Advances in Bioscience and Biotechnology
Vol.2 No.3(2011), Article ID:5041,5 pages DOI:10.4236/abb.2011.23019
Molecular cloning, expression and characterization of a novel geneβ-N-acetylglucosaminidase from Bombyx mori
![]()
Institute of Life Sciences, Jiangsu University, Zhenjiang, China.
Email: kpchen@ujs.edu.cn
Received 23 April 2011; revised 30 April 2011; accepted 4 May 2011
Keywords: Bombyx mori; β-N-acetylglucosaminidase2; Western Bolt; Sub-Cellular Localization
ABSTRACT
Previously, we have reported that a gene encoding Bombyxmoriβ-N-acetylglucosaminidase 2 (BmGlcNA case2) has been identified differentially expressed in the midgut of Bombyxmori strain NB resistant to nucleopolyhedrovirus (BmNPV), strain 306 susceptible to NPV and a near isogenic line BC9 with similar genetic background to 306 but resistant to NPV by two-dimensional gel electrophoresis (2-DE). To get more knowledge about the relationship between β-N- -acetylglucosaminidase and the resistance of NPV, in this study, the 1542 bp open reading frame of a putative bombyxmoriβ-N-acetylglucosaminidase 2 gene (BmGlcNAcase2) was amplified from a pool of bombyxmoricDNAs and inserted into the prokaryotic expression plasmid pET-30a(+). Western blotting analysis showed that BmGlcNAcase2 was expressed in hemolymph, ovary, testis, fat body, trachea, midgut and silk gland of fifth instar larvae respectively. Immunofluoresence analysis indicated that BmGlcNA case2 was mainly located to the cytoplasm or some structure in cytoplasm.
1. INTRODUCTION
Sericulture is an important component of ariculture in China, which has a history of over 5,000 years in raising silkworms (BombyxmoriL.). It is also widely practiced in the world, such as in Japan, the former Soviet Union and Brazil. Silkworm viral diseases are major diseases causing great loss in sericulture, and Bombyxmorinucleopolyhedrovirus (BmNPV) is one of the most disastrous. Therefore, it is a subject of intensive research to control silkworm NPV disease. The key to the sericulture is to develop pathogen-resistant silkworm strains [1]. Recently, comparative gene expression techniques, such as differential display, cDNA microarray assay and two-dimensional gel electrophoresis have become routine to examine changes in gene expression. Such methodologies provide useful approaches to identify differently expressed transcripts, because many genes can be examined simultaneously. To date, although a few BmNPV resistant genes have been reported, such as serine protease [2] and Bmlipase-1 [3], knowledge on the molecular mechanisms of Bombyx mori against this virus remains very limited.
β-N-acetylglucosaminidase(GlcNAcase) are widely distributed in various organisms. These enzymes catalyze the hydrolysis of an O-glycosidic bond in nonreducing terminal N-acetylglucosamine(GlcNAc) residues in an oligosaccharide chain. In insects, GlcNAcase plays an important role in the degradation of various oligosaccharides and glycoconjugates [4-6], a putative bombyxmoriβ-N-acetylglucosaminidase 2 showed broad substrate specificity, and cleaved terminal N-acetylglucosamine residues from the α-3 and α-6 branches of a biantennary N-glycan substrate, and also hydrolyzed chitotriose to chitobiose [7]. In our previous study, by comparison the proteomes of the resistant Bombyx mori strain NB, the susceptible strain 306 and the near isogenic line BC9 strain by two-dimensional gel electrophoresis (2- DE), many differential protein spots have been obtained, one of which was identified as bombyxmori β-N-acetylglucosaminidase 2 by mass .We speculate that the excessive expression of β-N-acetylglucosaminidase in hemolymph of the resistant silkworm can probably disturb the N-linked glycans of GP64 protein on the cell membrane which is an essential process for initiating second infections, and thus reduce the reproduction of infectious viruses [8,9]. In this paper,the open reading frame (ORF) of BmGlcNAcase2 was cloned and the recombinant enzyme was expressed in E. coli. The amino acid sequence of recombinant BmGlcNAcase2 was verified by mass spectroscopic analysis. Western blotting analysis showed that there is no obvious difference of BmGlcNAcase2 expression in hemolymph, fat body, trachea, ovary, midgut, silk gland and testis of fifth instar larvae. The subcellular localization study through immunofluoresence analysis indicated for the first time that the BmGlcNA case2 was located to cytoplasm or some structure in cytoplasm. The obtained results would facilitate further studies to elucidate molecular mechanisms of Bombyxmoriagainst BmNPV infection.
