World Journal of AIDS
Vol.2 No.4(2012), Article ID:25285,7 pages DOI:10.4236/wja.2012.24037
Thyroid Function and Depression in HIV-1 Infection
![]()
1Department of Psychiatry and Behavioral Medicine, Nova Southeastern University, Fort Lauderdale, USA; 2Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, USA; 3Department of Psychiatry and Behavioral Sciences, Miller School of Medicine, University of Miami, Miami, USA.
Email: ro71@nova.edu
Received October 1st, 2012; revised October 28th, 2012; accepted November 12th, 2012
Keywords: Thyroid; HIV; Depression
ABSTRACT
Thyroid abnormalities have been reported in persons with HIV infection, although data have been inconsistent with respect to its frequency and association with specific medications. The purpose of this study was to explore thyroid system response to thyroid releasing hormone stimulation in persons with and without HIV infection and determine the extent to which their response was associated with depression. As part of a larger study of neuroendocrine response persons with HIV-1 infection, control and HIV-1 infected individuals were evaluated. Participants’ response to TRH stimulation was evaluated via TSH, total T3, and T4 levels at baseline and 15, 30, 45, and 60 minutes after TRH stimulation. Participants with HIV infection had a more robust response to TRH stimulation as measured by higher levels of TSH, lower levels of T4 and modestly higher levels of T3. Depressed persons had a reduced TSH response to stimulation and lower levels of both T4 and T3, although the effect of depression on T4 was not statistically significant. These results suggest that TSH response to TRH-stimulation may be exaggerated in individuals with HIV infection but reduced in those with depression. They also suggest that the effects of depression and HIV infection may interact, and may provide a partial explanation for observed thyroid abnormalities in HIV-infected individuals. Results thus provide a partial explanation for findings on thyroid and depression in those affected by HIV infection.
1. Introduction
HIV-1 infection produces a wide range of effects in the human body including devastating effects on the immune system as well as on multiple other organ systems. HIV-1 affects the central nervous [1-3] producing significant cognitive and psychiatric morbidity. Current treatments for HIV infection may themselves result in metabolic dysfunction [4,5]. As treatment of HIV infection has shifted from a focus on helping patients to survive to its management as a chronic disease, the importance of detecting and managing all of its complications has increased.
Thyroid function in HIV-1+ individuals has increasingly been an area of concern due to reports of thyroid function abnormalities such as hypothyroidism in these patients. Data suggest that HIV-1 infection by itself is associated with thyroid abnormalities and that some antiretroviral (ARV) medications may also be related. Thyroid dysfunction may occur as the result of immune system reconstitution [6,7] and are associated with other viral infections in HIV-1+ patients such as hepatitis C [8]. Several studies have found evidence of abnormal thyroid function in persons with HIV-1 infection [9]. These studies show that patients with HIV-1 may have elevated rates of either overt or subclinical hypothyroidism defined as the presence of elevated TSH with reduced or normal levels of T3 and T4 [10-14]. The clinical manifestations of hypothyroidism, such as fatigue, apathy, and depression, have clear clinical significance for patients’ quality of life as well as their functional status [15,16].
Various authors have suggested that these abnormalities result from infection-related autoimmune processes or ARV therapy [17-20]. Some studies have suggested that treatment with the ARV medication stavudine is specifically associated with increased risk for hypothyroidism [17,20,21]. It should be noted, however, that other investigations have not found that thyroid dysfunction is associated with ARV therapy generally [18,22] or with specific ARV medications [23]. In a small case control study, hypothyroidism was not associated with ARV treatment [18], and another study found no relation between hypothyroidism and any ARV treatment or, specifically, treatment with stavudine [22].
Thyroid abnormalities have also been reported in persons with HIV-1 infection who have not been treated with ARV medications [10,24]. Bongiovanni [25], in a longitudinal study of thyroid function in HIV-1+ patients, found substantial rates of subclinical hypothyroidism in both treated and untreated HIV-1+ patients at baseline and the development of new cases of subclinical hypothyroidism in both untreated persons and patients beginning treatment with ARV medications as both groups were followed over 24 months. The overall picture is complicated by results of two studies of thyroid function in nonclinical samples of HIV-1+ individuals found no evidence of thyroid abnormalities [26,27]. The exact relation of thyroid abnormalities to HIV infection and its treatment is thus unclear.
Several possibilities exist to account for abnormal thyroid function in those affected by HIV-1 infection. Immune reconstitution after the initiation of antiretroviral treatment has been associated with autoimmune phenomena, including increases in thyroid antibodies [6,7]. Six patients with subclinical hypothyroidism in the report by Calza et al. [14], however, had normal levels of thyroid autoantibodies. As already noted, hypothyroidism may be associated with ARV medication use, although no specific mechanism has been elucidated.
A link between thyroid function mood disorders is well established [28,29]. Since the observations that depression can result from hypothyroidism and that antidepressant efficacy is enhanced through the administration of supplemental thyroid hormone [30-32], investigators and clinicians have been aware of the importance of understanding thyroid function in persons with depression.
