Green and Sustainable Chemistry
Vol.3 No.2(2013), Article ID:31287,5 pages DOI:10.4236/gsc.2013.32008
AlCl3 Supported Catalysts for the Isomerization of Endo-Tetrahydrodicyclopentadiene
1School of Chemistry, Dalian University of Technology, Dalian, China
2Laboratory of Nano-Green Catalysis and Nano Center for Fine Chemicals Fusion Technology, Department of Chemistry, Inha University, Incheon, South Korea
Email: *jimin@dlut.edu.cn
Copyright © 2013 Min Ji et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 21, 2013; revised March 22, 2013; accepted March 30, 2013
Keywords: Tetrahydrodicyclopentadiene; Isomerization; Zeolite; Immobilization; Aluminium Chloride
ABSTRACT
Two of zeolites such as Hβ and HY, and mesoporous HMCM-41 were used as supports to immobilize AlCl3 for the investigation of the isomerization of endo-tetrahydrodicyclopentadiene (endo-TCD). The dependences of porous structure and surface acidity of the AlCl3 immobilized catalysts on the activity and selectivity were studied. Based on characterization studies, both the large pore diameter and strong acid sites of the supported catalysts contributed to the high activities in the endo-TCD conversion. And specifically the stronger Lewis acid sites seems to be responsible for the higher selectivity onto exo-TCD, and the stronger Brönsted acid sites were crucial to the formation of adamantane through the further isomerization of exo-TCD.
1. Introduction
Endo-tetrahydrodicyclopentadiene (endo-TCD) has been typically formed through the dimerization followed by hydrogenation from dicyclopentadiene, and its isomers, both exo-tetrahydrodicyclopentadiene(exo-TCD) and adamantane(ADH), are very useful fine chemical products[1-3]. In industry, exo-TCD and ADH are produced through the rearrangement of endo-TCD by acid catalysis [4], as shown in Scheme 1. Due to the advantages of low reaction temperature and strong resistance to ward coke, AlCl3 has been used as the most widely used catalyst [5], but it causes environmental problems such as severe corrosion and difficulty in separation from the reaction mixture. In the 1980s, liquid type superacids, such as CF3SO3H and SbF5-graphite, were used to catalyze endo-TCD to exo-TCD [6]. However, the problems of separation and corrosion were still remained. Since the 2000s, ionic liquids [7] were used to isomerize the endo-TCD, which resulted in pretty good result, but the expensive price restricts its industrial application. Hence, there is a strong motivation to develop new catalyst system for the isomerization that has minimum environmental impact. On the other hand, during the past decades, zeolites have been drawn much attentions in the
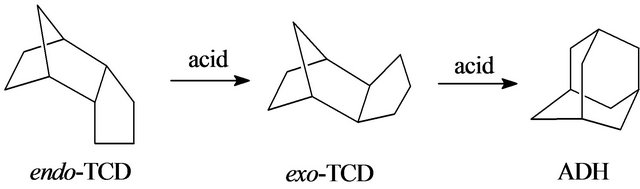
Scheme 1. Exo-TCD and ADH are produced through the rearrangement of endo-TCD by acid catalysis.
rearrangement of endo-TCD [8,9]. However, the low activity of zeolites limits its industrial application.
Since then, the immobilization of AlCl3 on inorganic solid supports has been proposed [10-12]. In our previous study [13,14], we developed the catalysts of AlCl3 immobilized on SiO2, Al2O3 and MCM-41, and found that it could keep the excellent reactivities of traditional AlCl3 catalyst at mild reaction conditions for endo-TCD isomerization, and the properties of supports strongly affected to the product selectivity. It is known that the reaction mechanism for the endo-TCD isomerization to exo-TCD and ADH is a carbenium ion skeleton rearrangement [4]. So, the pore structure and acid type of the catalyst seem to be important parameters in the conversion and product distribution. In the present report, Hβ and HY zeolites, and HMCM-41mesoporous material were used as catalyst supports to immobilize AlCl3 for the purpose of differentiating both pore structures and acidities. Based on the property changes of supports after AlCl3 immobilization, the influences of porous property, the type of acidities, and acidic strength of catalysts on both endo-TCD conversion and product selectivities in the endo-TCD rearrangement reaction were investigated.