2. MATERIALS AND METHODS
2.1. Insect, Cell and Virus
B. mori strain C108 (standard strain of silkworm) was maintained in our laboratory. All larvae were reared with fresh mulberry leaves at 27˚C under a 12 h light/12 h dark photoperiod.
The BmN cell line was maintained at 27˚C in TC-100 insect medium (Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco, USA) using standard techniques.
The expression vector pET-30a(+) and E. coli strains BL21 (DE3) were obtained from Novagen (CA, USA). All primers, RNase-free DNaseI, EX Taq polymerase, restriction enzymes, T4 DNA ligase and DNA the subcloning vector pMD18-T were purchased from TaKaRa (Dalian, China). Chemicals are all from sigma (MO, USA) or a domestic provider in China if not stated otherwise.
2.2. Cloning of the Bmglcnacase2 Gene
The BmGlcNAcase2 specific primers, forward primer (5’-CATGCCATGGCACCGGGACCCGAATAT-3’) with a NcoI site (underlined), and reverse primer (5’-ATAAGAATGCGGCCGCCTAAGCGCCTAGGCAGA-3’) with an NotI site (underlined) were designed to amplify the ORF of the putative BmGlcNAcase2 gene (GenBank accession no. AB286958).The PCR reaction was carried out with 30 amplification cycles (94˚C for 30 s, 55˚C for 30 s, and 72˚C for 90 s) in a Gene Amp 2400 System thermocycler. The PCR product was ligated into pMD 18-T vector using T4 DNA ligase and then transformed into E. coli TG1. A fragment between NcoI and NotI containing the BmGlcNAcase2 gene was excised from the recombinant plasmid. The purified fragment was subcloned into the pET-30a(+) expression vector and transformed into E. coli BL21 (DE3). DNA sequencing confirmed that the BmGlcNAcase2 gene was correctly fused to the N-terminal 6 × His-tag.
2.3. Expression and Purification of Recombinant Protein
To express recombinant protein, a freshly transformed colony was cultured in LB medium supplemented with kanamycin (50 μg/ml) at 37˚C overnight. This overnight culture was inoculated into fresh LB medium and cultured at 37˚C with vigorous shaking. When OD600 reach 0.5, the expression of BmGlcNAcase2 was induced with IPTG (final concentration 0.1-1 mM during optimization) and further cultured at 37˚C for another 8 hours and 16˚C for another 12 hours respectively. Cells were harvested by centrifugation (4500 g, 4˚C, 15 min) and SDSPAGE analysis on 15% gel was performed to estimate the expression level of BmGlcNAcase2. The cell pellet was resuspended in buffer A (50 mM sodium phosphate, 300 mMNaCl, 1 mM EDTA, 0.5 mM PMSF, pH 8.0), then the suspension was lysed by sonication. The lysate was clarified by centrifugation (16,000 g, 4˚C, 25 min). The supernatant was loaded onto a Ni-NTA affinity column (Qiagen). Purification conditions were standardized by optimizing pH, the concentration of salt and imidazole. After washing the captured column with 20 mM and 40 mM imidazole, the fusion protein was eluted with 250 mM imidazole. The eluted protein was dialyzed against buffer B (50 mM sodium phosphate, 150 mM NaCl, pH 7.5) at 4˚C.
2.4. Mass Spectrometry
The specific bands corresponding to BmGlcNAcase2 were excised manually from the gel with a sterile scalpel and digested with trypsin according to Li’s [10] method. The digested samples were analyzed by an ultraflex MALDI-TOF-TOF (BRUKER, GERMANY). Peptide mass fingerprinting (PMF) was performed by comparing the masses of peptides to NCBI protein database using the MASCOT search engine (http://www.matrixscience.com).