Depression is common in persons treated for HIV infection [33] and a link between thyroid function mood disorders is well established [28,29]. Estimates of the prevalence of depression in persons with HIV infection vary widely depending on clinical population and method of depression diagnosis, ranging from 0% to as high as 37% [34,35]. Since the observations that depression can result from hypothyroidism and that antidepressant efficacy is enhanced through the administration of supplemental thyroid hormone [30-32], investigators and clinicians have been aware of the importance of understanding thyroid function in persons with depression.
Given the reported incidence of subclinical hypothyroidism in HIV infection and the hypothyroidism to depression, it is possible that thyroid abnormalities may in part account for the prevalence of depression in this population. Most studies of thyroid function in persons with HIV-1 infection have employed a cross-sectional design with measurement of thyroid indices on a single occasion. As reviewed above, studies have yielded conflicting results. Few studies have employed the more sensitive strategy of evaluating individuals’ response to thyroid stimulation via thyrotropin releasing hormone (TRH). Beltran et al. [17] evaluated thyroid response to TRH stimulation in persons with HIV-1 infection and evidence of thyroid abnormalities. Although this study found some evidence of HIV-1 infection related thyroid abnormalities at baseline related to study selection criteria, no differences in thyroid response to TRH were observed.
The relation of the effects of HIV-1 infection and depression on thyroid function may thus be complex and interacting. HIV-1 infection, as well as other viral infections, can cause thyroid function abnormalities. The association of HIV infection and depression may exist for a number of reasons, ranging from the purely psychological to the neuroendocrinological [33]. A better understanding of how these factors interact might provide a better understanding of thyroid abnormalities and depression in this population. The purpose of this study was thus to investigate the relations of HIV infection, depression, and thyroid function. We hypothesized that both HIV-1 infection and depression would be related to indices of thyroid function.
2. Method
Data reported in this paper are drawn from a larger study that focused on immune and endocrine response in HIV-1 infected individuals, all of whom had a history of injecting drug use, and uninfected individuals with or without a history of injecting drug use (IDU). Preliminary analyses suggested that IDU status was not related to thyroid response but was closely related to mood. Analyses that included both depression and IDU status also showed that they were closely related but that inclusion of IDU status did not provide additional information with respect to thyroid response; in no models was IDU status significantly related to thyroid markers. For clarity’s sake, IDU status is thus not included in the analyses reported here. Data on participants’ use of specific ARV medications were not available for use in analyses. The occurrence of hepatitis C infection was not evaluated in this study, and the small number of individuals who reported their status on this variable limited its use in analyses. The presence of antithyroid antibodies among participants was not evaluated and thus data on this issue are not reported.
Participants: Men and women aged 18 to 50 years were enrolled for this study. All participants gave informed consent prior to initiation of the study and all participants were paid for their participation. Potential participants were excluded from participation if they reported a history of head injury with loss of consciousness, learning disability or a history of major psychiatric illness such as schizophrenia or bipolar disorder, hypertension or diabetes mellitus. The study was conducted under a protocol approved by the University of Miami Human Subjects Research Office.
HIV-1 Infection Inclusion and Exclusion Criteria: HIV-1-positive participants were required to bring evidence of their serostatus to the study. Additionally, their peripheral plasma viral load was determined using the PCR amplicor method (Roche Diagnostics; at the Clinical Immunology Laboratory in the Department of Medicine, the University of Miami School of Medicine). HIV-1-positive participants were free of any AIDSdefining infections at the time of the study. Verification of HIV-1 seronegative status was not done as part of this study.
TRH-Stimulation Challenge: On arrival in the morning, each participant was asked to relax in a sitting position in a chair and an intravenous line was inserted in a vein in their arm for collection of blood. Each patient received 100 µg of TRH (Thyril, obtained commercially) and blood samples were collected at baseline and at 15, 30, 45 and 60 minutes after TRH administration.
Depression: Participants’ mood was assessed using the Beck Depression Inventory [36]. The BDI is a widelyused self-report measure of depressive symptoms. Persons were characterized as depressed if they scored 12 or higher on this measure, based on previous studies of the sensitivity and specificity of this or similar cut off scores in other groups of normal and medically ill patients [37,38].
2.1. Laboratory Procedures
Plasma Isolation: Blood was collected in tubes with EDTA added as an anticoagulant. Samples were centrifuged at 1000 g within 30 minutes and stored at −70˚C until analyzed for TSH, total T4 and T3.
Assays: Plasma was assayed using RIA kits obtained commercially (DSL, Webster, Texas) as described by the vendors. The TSH kit used had a sensitivity of 0.04 µIU/ ml and its intraand inter-assay coefficients of variance (CV) were 3.46% and 6.63% respectively. The total T3 assay had a sensitivity of 4.3 ng/dL and intraand interassay CV were 2.2% to 9% and 5.62% to 12.4% respectively. Total T4 assay had a sensitivity of 0.4 µg/dL and its intraand inter-assay CVs were 1.5% to 8.5% and 2.8% to 17.3% respectively.
2.2. Data Analyses
The overall data analysis strategy was to evaluate the relation of HIV infection and depression to thyroid function over time using repeated measures analysis of covariance (ANCOVA) models. In order to control for possible confounding variables, models included age, gender, and body mass index (BMI). Interactions among the two main factors in these analyses (HIV, depression) were explored to evaluate the extent to which their interactions would be related to thyroid function.