2. Experiment
2.1. Catalyst Preparation
The Hβ and HY zeolites were purchased from Nankai Catalyst Co., China. HMCM-41 was prepared by the tetraethyl silicate (TEOS) in basic solution condensation with cetyltrimethylammonium bromide (CTAB) as a supramolecular template, and by ion-exchange with HNO3 [15]. The immobilized AlCl3 catalyst was prepared by a two-step method as described in our previous publications [13].
2.2. Catalyst Characterization
The BET surface areas and the pore properties of the catalysts were measured by nitrogen physisorption by using a Micromeritics Gemini 2395 instrument at 77 K. In-situ Fourier Transform Infrared Spectra (FT-IR) of chemically adsorbed pyridine were collected on a Nicolet 360 FT-IR spectrophotometer. All spectra were taken at a resolution of 2 cm−1 for 32 scans. Catalysts were activated in an IR cell at 550˚C under vacuum for 2 h, cooled down to room temperature, followed by exposure to pyridine at this temperature for 30 min. The samples were heated up to 150˚C or 250˚C and evacuated under vacuum (10−5 Torr) for 1 h to remove physically adsorbed pyridine. Finally, the spectra were recorded at the desired temperatures.
2.3. Catalyst Activity Test
The isomerization rearrangement reaction was carried out in a batch suspension reactor. The catalyst was charged into 20 ml n-heptane solution. The reaction temperature was controlled by an oil bath equipped with a thermostat and an electric stirrer. The reaction pressure of 1.0 MPa was set by the replacement of air by N2 to keep the solvent in liquid phase. The reaction time was 3 h. The products were analyzed by an Agilent 6890 GC equipped with an HP-5 capillary column and FID detector of HP 6890/5973 GC-MS.
3. Results and Discussion
The physico-chemical properties of the catalysts are summarized in Table 1. The specific surface area and pore diameter of HMCM-41 were 1001 m2/g and 2.33 nm, respectively, which were much higher than that of
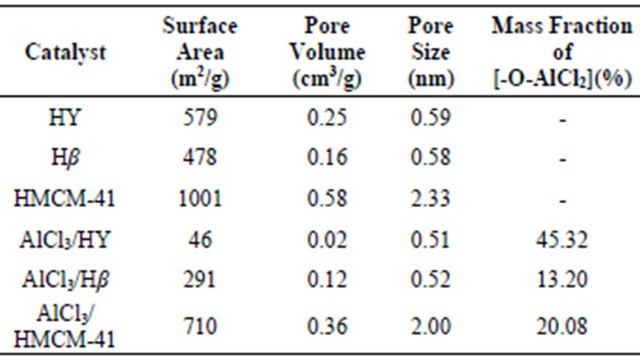
Table 1. Physico-chemical properties of supports and AlCl3 supported catalysts.
both HY and Hβ due to the mesoporosity. After AlCl3 was loaded, the decreasing surface area, pore volumes, and pore diameters were observed for both AlCl3/zeolites and AlCl3/HMCM-41 catalysts. This was primarily due to the penetration of AlCl3 into the pores of supports and located mostly inside of pores. Specially, HY showed the most drastic decresse in its surface area (46 m2/g) and pore volume (only 0.02 cm3/g) after AlCl3 immobilization, indicating that the pore channel of HY seemed to be greatly blocked by the large amounts of AlCl3 loading of 45.3%. However, the mass fractions of [-O-AlCl2] on the case of Hβ and HMCM-41 were 13.2% and 20.1%, respectively. So, they sustained pretty high pore volumes and surface areas even after immobilization of AlCl3. The mechanism of AlCl3 immobilization onto the supports were proposed via the surface reaction of AlCl3 with surface hydroxyl groups of the supports accompanied with HCl release [16,17]. Over 90% of the chloroaluminium species on the surface of the immobilized supports had the composition of [-O-AlCl2] [16]. So, the higher [-O-AlCl2] loading on HMCM-41 could be attributed to its higher surface area and more abundant surface hydroxyl groups.