2.5. Antibody Production and Western Blot Analysis
The antibody was prepared by standard techniques [11]. Briefly, purified BmGlcNAcase2 protein (about 2 mg) was injected subcutaneously to immunize New Zealand white rabbits in complete Freund’s adjuvant, followed by two booster injections in incomplete Freund’s adjuvant within a gap of 2 weeks before exsanguinations. Then, the polyclonal rabbit antibody against 6 × HisBmGlcNAcase2 was obtained and used for immunoassay.
After the SDS-PAGE (Bio-Rad Mini-Protean II, Hercules, CA) was finished, the proteins were transferred to a PVDF membrane with a Bio-Rad liquid transfer apparatus for Western blot. The rabbit anti-BmIDGF polyclonal antibodies (1:1,000 dilution) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (1:2,000 dilution) were used, and signals were detected by diaminobenzidine (DAB) (Sigma, USA). To detect if this protein expressed specially in tissues, total protein of each tissues was added to the lines of the SDS-PAGE, and the added protein concentration of each tissues are 50 ug.
2.6. Immunofluorescence Microscopy
BmN cells seeded onto coverslips were washed with PBS, and fixed with 2 ml of 4% paraformaldehyde for 15 min. Then cells were washed three times with PBS and permeabilized with 0.1% Triton X-100 in PBS for 15 min. After washing three times with PBS, cells were incubated with anti-BmGlcNAcase2 antibody (1:1000) as primary antibody, fluorescein isothiocyanate (FITC) conjugated goat anti-rabbit IgG antibody as secondary antibody (1:3000) (Qualex, Inc), and nuclei were stained with DAPI (Roche), then examined with a confocal laser scanning microscope (Zeiss lsm 5 live).
3. RESULTS AND DISCUSSION
3.1. Cloning of the BmGlcNAcase2 Gene
The recombinant BmGlcNAcase2 protein was expressed in E. coli BL21 (DE3) harboring the expression vector pET-30a(+)-BmGlcNAcase2. The BmGlcNAcase 2 proteins formed inclusion bodies when host E. coli was cultivated at 37°C and induced with 1 mM IPTG. The expression of 6 His-tagged Bm122 was also confirmed by anti-6xHis monoclonal antibody (Figure 1(b)). The soluble BmGlcNAcase2 was purified by a Ni-NTA column (Figure 1(a), lane 3).
3.2. Mass Spectrometry
To determine whether the amino acid sequence of recombinant BmGlcNAcase2 matches the one predicted from DNA sequencing results, the MALDI-TOF-TOF mass spectra of tryptic digest of recombinant BmGlcN Acase2 was characterized to identify the recombinant protein. 17 peptide fragments were identified. By comparing the masses of indentified peptides to the hypothetical tryptic peptides for proteins in non-redundant NCBI database using the MASCOT search engine, BmGlcNAcase2 was obviously identified with MOWSE score of 86. The identified 17 peptide fragments matched against the deduced amino acid sequence of BmGlcN Acase2 accounted for 41% peptide mass fingerprint sequence coverage (Figure 2).
