Journal of Environmental Protection
Vol. 4 No. 7 (2013) , Article ID: 34531 , 5 pages DOI:10.4236/jep.2013.47077
Experimental Study on a New Corrosion and Scale Inhibitor
![]()
School of Resource and Environmental Engineering, Wuhan University of Technology, Wuhan, China.
Email: 542785846@qq.com
Copyright © 2013 Defang Zeng, Huan Yan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 5th, 2013; revised March 6th, 2013; accepted April 5th, 2013
Keywords: Corrosion and Scale Inhibitor; Scale Inhibition Performance; Corrosion Inhibition
ABSTRACT
The mixture consisted of benzotriazole (BTA), chitosan (CTS), polyacrylic acid and zinc salt has been investigated as a corrosion and scale inhibitor of A3 carbon steel in cooling water. The scale and corrosion inhibition efficiency was evaluated by static anti-scaling teat together with rotary coupon test. Compared with the phosphorus corrosion and scale inhibitor, the corrosion inhibition rate and scale inhibition rate of it increased respectively by 2.51% and 1.16%. As the corrosion and scale inhibitor is phosphate-free, it won’t cause eutrophication, considering the product performance and environmental influence, the phosphate-free corrosion and scale inhibitor is superior to the traditional one.
1. Introduction
With the development of economy, the consumption of industrial cooling water is increasing rapidly. Because of evaporation, concentration, temperature rise and other reasons in the process of operation, dissolved salts will be separated out from cooling water and it can adsorb in pipe wall and equipment in the form of scale, the metal surface may form electrochemical corrosion because of irregularity, both of them will affect the equipment, service life of pipelines and use efficiency. Therefore people have to pay more attention to the treatment of the circulating cooling water [1,2].
Recently, dosing reagents into circulating cooling water is the most common method, and through the role of the pharmacy, the corrosion and scaling problem in the pipes will be solved effectively [3-6]. Now, phosphorus products are widely used in cooling water system. Because they can easily cause environmental pollution, an intense research effort is being undertaken to look for the replacement of phosphorus products by more environmentally friendly products [7-12]. The previous work has shown that Chitosan is a natural polymer material which has good resistance scale effect; benzotriazole and zinc salts both have corrosion inhibition effect.
The present work aims to study the performance of a new corrosion and scale inhibitor mixed by chitosan (CTS), polyacrylic acid, benzotriazole (BTA) and zinc salt in cooling water. The best formula has been found and it has obvious economic and environmental benefits.
2. Experimental
2.1. Materials and Instruments
Test specimens were 50 × 25 × 2 mm sheets prepared from A3 carbon steel. The exposed surfaces degreased with acetone. The corrosion products were eliminated by HCL at 10% for 3 minutes before tested.
The chitosan was made from shrimp. After removing impurities, cleaning the selected shrimp by water, soaking it into 10% hydrochloric acid for 3 days in order to remove the calcium in it. After filtration and washing, placed it into 10% NaOH, heated it to boiling for 3 hours, oxidated it with 4% KMnO4 for 2 hours, then added 0.2% NaHSO3 in order to fade the colour of KMnO4 completely. Finally dry it to get white flakes of chitin. Placed 5 g chitin into a three-necked flask equipped with 50 mL 50% sodium hydroxide solution, keeping the temperature 100˚C, stirring speed 50 r/min, stirring time 50 min under the conditions of the deacetylation reaction, then filtration, washing and drying to get chitosan products, finally taking the chitosan products to prepare CTS working fluid of 0.02%.
2.2. Experimental Methods
2.2.1. Rotating Hanging Plate Experiment
Corrosion inhibition efficiency was determined by weight loss of the tested specimens using rotating hanging plate method [13], afterward, the instruments used was RCC-type I rotate hang piece of test, the experiment was achieved in laboratory configuration water (Table 1) at temperature 45˚C ± 1˚C, time 72 h and rotation speed 75 r/min. The corrosion rate X and corrosion inhibition rate η were calculated respectively by Types (1) and (2):
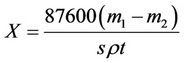 (1)
(1)
where m1 and m2 were the quality of A3 carbon steel before and after the test respectively. s is the surface area, cm2; ρ is the density. g/cm; t is the experimental time, h
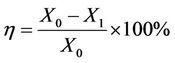 (2)
(2)
where X0 and X1 were the corrosion rate without and with the corrosion and scale inhibitor.
2.2.2. Static Scale Inhibition Experiment
Scale inhibition efficiency was tested by “Calcium carbonate deposition method” [14]. The principle was to heat water samples with and without the scale and corrosion inhibitor, then determined the concentration of Ca2+ and calculated the inhibition rate. The experiment was achieved at temperature 80˚C ± 1˚C and time 10 h. Scale inhibition rate η was calculated by Type (3):
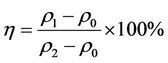 (3)
(3)
where ρ0 and ρ1 were the Ca2+ concentration without and with corrosion and scale inhibitor ,while ρ2 was Ca2+ concentration before experimenting.