Dependent variables (TSH, T3, and T4) were first natural log transformed to satisfy linearity assumptions underlying ANCOVA. Separate ANCOVA models were created for each outcome (TSH, T4 and T3) across assessments, with all possible twoand three-way interactions explored in preliminary analyses. No significant between-subjects interactions for TSH, T4, or T3 were found, and final models include only main effects for factors and covariates. The within-subjects effect for change over time was significant only for TSH response, as were several of the interactions of factors and covariates with time and are reported. None of the within-subjects effects was statistically significant for T4 or T3 response and are not reported here.
3. Results
Descriptive statistics for the sample are presented in Table 1 for both categorical and continuous variables. The final ANOVA model for TSH is presented in Table 2 and graphically in Figures 1 and 2 (all figures present model-corrected estimated values).
Both HIV-1 infection status and depression were associated with TSH response to TRH stimulation, but in
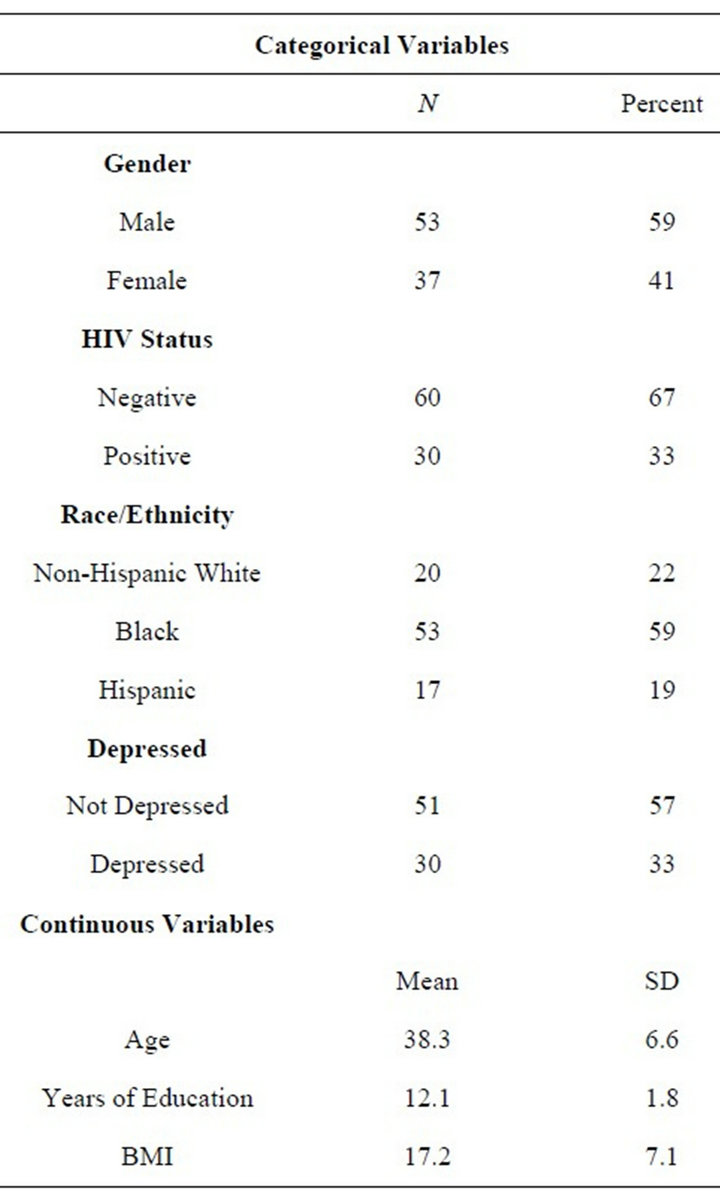
Table 1. Descriptive statics.
opposite directions. Persons who were HIV-1+ had a greater TSH response to stimulation (Figure 3) while those with depression had a reduced response (Figure 1) relative to those without either condition. Significant
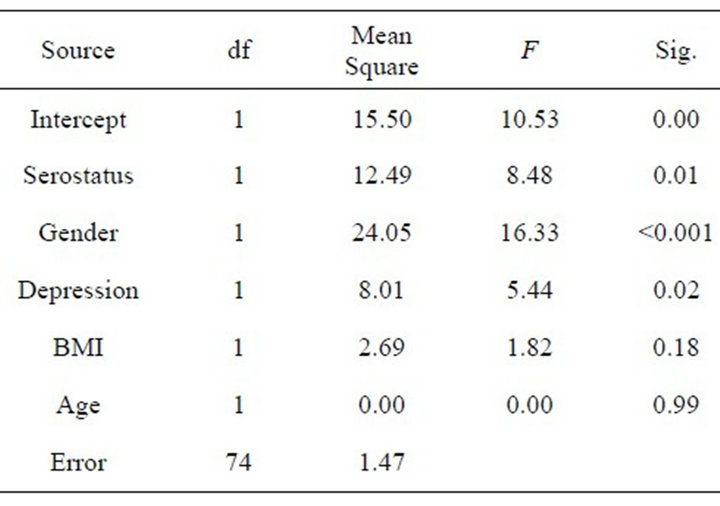
Table 2. Between-subjects effects for TSH.