3.3. Cellular Localization of BmGlcNAcase2 in BmN Cells
Confocal laser scanning fluorescence microscopy was utilized to determine the cellular localization of BmGlc NAcase2 protein in BmN cells. The result showed that the BmGlcNAcase2 protein was primarily located in the cytoplasm and was scarcely detectable in the nucleus, while no obvious fluorescence signal was observed in cells stained with pre-immune serum of rabbit as pri-
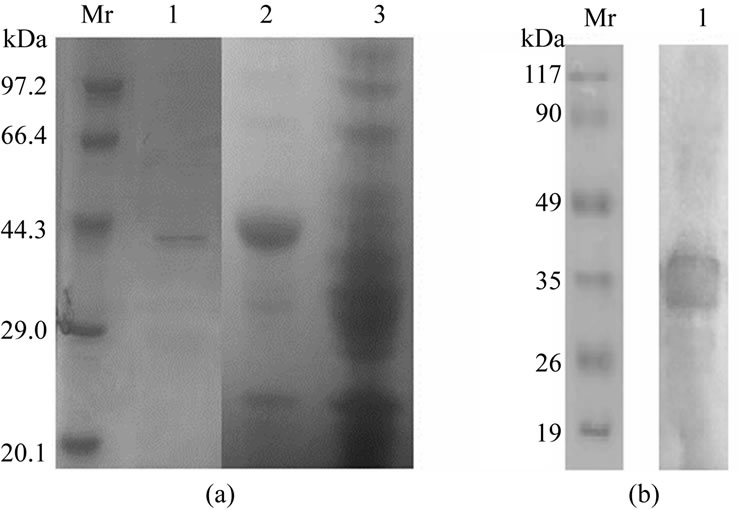
Figure 1. SDS-PAGE stained with commassie blue and western blotting analysis of recombinant Bm GlcNAcase2. (a) Lane1: recombinant BmGlcNAcase2 purified by Ni-NTA resin column; lane2: lysate of BL21 (DE3) harboring pET-30a(+)- -BmGlcNAcase2 induced with 0.5 mM IPTG; lane3: lysate of host cell transformed with empty vector. (b) Lane1: western blotting analysis of recombinant BmGlcNAcase2 with anti-6 his antibody.
mary antibody (Figure 3).
3.4. Western Blot Analysis
Antibody against BmGlcNAcase2 protein was used to perform western blot analysis Bombyx mori. The result showed that BmGlcNAcase2 was expressed in hemolymph, ovary, testis, fat body, trachea, midgut and silk gland of fifth instar larvae, and there was no obvious difference in expression of these tissues(Figure 4).
4. DISCUSSION
β-N-acetylglucosaminidase of Bombyx mori plays an important role in several physiological process, the enzyme is essential to hydrolyze chitooligosaccharides to their constitutive monomer and contribute to recycling GlcNAc pools for remodeling of the exoskeleton during metamorphosis, as indicated previously [12,13]. It was reported recently that beta-N-acetylglucosaminidase activity in BmNPV resistant strains NB and the near-isogenic line BC9 was significantly higher than that in the BmNPV susceptible strain 306 [9] so it is interesting to find out the relationship between this enzyme and silkworm NPV disease.
In this report, we have successfully cloned, optimized the expression and purifiedβ-N-acetylglucosaminidase of Bombyx mori in the E.coli strains BL21 (DE3) and we showed that the expression of BmGlcNAcase2 can be detected from almost all tissues(hemolymph, ovary, testis, fat body, trachea, midgut and silk gland) of the fifth instar larvae. Identifying the subcellular localization of protein is particularly helpful in the functional annotation of gene products. The result showed that BmGlcN
Figure 2. MALDI spectra of tryptic digest of recombinant BmGlcNAcase2. The identified protein, score, amino acid sequence coverage and the number of identified peptides are shown. Matched peptide sequences are showed in red.
Figure 3. Subcellular localization of BmGlcNAcase2 in BmN cells treated with anti-BmGlcNAcase2 antibody, followed by treatment with FITC-conjugated goat anti-rabbit IgG, and examined in confocal laser microscope. From left to right: green fluorescence for BmGlcNAcase2, DAPI and the overlay images. For the control, pre-immune serum was used as the primary antibody. Nuclei were stained with DAPI (blue). Samples were observed under a confocal laser scanning microscope.
Figure 4. Expression profile of BmGlcNAcase2 in different tissues of Bombyx mori. Lanes 1 to 7 represented fat body, Malpighian tubule,midgut,ovary, hemocytes, testis, and silk gland.
Acase2 was a protein distributed mostly in cytoplasm firstly, which is consistent with prediction by computer tool of PLOC (http://www.genome.jp/SIT/plocdir/), whereas the precise biochemical function and the possible role of BmGlcNAcase2 in this process remain to be determined.