3. Results and Discussion
3.1. Best Formula of the New Phosphate-Free Corrosion and Scale Inhibitor
Adequate experimental studies have been made to determine the preliminary formula. In this paper, Table 2 shows the four factors and their levels .The final formula would been determined by further experiments.
The best formula would be determined through orthogonal experiment used calcium carbonate deposition method (GB/T16632-2008). The initial Ca2+ concentra-

Table 1. Water quality index.
tion was 240 mg∙L−1, scale inhibition rate was calculated by Type (3). Table 3 shows the importance of factors as polyacrylic acid > CTS > BTA > zinc salt, the best formula was B3C2A2D2.
3.2. Corrosion Inhibition Performance Analysis
Table 4 contains the corrosion inhibition efficiency of A3 carbon steel samples in colling water with 0PPM, 10 PPM, 20 PPM, 30 PPM and 40 PPM corrosion and scale inhibitor .In all cases the typical behavior was observed, corrosion inhibitor rate of the phosphorus—free corrosion inhibitor increased as the concentration elevated. The corrosion inhibition rate could reach 96.14% when the dosage was 30 PPM .
3.3. Scale Inhibition Performance Analysis
Table 5 shows the scale inhibition performance respectively with 0 PPM, 10 PPM, 20 PPM, 30 PPM and 40 PPM corrosion and scale inhibitor. The scale inhibition rate increased with the concentration elevated. The scale inhibition rate could reach 94.48% when the dosage was 30 PPM.
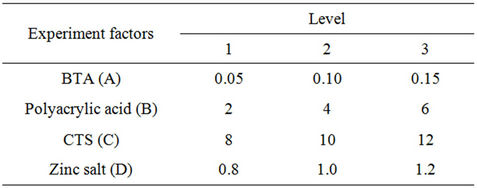
Table 2. Factors and levels of orthogonal experiment.
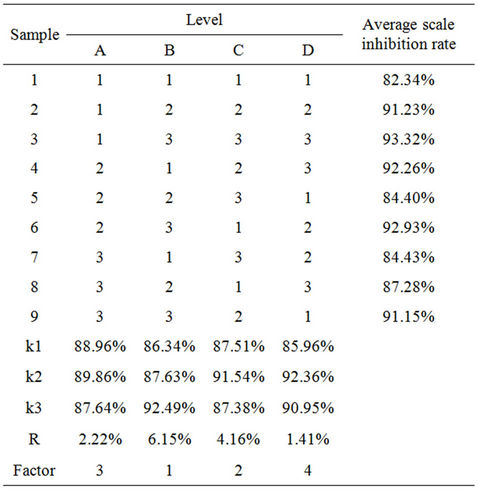
Table 3. Orthogonal experiment of L9 (34).
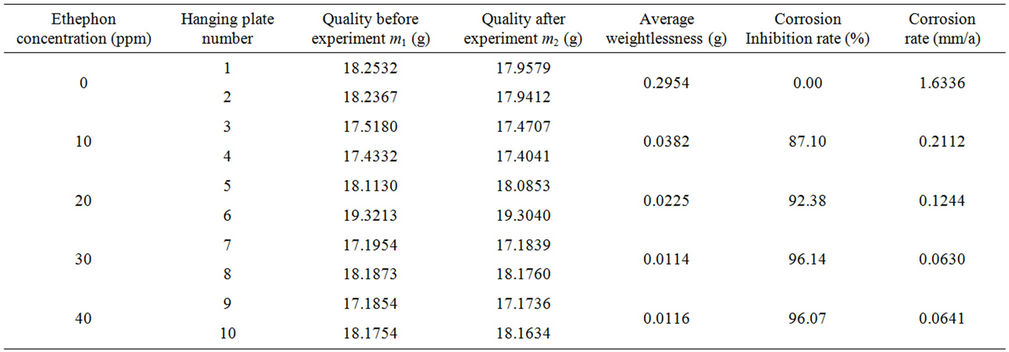
Table 4. Results of corrosion inhibition rate.
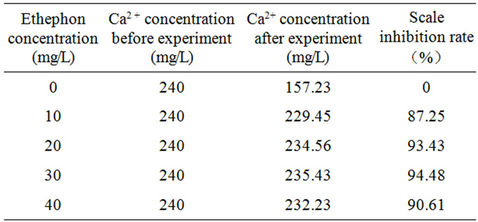
Table 5. Test results of scale inhibition.