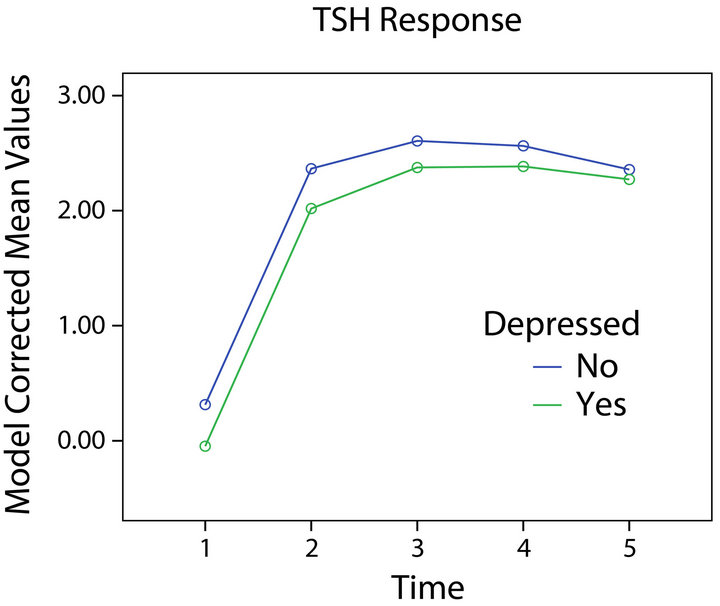
Figure 1. TSH response and depression.
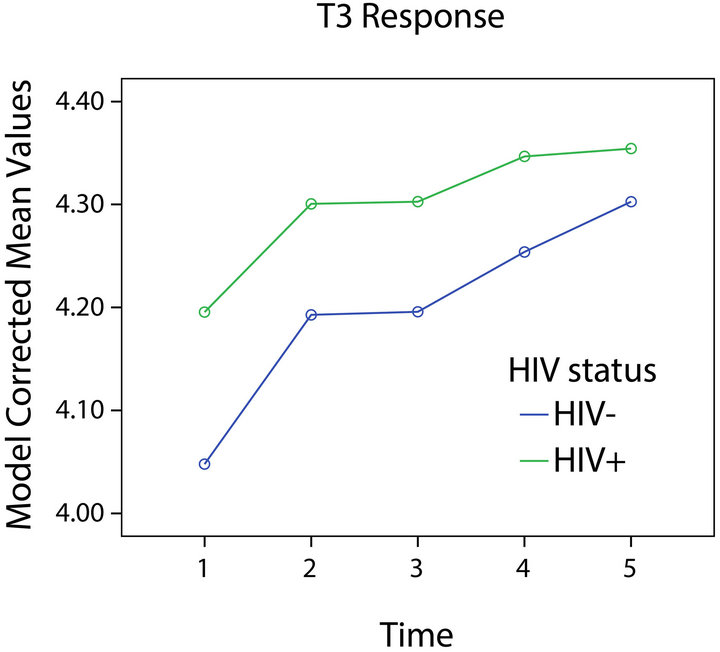
Figure 2. T3 response and HIV status.
interactions of time course of TSH response with HIV-1 infection status (F = 2.99, df = 4, 296, p = 0.02), gender (F = 3.30, df = 4, 296, p = 0.01), depression (F = 2.79, df = 4, 296, p = 0.03), and age (F = 3.01, df = 4, 296, p = 0.02) were found, suggesting that the pattern of individuals’ TSH response over time varied with respect to these variables.
The model for T3 is presented in Table 3 and Figures 2 and 4. Significant effects for depression, BMI, and age were found. No significant interaction of time with any variable was found. Depressed participants had lower levels of T3, while those with and without HIV-1 infection did not differ significantly.
Neither HIV status nor depression was significantly related to T4 response (Full model not shown; HIV status F[1,65] = 0.49, p = 0.49; Depression F[1,65] = 1.00, p = 0.32). There was a significant effect for gender (with women having higher levels of T4). No significant interactions of time with other variables were obtained.
Inspection of the plot of T4 response for those with
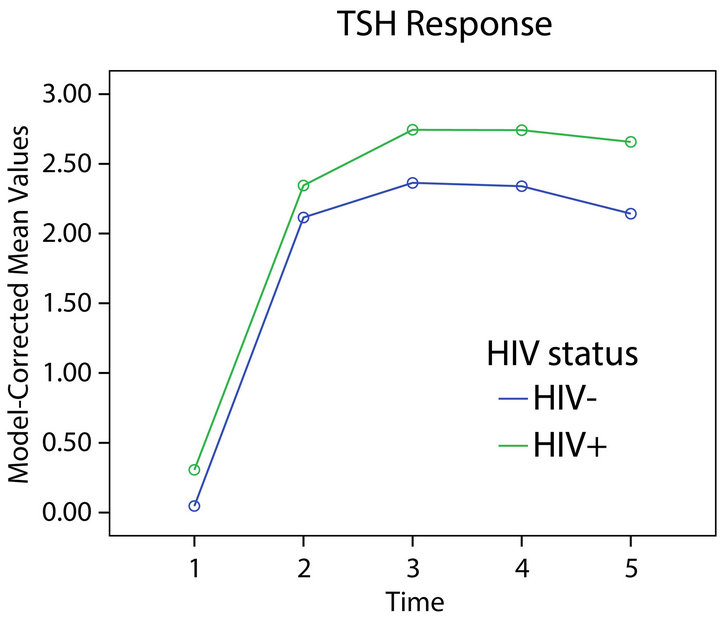
Figure 3. TSH response and HIV status.