5. ACKNOWLEDGEMENTS
This work was supported by the grants from Jiangsu SciTech Support Project-Agriculture (No. BE2008379).
REFERENCES
- Chen, H.Q., Chen, K.P., Yao, Q., Guo, Z.J. and Wang, L.L. (2007) Characterization of a late gene, ORF67 from bombyx mori nucleopolyhedrovirus. FEBS Letters, 581, 5836-5842. doi:10.1016/j.febslet.2007.11.059
- Nakazawa, H., Tsuneishi, E., Ponnuvel, K.M., Furukawa, S., Asaoka, A., et al. (2004) Antiviral activity of a serine protease from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus. Virology, 321, 154-162. doi:10.1016/j.virol.2003.12.011
- Ponnuvel, K.M., Nakazawa, H., Furukawa, S., Asaoka, A., Ishibashi, J., et al. (2003) A lipase isolated from the silkworm Bombyx mori shows antiviral activity against nucleopolyhedrovirus. Journal of Virology, 77, 10725- 10729. doi:10.1128/JVI.77.19.10725-10729.2003
- Aumiller, J.J., Hollister, J.R. and Jarvis, D.L. (2006) Molecular cloning and functional characterization of beta-N-acetylglucosaminidase genes from Sf9 cells. Protein Expression and Purification, 47, 571-590. doi:10.1016/j.pep.2005.11.026
- Cattaneo, F., Pasini, M.E., Intra, J., Matsumoto, M., Briani, F., et al. (2006) Identification and expression analysis of Drosophila melanogaster genes encoding β- -hexosaminidases of the sperm plasma membrane. Glycobiology, 16, 786-800. doi:10.1093/glycob/cwl007
- Nagamatsu, Y., Yanagisawa, I., Kimoto, M., Okamoto, E. and Koga, D. (1995) Purification of a chitooligosaccharidolytic beta-N-acetylglucosaminidase from Bombyx mori larvae during metamorphosis and the nucleotide sequence of its cDNA. Bioscience, Biotechnology, and Biochemistry, 59, 219-225. doi:10.1271/bbb.59.219
- Takahiro, O., Seiji, I., Hideki, S., Akihiro, U., Toshiki, T., et al. (2007) Molecular cloning and expression of two novel β-n-acetylglucosaminidases from silkworm Bombyx mori. Bioscience, Biotechnology, and Biochemistry, 71, 1626-1635. doi:10.1271/bbb.60705
- Jarvis, D.L., Wills, L., Burow, G. and Bohlmeyer, D.A. (1998) Mutational analysis of the N-linked glycans on autographa californica nucleopolyhedrovirus gp64. Journal of Virology, 72, 9459-9469.
- Liu, X.Y., et al. (2010) Proteomic analysis of nucleopolyhedrovirus infection resistance in the silkworm, Bombyx mori (Lepidoptera: Bombycidae). Journal of Invertebrate Pathology, 105, 84-90.
- Li, X.H., Wu, X.F., Yue, W.F., Liu, J.M., Li, G.L., et al. (2006) Proteomic analysis of the silkworm (Bombyx mori L.) hemolymph during developmental stage. Journal of Proteome Research, 5, 2809-2814. doi:10.1021/pr0603093
- Kothari, H., Kumar, P. and Singh, N. (2006) Prokaryotic expression, purification, and polyclonal antibody production against a novel drug resistance gene of Leishmania donovani clinical isolate. Protein Expression and Purification, 45, 15-21. doi:10.1016/j.pep.2005.10.002
- Bade, M.L. and Wyatt, G.R. (1962) Metabolic conversions during putation of the cecropia silkworm. 1. Deposition and utilization of nutrient. Biochemical Journal, 83, 470-478.
- Zen, K.C., Choi, H.K., Krishnamachary, N., Muthukrishnan, S. and Kramer, K.J. (1996) Cloning, expression and hormonal regulation of an insect β-N-acetylglucosaminidase gene. Insect Biochemistry and Molecular Biology, 26, 435-444. doi:10.1016/0965-1748(95)00111-5

