World Journal of Vaccines
Vol.3 No.2(2013), Article ID:31501,7 pages DOI:10.4236/wjv.2013.32006
Identification of Growth Promoting Effect of rBCG/BCG Culture Supernatant and Its Potential Applications
![]()
Aeras, Rockville, USA.
Email: *zjin@aeras.org
Copyright © 2013 Tom H. Jin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 27th, 2013; revised March 30th, 2013; accepted April 10th, 2013
Keywords: Resuscitation-Promoting Factors (Rpfs); rBCG/BCG; Culture
ABSTRACT
Background: Although BCG is the most widely administered vaccine in the world, there have never been as many cases of TB as there are now. Globally, more than 8.8 million people developed active TB and 1.4 million—many of them—died in 2010. It is estimated that half of pulmonary TB cases arise from latent Mtb infection, making the study of latency and reactivation of utmost importance. Methods: Widely administered BCG vaccines and a gene modified recombinant BCG (rBCG) strain, AERAS-422, were used as models to investigate the growth promoting function of resuscitation-promoting factors (Rpfs) in different bacilli culture phases. Different supernatant fractions were prepared by ultrafiltration, and the promoting function of each fraction containing secreted Rpf(s) was evaluated by growth curve monitoring and colony counting on 7H10 agar plates. Results: The promoting effect of culture supernatants was mainly associated with the high molecular weight fraction (>30 kDa), which stimulated bacterial growth, but did not extend the exponential phase of stimulated culture. Anti-RpfB antibody showed significant growth restriction of the tested cultures. When comparing rBCG cultures containing 7H9 medium, the 10 - 30 kDa fraction, or the >30 kDa fraction, only the >30 kDa fraction was displayed with down-regulation of the secretion of RpfC, D and E. In colony counting tests, the plates containing the >30 kDa fraction had total countable colony numbers 2 to 3 fold higher than the plates with the 10 - 30 kDa fraction, and colonies appeared one to two weeks earlier than on the regular plates. The potential applications of the prepared supernatant fractions containing RpfA and RpfB are discussed, which may include accelerating diagnosis of Mtb infection and future TB vaccine development.
1. Introduction
Tuberculosis (TB) is a serious and highly contagious disease affecting millions of people worldwide. Despite efforts to control and treat tuberculosis, in 2010, more than 8.8 million people developed active TB and 1.4 million—many of them—died [1]. Mycobacterium tuberculosis (M. tuberculosis or Mtb), the bacterium that causes tuberculosis, is estimated to be harbored by one-third of the world’s population [2]. It is believed that the bacilli of Mtb are able to persist in vivo for long periods by entering into a latent state from which they may reactivate to cause the active pulmonary form of TB and also transmit the infection to others. It is estimated that globally half of pulmonary TB cases arise from latent Mtb infection, making the study of latency and reactivation of utmost importance [3,4].
A major breakthrough has been the discovery of resuscitation-promoting factor (Rpf) from Micrococcus luteus, which is an extremely potent anti-dormancy factor [5-9]. It was shown recently that Mtb also expresses 5 Rpf proteins (RpfA, B, C, D, and E) that are homologous to Rpf, and have a strong similarity to a lysozyme-like enzyme previously implicated in reactivation of dormant bacteria both in vitro and in vivo [10-12]. The Rpf-like proteins of M. tuberculosis stimulate bacterial growth in laboratory culture at very low (pM) concentrations, which effectively excludes the possibility that they are simply being used as nutrients [11]. The rpf gene homologues of M. tuberculosis, Rv0867c (rpfA), Rv1009 (rpfB), Rv1884c (rpfC), Rv2389c (rpfD), and Rv2450c (rpfE), have been demonstrated to stimulate the growth of stationary-phase mycobacteria, including Mycobacterium bovis BCG and M. tuberculosis [13-15]. However, the growth promoting function of each Rpf to latency bacilli, and the relationship of the five Rpfs to M. tuberculosis growth, have not been clearly elucidated. The application of the promoting function of these proteins has not been extensively studied. We propose that the widely used TB vaccine, the attenuated strain named bacilli of Calmette and Guérin (BCG), and a gene modified recombinant BCG (rBCG) strain, might be good models to fulfill this purpose. In this study, different supernatant fractions containing secreted Rpf(s) were prepared from BCG or rBCG culture, and the promoting function of each fraction with respect to different stages of bacterial growth was evaluated. The potential applications of Rpfs identified by Western Blot, especially RpfA and RpfB, are discussed.
2. Materials and Methods
2.1. BCG Strains and Growth Condition
AERAS-422 rBCG (Ag85A, Ag85B, Rv3407, ∆ureC: pfoAG137Q, ∆panCD) is a live attenuated rBCG strain being developed by Aeras [16,17]. Danish BCG 1331 was bought from Statens Serum Institut, Denmark. Chinese BCG strain was a gift from Shanghai Institute of Biological Products, China. Bacteria were inoculated in Middle brook 7H9 medium (BD, USA) plus the following components: 0.2% Na glutamate (EMD), 0.05% Tyloxapol (Sigma), 2% Glycerol, 0.03% ZnSO4, 2% MgSO4 (J T Baker) and 2% Dextrose (Fisher Scientific). Baffled bottom conical flasks were used with 125 rpm at 37 ˚C. 7H10 agar plates were bought from BD, USA.