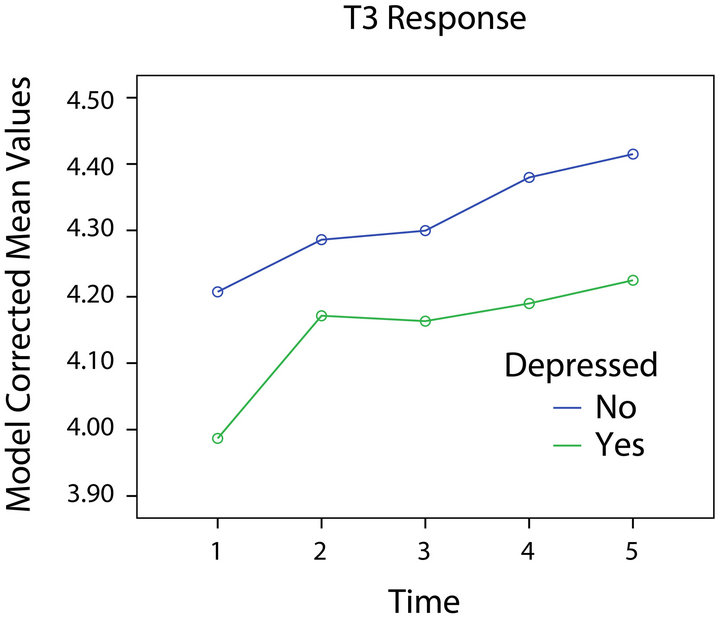
Figure 4. T3 response and depression.
and without depression suggested that depressed participants had lower levels of T4 across times (Figure 5). The plot of T4 response in relation to HIV status was similar. These observations raise the possibility that the failure to find statistical significance might have been the result of low power due to our sample size.
4. Discussion
The purpose of this study was to evaluate the effects of HIV-1 infection and depression on thyroid function in a clinically relevant population. Our results show that both HIV-1 infection and depression may have significant but contrasting impacts on thyroid function. These results are summarized in Table 4.
These findings present the possibility that two independent processes affect thyroid response to stimulation among persons with HIV-1 infection. The infection itself is associated with a pattern of thyroid function consistent with increased level of T3 but a reduced production of T4 in response to stimulation (an increased response of TSH to TRH stimulation with reduced T4 response). This pattern would be consistent with intact increases in T3 in response to TSH stimulation but reduced effectiveness of conversion of T4 to T3 in the periphery. In contrast, in this study depression was associated with a reduced TSH response to stimulation and lower levels of both T3 and T4. These findings might be consistent with a central effect for depression and a peripheral effect of HIV infection on T4 conversion. This might reflect the direct action of HIV infection of peripheral tissues and the hypothesized link between the hypothalamic-pituitary (HPA) axis and thyroid function, possibly via an autoimmune process [39,40]. This interpretation must be advanced tentatively, as several of the observed effects were not statistically significant.
These findings are consistent with the only other read-
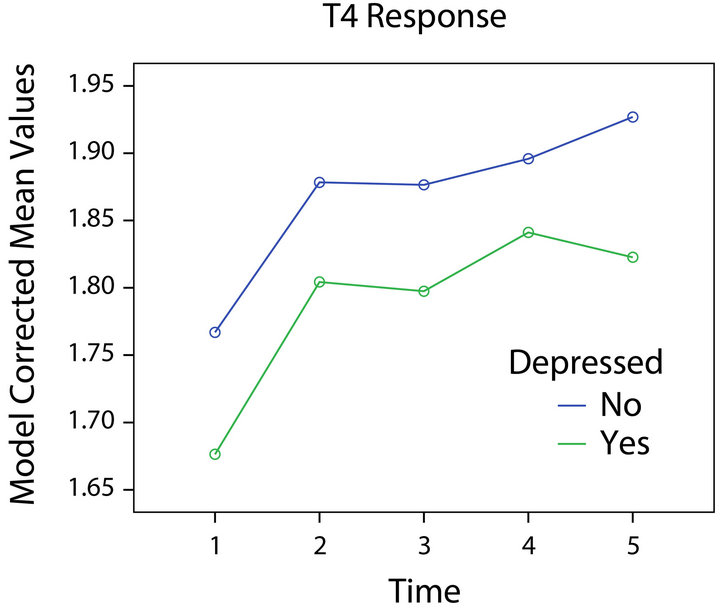
Figure 5. T4 response and depression.