The types of acid sites (Lewis and Brönsted) were studied by in-situ FT-IR adsorption of pyridine. The infrared spectra of the pyridine-adsorbed Hβ and HY (in Figure 1) revealed three peaks at ca. 1449 and 1540 cm−1 due to pyridine adsorbed on Lewis and Brönsted acid sites, respectively, and ~1480 cm−1 band corresponded to both Lewis and Brönsted (L+B) sites [18,19]. The infrared spectrum of the pyridine adsorbed HMCM-41 was somewhat different from those on HY and Hβ since it only showed Lewis acid sites at 1449 cm−1. After immobilization of AlCl3, the amounts of acid sites on catalyst surfaces increased remarkablely in all the cases. The infrared spectra of all the samples recorded after evacuation at 150˚C and 250˚C are indicating total acid sites and only strong acid sites, respectively, and the difference between them could be presumed to show weak acid sites of the catalysts. Based on the spectra in Figure 1, the adsorption capacities were the planimetered ac-
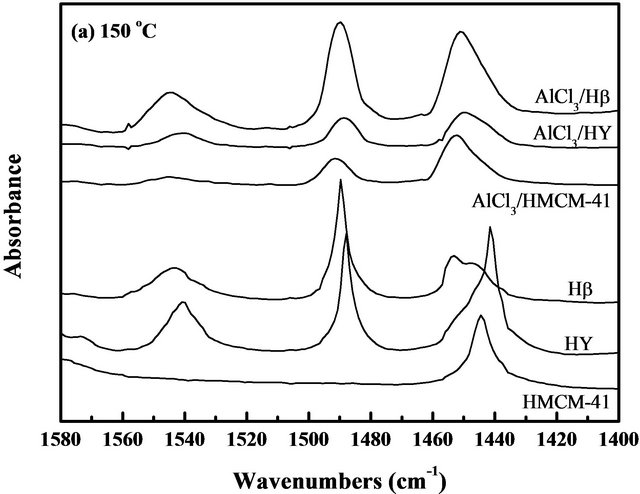 (a)
(a)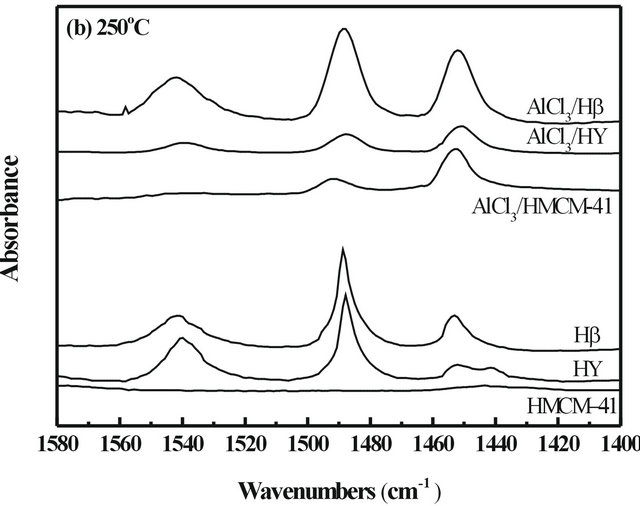 (b)
(b)
Figure 1. The infrared spectra of the pyridine-adsorbed over catalyst.
cording to the peak areas and normalized with both surface areas and wafer thickness of the catalysts [20], and the calculation results are listed in Table 2. It can be found that the pure HMCM-41 possessed only weak Lewis acid sites. After immobilization of AlCl3, it still lacked of strong Brönsted acid sites but owned abundant strong Lewis acid sites, which was as high as 11.50 (a.u.)·cm−1·cm2·mg−1. HY showed fewer strong Brönsted acid sites and strong Lewis acid sites, but after immobilization of AlCl3 on HY by forming AlCl3/HY, both strong Brönsted acid sites and strong Lewis acid sites increased simultaneously. For Hβ, it exhibited both the strongest Brönsted and Lewis acidities among the three supports, and those properties were also enhanced after AlCl3 immobilized on Hβ to form AlCl3/Hβ. It is interesting that the amount of AlCl3 loading on HY was much more than that on both HMCM-41 and Hβ, as shown in Table 1, but the number of total acid sites on AlCl3/HY was lower than that on AlCl3/Hβ, and AlCl3/HMCM-41.
It is mainly because of huge pore block in the case of AlCl3/HY.