3.4. Effect of Ca2+ Concentration
The relateship between inhibition rate and Ca2+ concentration was tested at scale inhibitor concentration 30 PPM, pH 7, temperature 80˚C and heating temperature 10 hours. Figure 1 showed that scale inhibition rate increased with Ca2+ concentration elevated in the scope of 100 mg∙L−1 to 220 mg∙L−1, so this corrosion and scale inhibitor was applied to the system of Ca2+ concentration in 100 to 220 mg∙L−1.
3.5. Effect of pH Values
The curves included in Figure 2 allowed to evaluate pH related to corrosion and scale inhibition. Calcium carbonate deposition method (GB/T16632-2008) was used. Figure 2 showed that pH had great influence on corrosion and scale rate. Scale inhibition rate was less than 93% when pH was less than 7, it could reach 94.48% while the pH value was between 7 and 8, after PH researched 8, scale inhibition rate declined. In alkaline system calcium carbonate will generated Ca (HCO3)2 and OH−, they would make scale inhibition rate declined. In acidic condition, a protective and dense calcium carbonate scale layer could not been easily formed on surface of A3 carbon steel, zinc salt would sediment and influence the corrosion inhibition rate on alkaline conditions when pH was greater than 8 [15-17]. This showed the corro-
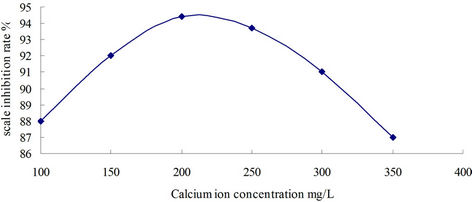
Figure 1. Influence of Ca2+ Concentration to scale inhibition rate.
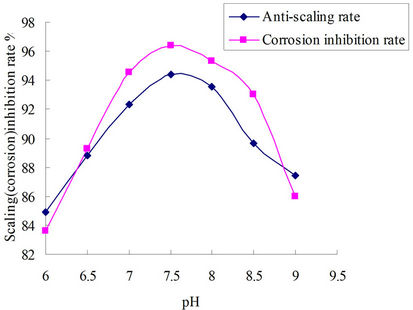
Figure 2. Influence of pH to corrosion and scale inhibition rate.
sion inhibitor was applicable to the system in which pH value was between 7 to 8.
Table 6 showed the comparisons between the new and traditional corrosion and scale inhibitors were showed the corrosion inhibition rate and scale inhibition rate of the optimum formula increased respectively by 2.51% and 1.16%. It is an ideal corrosion inhibitor with phosphorus—free and would not cause water eutrophication.
4. Conclusions
From the whole set of datas obtained in this study, it can
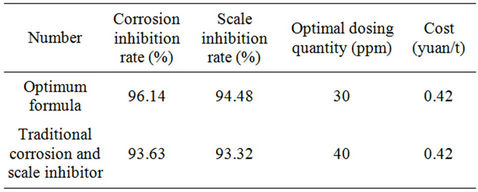
Table 6. Comparisons between the new and traditional corrosion inhibitors.
be concluded that a phosphorus-free corrosion and scale inhibitor consist of benzotriazole (BTA), chitosan (CTS), polyacrylic acid and zinc salt showed a better performance than the traditional one.
Scale and corrosion inhibition rates could reach respectively 96.14% and 94.48% when dosage was 30 ppm. The phosphorus-free corrosion and scale inhibitor can apply to system at pH between 7 and 8.1 and Ca2+ concentration between 100 and 220 mg∙L−1.
Compared with the traditional one, the corrosion inhibition rate and scale inhibition rate increased respectively by 2.51% and 1.16%. As the corrosion and scale inhibitor was phosphate-free, it wouldn’t cause eutrophication, considering the economic cost, product performance and environmental influence, the new phosphate-free corrosion and scale inhibitor was significantly superior to the traditional one and had obvious environmental benefits.
5. Acknowledgements
This project was supported by Ministry of Science and Technology in China of SME Technology Innovation Foundation and Department of Science and Technology in Hubei Province, China of Science and Technology Research Foundation, express our thanks to those provided guidance and assistance.