2.2. Preparation of Culture Supernatant and Fractions
2.2.1. Centrifugation and Filter Sterilization
Supernatant was prepared from the fifth day culture of rBCG/BCG when Optical Density (OD) reached 4.5 ± 0.5. The culture was centrifuged at 2400 × g for 20 min. The supernatant was filter sterilized on a 0.22 µm member after being slowly poured out in a Biological Safety cabinet.
2.2.2. One Step Cut-Off Ultrafiltration
A one step cut-off ultrafiltration was used to prepare different fractions of molecular weight (MW) greater than 10 kDa, 30 kDa and 100 kDa (>10 kDa, >30 kDa, and >100 kDa, respectively) of the filter-sterilized supernatant. The supernatant was transferred to a 200 mL cup ultrafiltration device (Amicon, Millipore) equipped with a 10 kDa, 30 kDa or 100 kDa membrane. Nitrogen gas was connected to force the supernatant through the membrane. When retentate volume was reduced to around 10 mL, the ultrafiltration cup was gently washed and refilled up to 200 mL with fresh 7H9 Middlebrook medium broth (VWR). The ultrafiltration process was repeated two more times. The final retentate volume was adjusted to achieve 25-fold concentration based on the starting volume. The ultrafiltration process was performed in a 2˚C to 8˚C room. The final prepared fractions were filtersterilized using a 0.22 µm membrane.
2.2.3. Two Step Cut-Off Ultrafiltration
The supernatant fraction with MW between 10 and 30 kDa was prepared the same way as the one step cut-off ultrafiltration, except 30 kDa and 10 kDa membranes were used in sequence. After the filter-sterilized supernatant passed through the 30 kDa membrane, the filtrate containing < 30 kDa fraction of the supernatant was continually ultrafiltered on the 10 kDa membrane. The final retentate of the 10 to 30 kDa fraction (10 - 30 kDa) was adjusted to achieve 25-fold concentration based on the starting supernatant volume, and then filter-sterilized.
2.3. Growth Promoting Test with Prepared Fractions of Culture Supernatant
10 mL of each filter-sterilized supernatant fractions (MW of >10 kDa, 10 - 30 kDa, >30 kDa, and >100 kDa) were added to 190 mL 7H9 medium. rBCG or BCG was inoculated at a starting concentration of 107 CFU/mL. Two hundred mL of 7H9 broth only was used as a control. To eliminate the growth interference of left-over nutrition, the target fraction was concentrated and re-suspended with 7H9 medium in triplicate during the ultrafiltration process. OD at 600 nm of each culture was measured daily from 48 hr post-inoculation.
To select the optimum promoting volume of the >30 kDa fraction for bacterial growth, rBCG was inoculated and cultured 7H9 medium containing 5, 10 or 20 mL of the >30 kDa fraction.
2.4. Growth Promoting Test on 7H10 Agar Plate
Thawed rBCG frozen bulk was serially diluted to 10−6, 10−7 and 10−8 with 7H9 medium broth. 100 µL diluted bacteria were mixed with 50 µL of the >30 kDa or 10 - 30 kDa filter-sterilized fraction, or 50 µL 7H9 broth as a control. Then 100 µL of the mixture was directly dropped or spread by sterile glass beads on 7H10 plates. The resulting colonies were counted on day 14 or 21, and representative photographs were taken by Protocol Colony Counter (Microbiology Inc.) (12). There were a total of 27 plates used in three dilutions for each sample lot; the average CFU counting from each lot was calculated, and representative plates are shown.
2.5. Antibody Inhibition Assay
The antibody inhibition assay was executed with antiRpfA and/or anti-RpfB rabbit serum. The process is the same as “Growth promoting test on 7H10 agar plate,” described above, except that 2 µL or 5 µL of anti-RpfB rabbit serum, 5 µL of anti-RpfA rabbit serum, or 5 µL of both anti-RpfA and anti-RpfB rabbit sera were first mixed with 998 µL (1:500) or 995 µL (1:200) diluted bacteria (E + 6 or E + 7), then 300 µL of the mixture was transferred and mixed with 150 µL of the >30 kDa or 10 - 30 kDa supernatant fraction, or 150 µL 7H9 broth as a control, with a final volume of plating at 100 µL, plated in triplicate. The plates were incubated at 37˚C for 21 days; the resulting colonies were counted by Protocol Colony Counter. The average CFU counting from each group was calculated.
2.6. Inter-Regulation Function among Rpfs
The bacteria were grown as described above in “Growth promoting test with prepared fractions of culture supernatant”. From the fourth to the eleventh day, samples were taken from each culture (7H9 medium, 7H9 medium with the >30 kDa fraction or 10 - 30 kDa fraction) to detect the inter-regulation function of different Rpfs by Western Blotting.