Since the isomerization reaction of endo-TCD → exoTCD → ADH is a consecutive reaction, the pore structure and surface acidity of solid acid catalyst were speculated to play important roles in the endo-TCD conversion and product selectivity. Table 3 shows the catalytic properties of supports and supported AlCl3 catalysts for the isomerization of endo-TCD at 150˚C and 250˚C. Three pure supports showed no activity at 150˚C. When reaction temperature was risen up to 250˚C, HY and Hβ showed a certain degree of activity increase, while HMCM-41 still kept no activity. The results of FT-IR of pyridine adsorption listed in Table 2 shows that HMCM- 41 had only weak Lewis acid sites, instead HY and Hβ owned both weak and strong acid sites. So, it can be suggested that the weak Lewis acid sites of solid acid catalyst might contribute insignificantly to the reaction.
For all the of AlCl3 immobilized catalysts, the catalytic activities were greatly increased whether HY, Hβ and HMCM-41 as supports. Although the pores of AlCl3/HY catalyst were largely blocked due to high AlCl3 loading compared with the pure zeolites, it still allowed the reaction by the contribution of AlCl3 on external surface.
The greatly enhanced activity over AlCl3/Hβ could be ascribed to the increase in strong acidity of the catalyst as well as low blocking due to low loading of AlCl3. The order of the strong acidity of the supported AlCl3 catalysts are listed in Table 2 ranked as AlCl3/Hβ > AlCl3/ HMCM-41 ≈ AlCl3/HY, and the highest endo-TCD conversion of 95.7% was observed over AlCl3/Hβ catalyst. It is noteworthy that the total strong acid sites on AlCl3/HY and AlCl3/HMCM-41 was quite similar, but the endoTCD conversion of AlCl3/HMCM-41 was much higher than that of AlCl3/HY, which might be caused by the the diffusional limitation of partial pore blocking in the AlCl3/HY catalyst. Therefore, the mesoporous structure of AlCl3/HMCM-41 catalyst seems to be quite beneficial to the reactant diffusion to overcome the small amounts of Lewis acidity for the reaction.
The data in Tables 2 and 3 also show the dependence of surface acidity of catalyst on the product selectivity.
The ADH selectivities obtained at 250˚C shows AlCl3/ Hβ > AlCl3/HY ≈ Hβ > HY ≈ AlCl3/HMCM-41, which seemed to be corresponding to the amounts of strong Brönsted acid sites of catalysts (see Table 2). AlCl3/Hβ possessed the large number of strong Brönsted acid sites, and its ADH yield reached 25.6%, while the others were lower than 4%. The AlCl3/HY catalyst and Hβ support itself provided nearly the same amounts of strong Brönsted acid sites, and their ADH selectivities were almost same, which were 10.9% and 9.8%, respectively.
In contrast, AlCl3/HMCM-41 showed only strong
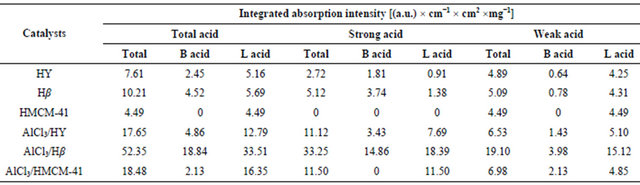
Table 2. Absorption intensities of FT-IR of pyridine adsorbed on acid sites of the catalysts.
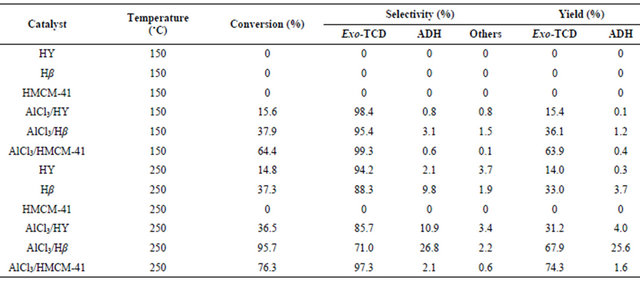
Table 3. Comparison of the catalyst performances in the isomerization of endo-TCD.