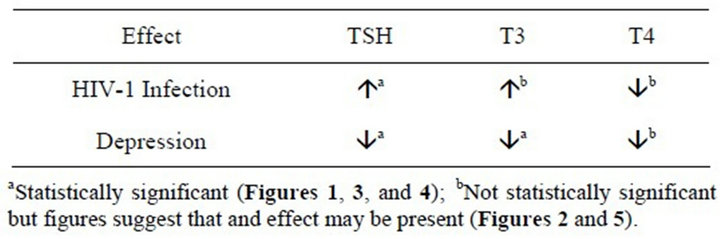
Table 4. Summary of findings.
ily-identifiable study of stimulated thyroid function in persons with HIV-1 infection [17]. In this study, Beltran and colleagues found high TSH combined with low T4 levels in some patients with HIV-1 infection, but her results were based only on participants preselected as having either subclinical hypothyroidism or normal TSH levels with low T4 compared with a euthyroid control group. This study, however, did not show significant differences in thyroid response. Due to their small sample size, Beltran et al. used a nonparametric statistical analysis strategy that did not allow them to control for potential confounding variables such as gender and BMI, as done in our study.
Our results provide a possible explanation for conflicting findings in cross sectional studies of thyroid function in HIV-1 infected individuals. This study’s statistical design allowed us to control for several possible confounders, including age, gender, and BMI as well as to take the effects of depression into account while evaluating the effects of HIV-1 infection on thyroid function. These results show that factors such as BMI and gender may have a significant impact on indices of thyroid function and should be considered in future studies. Further, the significant and contrasting effects of HIV-1 infection and depression on thyroid function show that mood may be an important confounder in studies of thyroid function in persons with HIV-1 infection.
Limitations of this study include our inability to evaluate the effects of specific ARV medications on thyroid status, the complex nature of our sample that included persons with and without a history of IDU, and the small number of persons with data on hepatitis C infection that limited our ability to take this factor into account. Further, as with IDU status, hepatitis C infection was closely tied to whether participants were depressed (data not presented), raising the possibility that all of these factors are interrelated. Our sample size was not sufficient to permit us to statistically disentangle the effects of each of these factors.
Another limitation is our lack of data on the presence of antithyroid antibodies in participants. Increased occurrence of antithyroid antibodies has been linked both to depression and HIV-1 infection [41,42] and thus might account in part for the current findings. Increased frequency of antithyroid antibodies has been found in several samples of HIV-1 infected individuals [6,7] as part of immune reconstitution. On the other hand, Beltran and her colleagues [17] in her sample found no evidence of antithyroid antibodies in her sample of HIV-1 infected persons with either subclinical hypothyroidism or low T4 levels. The possible role of antithyroid antibodies in HIV-related thyroid dysfunction is thus unclear.
In summary, results of this study suggest that both HIV infection and depression may be related to indices of thyroid function in persons with HIV-1 infection but perhaps by difference mechanisms. Analyses also indicate that several other variables, including gender, age, and body mass index may be important in understanding the relations among these variables. These results thus confirm others’ observations of a relation of thyroid function to HIV-1 infection and the well-established relation of thyroid function to depression. Our findings emphasize the importance of thyroid function in HIV-1 infection and highlight the possibly complex interaction between the infection and depression.
5. Acknowledgements
This study was supported by a grant from the National Institute on Drug Abuse to Dr. Mahendra Kumar (RO1 DA13550).
REFERENCES
- A. Antinori, G. Arendt, J. T. Becker, et al., “Updated Research Nosology for HIV-Associated Neurocognitive Disorders,” Neurology, Vol. 69, No. 18, 2007, pp. 1789- 1799. doi:10.1212/01.WNL.0000287431.88658.8b
- I. Grant, “Neurocognitive Disturbances in HIV,” International Review of Psychiatry, Vol. 20, No. 1, 2008, pp. 33-47. doi:10.1080/09540260701877894
- M. Reger, R. Welsh, J. Razani, et al., “A Meta-Analysis of the Neuropsychological Sequelae of HIV Infection,” Journal of the International Neuropsychological Society, Vol. 8, No. 3, 2002, pp. 410-424. doi:10.1017/S1355617702813212
- J. A. Aberg, “Cardiovascular Risk Among HIV-Positive Patients on Antiretroviral Therapy,” Journal of International Association of Physicians in AIDS Care (Chic Ill), Vol. 2, Suppl. 2, 2003, pp. S24-S39.