REFERENCES
- R. Touir, N. Dkhireche, M. Ebn Touhami, M. Lakhrissi, B. Lakhrissi and M. Sfaira, “Corrosion and Scale Processes and Their Inhibition in Simulated Cooling Water Systems by Monosaccharides Derivatives: Part I: EIS Study,” Desalination, Vol. 249, No. 3, 2009, pp. 922-928. doi:10.1016/j.desal.2009.06.068
- M. A. Quraishi, A. Singh and V. K. Singh, “Green Approach to Corrosion Inhibition of Mild Steel in Hydrochloric Acid and Sulphuric Acid Solutions by the Extract of Murraya koenigii Leaves” Materials Chemistry and Physics, Vol. 122, No. 1, 2010, pp. 114-122. doi:10.1016/j.matchemphys.2010.02.066
- P. Kalaiselvi, S. Chellammal, et al., “Artemisia Pallens as Corrosion Inhibitor for Mild Steel in HCl Mediun,” Materials Chemistry and Physics, Vol. 120, No. 2, 2010, pp. 643-648. doi:10.1016/j.matchemphys.2009.12.015
- R. Touir, N. Dkhireche, M. Ebn Touhami, M. Sfaira, O. Senhaji, J. J. Robin, B. Boutevin and M. Cherkaoui, “Study of Phosphonate Addition and Hydrodynamic Conditions on Ordinary Steel Corrosion Inhibition in Simulated Cooling Water,” Materials Chemistry and Physics, Vol. 122, No. 1, 2010, pp. 1-9. doi:10.1016/j.matchemphys.2010.02.063
- L. G. Qing, H. J. Yi, Z. Y. Ming, et al., “Acrylic AcidAllylpolyethoxy Carboxylate Copolymer Dispersant for Calcium Carbonate and Iron(III) Hydroxide Scales in Cooling Water Systems,” Tenside Surfactants Detergents, Vol. 49, No. 3, 2012, pp. 216-224.
- D. Lzydor and F. Piotr, “Industrial Cooling Water Systems. Exploitation and Environmentally Benign Total Inhibitive Protection,” Przemysl Chemiczny, Vol.90, No. 5, 2011, pp. 737-741.
- X. Y. He, Y. H. Cheng, L. X. Wang and P. Huo, “Study of Corrosion and Scale Inhibition Performances of PASP Complex Water Treatment Agents,” CAS, Vol. 30, No. 8, pp. 64-66.
- A. Y. Hu, “Analysis of Water and Energy Saving Measures in Industrial Circulating Cooling Water System,” Industry Water and Wastewater, Vol. 42, No. 3, 2011, pp. 1-4.
- R. Touir, M. Cenoui, M. El Bakri and M. Ebn Touhami, “Sodium Gluconate as Corrosion and Scale Inhibitor of Ordinary Steel in Simulated Cooling Water,” Corrosion Science, Vol. 50, No. 6, 2008, pp. 1530-1537. doi:10.1016/j.corsci.2008.02.011
- B. Labriti, N. Dkhireche, R. Touir, M. Ebn Touhami, M. Sfaira, A. El Hallaoui, B. Hammouti and A. Alami, “Synergism in Mild Steel Corrosion and Scale Inhibition by a New Oxazoline in Synthetic Cooling Water,” Arabian Journal for Science and Engineering, Vol. 37, No. 5, 2012, pp. 1293-1303. doi:10.1007/s13369-012-0257-7
- A. Weisenburger, G. Müller, A. Heinzel, A. Jianu, H. Muscher and M. Kieser, “Corrosion, Al Containing Corrosion Barriers and Mechanical Properties of Steels Foreseen as Structural Materials in Liquid Lead Alloy Cooled Nuclear Systems,” Nuclear Engineering and Design, Vol. 241, No. 5, 2011, pp. 1329-1334. doi:10.1016/j.nucengdes.2010.08.005
- L.-J. Gao, J.-Y. Feng, B. Jin, Q.-N. Zhang, T.-Q. Liu, Y.- Q. Lun and Z.-J. Wu, “Carbazole and Hydroxy GroupsTagged Poly (Aspartic Acid) Scale Inhibitor for Cooling Water System,” Chemistry Letters, Vol. 40, No. 12, 2011, pp. 1392-1394. doi:10.1246/cl.2011.1392
- GB/T 18175-2000, “Water Treatment Agent Corrosion Inhibition Performance of the Determination of Rotation Coupon Method.”
- GB/T 16632-2008, “Performance of Water Treatment Agent and Scale Determination of Calcium Carbonate Deposition (CVD).”
- X. P. Ouyang, X. Q. Qiu, H. M. Lou and D. J. Yang, “Corrosion and Scale Inhibition Properties of Sodium Lignosulfonate and Its Potential Application in Recirculating Cooling Water System,” Industrial & Engineering Chemistry Research, Vol. 45, No. 16, 2006, pp. 5716- 5721. doi:10.1021/ie0513189
- Y. Sürme, A. Ali Gürten and E. Bayol “Corrosion Behavior of Mild Steel in the Presence of Scale Inhibitor in Sulfuric Acid Solution,” Protection of Metals and Physical Chemistry of Surfaces, Vol. 47, No. 1, 2011, pp. 117-120
- D. Hasson, H. Shemer and A. Sher, “State of the Art of Friendly ‘Green’ Scale Control Inhibitors,” Industrial & Engineering Chemistry Research, Vol. 50, No. 12, 2011, pp. 7601-7607. doi:10.1021/ie200370v