Lewis acidic sites and had no strong Brönsted acidity, then it exhibited the highest exo-TCD selectivity but the lowest ADH selectivity. Similar relationship between ADH selectivity and the amount of strong Brönsted acid sites could be found at the reaction temperature of 150˚C. These observations obviously indicate that strong Lewis acid sites of catalysts are attributed to the increases of selectivity of intermediate isomerization product, exoTCD, and the strong Brönsted acid sites are responsible for the farther ADH formation.
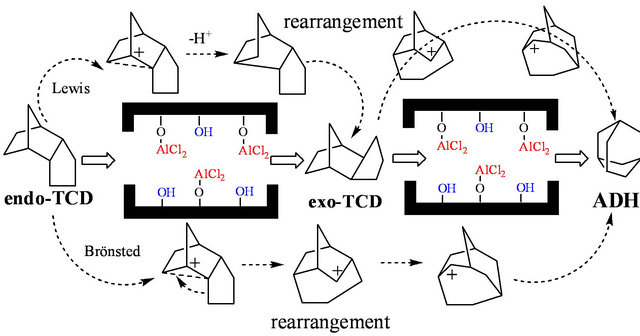
The synthesis mechanism of exo-TCD and ADH goes through a carbenium ion rearrangement (Scheme 2). Endo-TCD is isomerized on a acid by two consecutive reactions: endo-TCD is first isomerized to form exo-TCD, and then ADH was formed through the isomerization of exo-TCD. The first isomerization of endo-TCD can be catalyzed by strong Lewis acids, while the second step of exo-TCD to ADH requires strong Bronsted acids.
4. Conclusion
We have prepared a series of AlCl3 supported catalysts onto Hβ, HY, and HMCM-41 having different both po-
Scheme 2. The synthesis mechanism of exo-TCD and ADH.
rous structures and surface acidities. The dependence of the activity and selectivity in the conversion of endoTCD to exo-TCD and ADH was studied by correlation with both porous property and surface acidity of the catalysts. The suitable pore diameter and strong acid sites of catalyst are requisite for the isomerization of endoTCD. And the weak Lewis acid center of catalyst surface seemed to be no use to the catalytic activity for isomerization even at the high reaction temperature of 250˚C. However, the strong acid sites controlled the distribution of the products distinctly. Strong Lewis acid sites are responsible to get the intermediate isomerization product of exo-TCD, and the strong Brönsted acid sites of catalyst are crucial for the formation of ADH. The result found herein is believed to be potentially important for the selective formation of exo-TCD or ADH by controlling acidity and its strength.
5. Acknowledgements
Supports of this work by the National Natural Science Foundation of China (21076031) and the Fundamental Research Funds for the Central Universities (DUT12- ZD219) are gratefully acknowledged. Sang-Eon Park thanks Dalian University of Technology for a “Seasky” professorship.
REFERENCES
- H. S. Chung, C. S. H. Chen, R. A. Kremer, J. R. Boulton and G. W. Burdette, “Recent Developments in High-Energy Density Liquid Hydrocarbon Fuels,” Energy and Fuels, Vol. 13, No. 3, 1999, pp. 641-649. doi:10.1021/ef980195k
- G. Lamoureux and G. Artavia, “Use of the Adamantane Structure in Medicinal Chemistry,” Current Medicinal Chemistry, Vol. 17, No. 26, 2010, pp. 2967-2978. doi:10.2174/092986710792065027
- A. Masaaki, G. Akira and M. Shinji, EP Patent 2372799, 2011.
- E. M. Engler, M. Farcasiu, A. Sevin, J. M. Cense and P. V. R. Schleyer, “Mechanism of Adamantane Rearrangements,” Journal of the American Chemical Society, Vol. 95, No. 17, 1973, pp. 5769-5771. doi:10.1021/ja00798a059
- “Société des Usines Chimiques Rhǒne-Poluenc,” FR Patent 1431816, 1966.