- G. Barbaro and G. Barbarini, “Highly Active Antiretroviral Therapy-Associated Metabolic Syndrome and Cardiovascular Risk,” Chemotherapy, Vol. 52, No. 4, 2006, pp. 161-165. doi:10.1159/000093034
- F. Chen, S. L. Day, R. A. Metcalfe, et al., “Characteristics of Autoimmune Thyroid Disease Occurring as a Late Complication of Immune Reconstitution in Patients With Advanced Human Immunodeficiency Virus (HIV) Disease,” Medicine (Baltimore), Vol. 84, No. 2, 2005, pp. 98-106. doi:10.1097/01.md.0000159082.45703.90
- V. Jubault, A. Penfornis, F. Schillo, et al., “Sequential Occurrence of Thyroid Autoantibodies and Graves’ Disease after Immune Restoration in Severely Immunocompromised Human Immunodeficiency Virus-1-Infected Patients,” Journal of Clinical Endocrinology & Metabolism, Vol. 85, No. 11, 2000, pp. 4254-4257. doi:10.1210/jc.85.11.4254
- A. Antonelli, C. Ferri, P. Fallahi, et al., “Thyroid Disorders in Chronic Hepatitis C Virus Infection,” Thyroid, Vol. 16, No. 6, 2006, pp. 563-572. doi:10.1089/thy.2006.16.563
- C. J. Hoffmann and T. T. Brown, “Thyroid Function Abnormalities in HIV-Infected Patients,” Clinical Infectious Diseases, Vol. 45, No. 4, 2007, pp. 488-494. doi:10.1086/519978
- T. Quirino, M. Bongiovanni, E. Ricci, et al., “Hypothyroidism in HIV-Infected Patients Who Have or Have Not Received HAART,” Clinical Infectious Diseases, Vol. 38, No. 4, 2004, pp. 596-597. doi:10.1086/381442
- W. W. Tang and E. M. Kaptein, “Thyroid Hormone Levels in the Acquired Immunodeficiency Syndrome (AIDS) or AIDS-Related Complex,” West Journal of Medicine, Vol. 151, No. 6, 1989, pp. 627-631.
- S. Beltran, F. X. Lescure, R. Desailloud, et al., “Increased Prevalence of Hypothyroidism among Human Immunodeficiency Virus-Infected Patients: A Need for Screening,” Clinical Infectious Diseases, Vol. 37, No. 4, 2003, pp. 579-583. doi:10.1086/376626
- M. Wiener, Y. Lo and R. S. Klein, “Abnormal Thyroid Function in Older Men with or at Risk for HIV Infection,” HIV Medicine, Vol. 9, No. 7, 2008, pp. 544- 549. doi:10.1111/j.1468-1293.2008.00601.x
- L. Calza, R. Manfredi and F. Chiodo, “Subclinical Hypothyroidism in HIV-Infected Patients Receiving Highly Active Antiretroviral Therapy,” Journal of Acquired Immune Deficiency Syndromes, Vol. 31, No. 3, 2002, pp. 361-363. doi:10.1097/00126334-200211010-00014
- J. Barroso, “A Review of Fatigue in People with HIV Infection,” Journal of the Association of Nurses in AIDS Care, Vol. 10, No. 5, 1999, pp. 42-49. doi:10.1016/S1055-3290(06)60342-7
- J. Barroso, J. R. Carlson and J. Meynell, “Physiological and Psychological Markers Associated with HIV-Related Fatigue,” Clinical Nursing Research, Vol. 12, No. 1, 2003, pp. 49-68. doi:10.1177/1054773803238740
- S. Beltran, F. Lescure, I. El Esper, et al., “Subclinical Hypothyroidism in HIV-Infected Patients Is Not an Autoimmune Disease,” Hormone Research, Vol. 66, No. 1, 2006, pp. 21-26. doi:10.1159/000093228
- S. Afhami, V. Haghpanah, R. Heshmat, et al., “Assessment of the Factors Involving in the Development of Hypothyroidism in HIV-Infected Patients: A CaseControl Study,” Infection, Vol. 35, No. 5, 2007, pp. 334- 338. doi:10.1007/s15010-007-6163-3
- M. Nelson, T. Powles, A. Zeitlin, et al., “Thyroid Dysfunction and Relationship to Antiretroviral Therapy in HIV-Positive Individuals in the HAART Era,” Journal of Acquired Immune Deficiency Syndromes, Vol. 50, No. 1, 2009, pp. 113-114. doi:10.1097/QAI.0b013e31818ce835
- G. Madeddu, A. Spanu, F. Chessa, et al., “Thyroid Function in Human Immunodeficiency Virus Patients Treated with Highly Active Antiretroviral Therapy (HAART): A Longitudinal Study,” Clinical Endocrinology (Oxf), Vol. 64, No. 4, 2006, pp. 375-383.
- M. Grappin, L. Piroth, B. Verges, et al., “Increased Prevalence of Subclinical Hypothyroidism in HIV Patients Treated with Highly Active Antiretroviral Therapy,” AIDS, Vol. 14, No. 8, 2000, pp. 1070-1072. doi:10.1097/00002030-200005260-00026
- S. Madge, C. J. Smith, F. C. Lampe, et al., “No Association between HIV Disease and Its Treatment and Thyroid Function,” HIV Medicine, Vol. 8, No. 1, 2007, pp. 22-27. doi:10.1111/j.1468-1293.2007.00422.x
- J. Collazos, S. Ibarra, and J. Mayo, “Thyroid Hormones in HIV-Infected Patients in the Highly Active Antiretroviral Therapy Era: Evidence of an Interrelation between the Thyroid Axis and the Immune System,” AIDS, Vol. 17, No. 5, 2003, pp. 763-765. doi:10.1097/00002030-200303280-00019
- G. Jain, G. Devpura and B. S. Gupta, “Abnormalities in the Thyroid Function Tests as Surrogate Marker of Advancing HIV Infection in Infected Adults,” Journal of the Association of Physicians of India, Vol. 57, 2009, pp. 508-510.
