi:10.1038/ni1254
- Iwakura, Y., Nakae, S., Saijo, S. and Ishigame, H. (2008) The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunological Review, 226, 57-79.
- Stockinger, B. and Veldhoen, M. (2007) Differentiation and function of Th17 cells. Current Opinion in Immunology, 19, 281-286. doi:10.1016/j.coi.2007.04.005
- Vilas-Boas, W., Cerqueira, B.A., Zanette, A.M., Reis, M.G., Barral-Netto, M. and Goncalves M.S. (2010) Arginase levels and their association with Th17-related cytokines, soluble adhesion molecules (sICAM-1 and sVCAM-1) and hemolysis markers among steady-state sickle cell anemia patients. Annals of Hematolology, 89, 877-882. doi:10.1007/s00277-010-0954-9
- O’Shea, J.J and Paul W.E. (2002) Regulation of T(H)1 differentiation-controlling the controllers. Nature Immunology, 3, 506-508. doi:10.1038/ni0602-506
- Hunter, C.A. (2005) New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nature Reviews Immunology, 5, 521-531. doi:10.1038/nri1648
- Bettelli, E., Carrier, Y., Gao, W., Korn, T., Strom, T.B., Oukka, M., Weiner, H.L. and Kuchroo, V.K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 andregulatory T cells. Nature, 11, 235-238. doi:10.1038/nature04753
- Vignali, D.A. and Kuchroo, V.K. (2012) IL-12 family cytokines: Immunological playmakers. Nature Immunology, 13, 722-728. doi:10.1038/ni.2366
- Taylor, S.C., Shacks, S.J. and Qu, Z. (1999) In vivo production of type 1 cytokines in healthy sickle cell disease patients. Journal of the National Medical Association, 91, 619-624.
- Hassan, D.A., Marques, C., Santos-Gomes, G.M., do Rosario, V.E., Mohamed H.S., Elhussein, A.M., Ibrahim, M.E. and Abdulhadi, N.H. (2009) Differential expression of cytokine genes among sickle-cell-trait (HbAS) and normal (HbAA) children infected with Plasmodium falciparum. Annals of Tropical Medicine and Parasitology, 103, 283-295. doi:10.1179/136485909X435049
- Taylor, SC., Shacks, S.J., Qu, Z. and Wiley, P. (1997) Type 2 cytokine serum levels in healthy sickle cell disease patients. Journal of the National Medical Association, 89, 753-757.
- Oh, C.K., Geba, G.P. and Molfino, N. (2010) Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. European Respiratory Review, 115, 46-54. doi:10.1183/09059180.00007609
- Luzina, I.G., Keegan, A.D., Heller, N.M., Rook, G.A., Shea-Donohue, T. and Atamas, S.P. (2012) Regulation of inflammation by interleukin-4: A review of “alternatives”. Journal of Leukocyte Biology, 92, 753-764. doi:10.1189/jlb.0412214
- Raghupathy, R., Haider, M.Z., Azizieh, F., Abdelsalam, R., D’Souza, T.M. and Adekile, A.D. (2000) Th1 and Th2 cytokine profiles in sickle cell disease. Acta Haematologica, 103, 197-202. doi:10.1159/000041049
- Knight-Madden, J., Vergani, D., Patey, R., Sylvester, K., Hussain, M.J., Forrester, T. and Greenough, A. (2012) Cytokine levels and profiles in children related to sickle cell disease and asthma status. Journal of Interferon & Cytokine Research, 32, 1-5. doi:10.1089/jir.2011.0030
- Moore, K.W., Vieira, P., Fiorentino, D.F., Trounstine, M.L., Khan, T.A. and Mosmann, T.R. (1990) Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science, 248, 1230-1234. doi:10.1126/science.2161559
- Barbosa, M.C., Dos Santos, T.E., de Souza, G.F., de Assis, L.C., Freitas, M.V. and Gonçalves, R.P. (2013) Impact of iron overload on interleukin-10 levels, biochemical parameters and oxidative stress in patients with sickle cell anemia. Revista Brasileira de Hematologia e Hemoterapia, 35, 29-34. doi:10.5581/1516-8484.20130011
NOTES
*Corresponding author.