- G. A. Olah and O. Farooq, “Chemistry in Superacids. 7. Superacid-Catalyzed Isomerization of Endoto Exo-Trimethylenenorbornane (Tetrahydrodicyclopentadiene) and to Adamantine,” Journal of Organic Chemistry, Vol. 51, No. 26, 1986, pp. 5410-5413. doi:10.1021/jo00376a067
- M. Y. Huang, J. C. Wu, F. S. Shieu and J. J. Lin, “Isomerization of Exo-Tetrahydrodicyclopentadiene to Adamantane Using an Acidity-Adjustable Chloroaluminate Ionic Liquid,” Catalysis Communications, Vol. 10, No. 13, 2009, pp. 1747-1751. doi:10.1016/j.catcom.2009.05.030
- K. Honna, M. Sugimoto, N. Shimizu and K. Kurisaki, “Catalytic Rearrangement of Tetrahydrodicyclotadiene to Adamantine over Y-Zeolite,” Chemistry Letters, Vol. 3, No. 3, 1986, pp. 315-318.
- M. Navrátilová and K. Sporka, “Synthesis of Adamantane on Commercially Available Zeolitic Catalysts,” Applied Catalysis A: General, Vol. 203, No. 1, 2000, pp. 127-132. doi:10.1016/S0926-860X(00)00477-4
- J. H. Clark, K. Martin, A. J. Teasdale and S. J. Barlow, “Environmentally Friendly Catalysis Using Supported Reagents: Evolution of a Highly Active Form of Immobilised Aluminium Chloride,” Journal of the Chemical Society, Chemical Communications, Vol. 19, No. 19, 1995, pp. 2037-2040. doi:10.1039/c39950002037
- C. DeCastro, E. Sauvage, M. H. Valkenberg and W. F. Hölderich, “Immobilised Ionic Liquids as Lewis Acid Catalysts for the Alkylation of Aromatic Compounds with Dodecene,” Journal of Catalysis, Vol. 196, No. 1, 2000, pp. 86-94. doi:10.1006/jcat.2000.3004
- Y. Takefumi, I. Tokuji, I. Shigeru, S. Michimasa, K. Yoshiyuki and T. Masanori, “Highly Active Supported Catalysts for Olefin Polymerization: Preparation and Characterization of the Catalyst,” Journal of Polymer Science, Part A: Polymer Chemistry, Vol. 26, No. 2, 1988, pp. 477-489. doi:10.1002/pola.1988.080260212
- L. M. Wu, M. Ji, M. He and T. X. Cai, “Synthesis of Adamantane Catalyzed by an Active Immobilized Aluminium Chloride Catalyst,” Chinese Journal of Catalysis, Vol. 28, No. 7, 2007, pp. 585-587. doi:10.1016/S1872-2067(07)60049-7
- L. L. Qi, M. Ji, M. He and T. X. Cai, “AlCl3/MCM-41 as a Catalyst for Isomerization of Endo-Tricyclodecane,” Chinese Journal of Catalysis, Vol. 31, No. 4, 2010, pp. 383-385. doi:10.1016/S1872-2067(09)60057-7
- W. H. Zhang, J. L. Shi, L. Z. Wang and D. S. Yan, “Preparation and Characterization of ZnO Clusters inside Mesoporous Silica,” Chemistry of Materials, Vol. 12, No. 5, 2000, pp. 1408-1413. doi:10.1021/cm990740a
- R. S. Drago and E. E. Getty, “Preparation and Catalytic Activity of a New Solid Acid Catalyst,” Journal of the American Chemical Society, Vol. 110, No. 10, 1988, pp. 3311-3312. doi:10.1021/ja00218a057
- T. Xu, N. Kob, R. S. Drago, J. B. Nicholas and J. F. Haw, “A Solid Acid Catalyst at the Threshold of Superacid Strength: NMR, Calorimetry, and Density Functional Theory Studies of Silica-Supported Aluminum Chloride,” Journal of American Chemical Society, Vol. 119, No. 50, 1997, pp. 12231-12239. doi:10.1021/ja970850n
- E. P. Parry, “An Infrared Study of Pyridine Adsorbed on Acidic Solids. Characterization of Surface Acidity,” Journal of Catalysis, Vol. 2, No. 5, 1963, pp. 371-379. doi:10.1016/0021-9517(63)90102-7
- T. R. Hughes and H. M. White, “A Study of the Surface Structure of Decationized Y Zeolite by Quantitative Infrared Spectroscopy,” Journal of Physical Chemistry, Vol. 71, No. 7, 1967, pp. 2192-2201. doi:10.1021/j100866a035
NOTES
*Corresponding author.