- M. Bongiovanni, F. Adorni, M. Casana, et al., “Subclinical Hypothyroidism in HIV-Infected Subjects,” Journal of Antimicrobial Chemotherapy, Vol. 58, No. 5, 2006, pp. 1086-1089. doi:10.1093/jac/dkl360
- J. Barroso, B. W. Pence, N. Salahuddin, et al., “Physiological Correlates of HIV-Related Fatigue,” Clinical Nursing Research, Vol. 17, No. 1, 2008, pp. 5-19. doi:10.1177/1054773807311382
- N. H. Brockmeyer, A. Kreuter, A. Bader, et al., “Prevalence of Endocrine Dysfunction in HIV-Infected Men,” Hormone Research, Vol. 54, No. 5-6, 2000, pp. 294-295. doi:10.1159/000053274
- I. M. Jackson, “The Thyroid Axis and Depression,” Thyroid, Vol. 8, No. 10, 1998, pp. 951-956. doi:10.1089/thy.1998.8.951
- C. Kirkegaard and J. Faber, “The Role of Thyroid Hormones in Depression,” European Journal of Endocrinology, Vol. 138, No. 1, 1998, pp. 1-9. doi:10.1530/eje.0.1380001
- R. Cooper-Kazaz, J. T. Apter, R. Cohen, et al., “Combined Treatment With Sertraline and Liothyronine in Major Depression: A Randomized, Double-Blind, Placebo-Controlled Trial,” Archives of General Psychiatry, Vol. 64, No. 6, 2007, pp. 679-688. doi:10.1001/archpsyc.64.6.679
- T. Kelly and D. Z. Lieberman, “The Use of Triiodothyronine as an Augmentation Agent in TreatmentResistant Bipolar II and Bipolar Disorder NOS,” Journal of Affective Disorders, Vol. 116, No. 3, 2009, pp. 222- 226. doi:10.1016/j.jad.2008.12.010
- T. F. Kelly and D. Z. Lieberman, “Long Term Augmentation with T3 in Refractory Major Depression,” Journal of Affective Disorders, Vol. 115, No. 1-2, 2009, pp. 230-233. doi:10.1016/j.jad.2008.09.022
- R. L. Ownby, R. J. Jacobs, D. Waldrop-Valverde, et al., “Depression Care and Prevalence in HIV-Positive Individuals,” Journal of Neurobehavioral HIV Medicine, Vol. 2, 2010, pp. 73-83. doi:10.2147/NBHIV.S7296
- J. A. Ciesla and J. E. Roberts, “Meta-Analysis of the Relationship between HIV Infection and Risk for Depressive Disorders,” American Journal of Psychiatry, Vol. 158, No. 5, 2001, pp. 725-730. doi:10.1176/appi.ajp.158.5.725
- E. G. Bing, M. A. Burnam, D. Longshore, et al., “Psychiatric Disorders and Drug Use among Human Immunodeficiency Virus-Infected Adults in the United States,” Archives of General Psychiatry, Vol. 58, No. 8, 2001, pp. 721-728. doi:10.1001/archpsyc.58.8.721
- A. T. Beck, C. H. Ward, M. Mendelson, et al., “An Inventory for Measuring Depression,” Archives of General Psychiatry, Vol. 4, No. 6, 1961, pp. 561-571. doi:10.1001/archpsyc.1961.01710120031004
- T. Forkmann, T. Vehren, M. Boecker, et al., “Sensitivity and Specificity of the Beck Depression Inventory in Cardiologic Inpatients: How Useful Is the Conventional Cut-Off Score?” Journal of Psychosomatic Research, Vol. 67, No. 4, 2009, pp. 347-352. doi:10.1016/j.jpsychores.2009.04.003
- P. J. Lustman, R. E. Clouse, L. S. Griffith, et al., “Screening for Depression in Diabetes Using the Beck Depression Inventory,” Psychosomatic Medicine, Vol. 59, No. 1, 1997, pp. 24-31.
- K. N. Fountoulakis, A. Iacovides, P. Grammaticos, et al., “Thyroid Function in Clinical Subtypes of Major Depression: An Exploratory Study,” BMC Psychiatry, Vol. 4, 2004, p. 6. doi:10.1186/1471-244X-4-6
- M. P. Hage and S. T. Azar, “The Link between Thyroid Function and Depression,” Journal of Thyroid Research, Vol. 2012, 2012, Article ID: 590648. doi:10.1155/2012/590648
- C. B. Nemeroff, J. S. Simon, J. J. Haggerty Jr., et al., “Antithyroid Antibodies in Depressed Patients,” American Journal of Psychiatry, Vol. 142, No. 7, 1985, pp. 840-843.
- G. Zandman-Goddard and Y. Shoenfeld, “HIV and Autoimmunity,” Autoimmunity Reviews, Vol. 1, No. 6, 2002, pp. 329-337. doi:10.1016/S1568-9972(02)00086-1