3. Results
3.1. Growth Promoting (GP) Effect of Ultrafiltration Treated Fractions from Culture Supernatant
3.1.1. Fraction with MW of >10 kDa or >100 kDa
The GP effect of culture supernatant was first tested using the >10 kDa or >100 kDa fraction. rBCG was inoculated in parallel in culture medium of 7H9 or 7H9 plus the >10 kDa or >100 kDa supernatant (Figure 1). The culture inoculated with the >10 kDa supernatant fraction grew faster than the regular 7H9 medium and medium containing the >100 kDa fraction after inoculation. At the 125th hour, the OD600 nm of rBCG was 6.44 for the culture
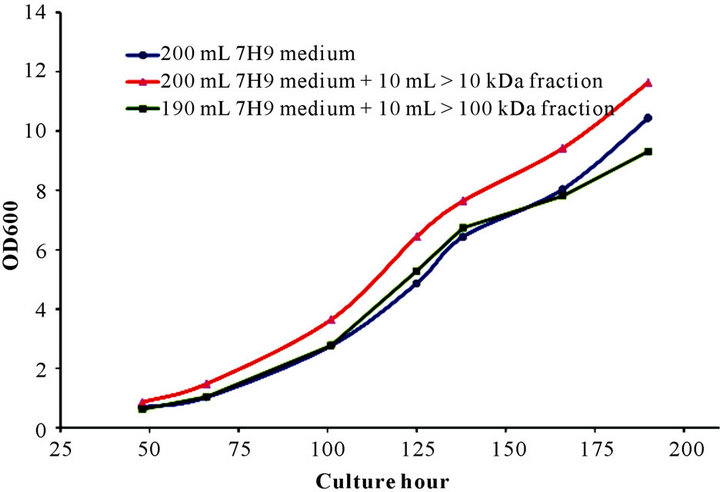
Figure 1. Growth promoting effect of >10 kDa or >100 kDa fraction of culture supernatant. Recombinant BCG was inoculated and cultured in 200 mL 7H9 medium or 190 mL 7H9 medium plus 10 mL of >10 kDa or >100 kDa fraction generated from culture supernatant.
with the >10 kDa fraction compared to 4.86 for the 7H9 medium only and 5.28 for the culture containing the >100 kDa supernatant. The same trend was maintained until the endpoint of testing. The growth curves of rBCG cultured in medium and medium containing the >100 kDa fraction were similar.
3.1.2. Fraction with MW of >30 kDa or 10 to 30 kDa
The GP effect of culture supernatant was narrowed down to fractions with MW of 10 to 30 kDa and >30 kDa (Figure 2) (rBCG, Danish BCG 1331 and Chinese BCG displayed the same growth pattern, data not shown). From around 95 hr, the culture inoculated with the >30 kDa fraction started to grow faster than medium alone or medium with the 10 to 30 kDa fraction. At 140 hr, its OD600 nm reached 8.6, which was significantly higher than the other two cultures (6.45 for the medium only and 6.72 for the culture with 10 to 30 kDa fraction). This faster growth pattern was maintained during the entire exponential phase. The culture inoculated with the 10 - 30 kDa fraction showed a delayed fast growth tendency from around 200 to 245 hr, however, this fast growth only lasted a short period of time. During 245 to 260 hr, all three cultures quickly fell into their death phase. However, the bacterial growth with the >30 kDa fraction dropped significantly faster compared to the other two cultures.
3.1.3. Optimum Promoting Volume of >30 kDa Fraction
The optimum promoting volume of the >30 kDa fraction for growth of rBCG in 200 mL culture was tested in 7H9 medium containing 5, 10 or 20 mL of the prepared >30 kDa fraction (Figure 3). All cultures inoculated with the >30 kDa fraction components grew consistently faster
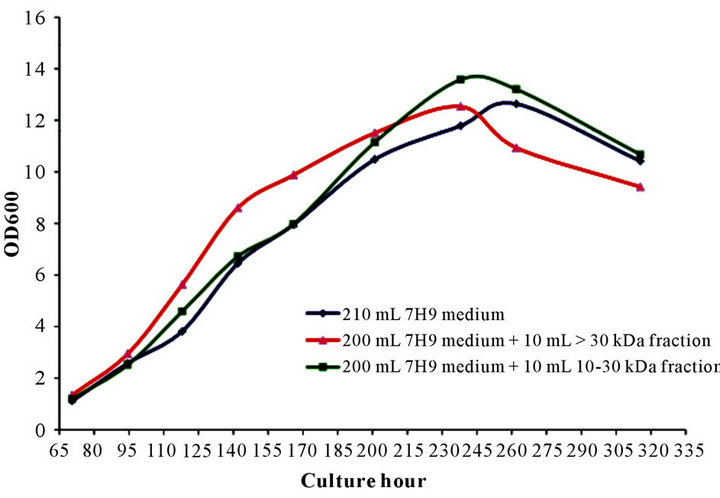
Figure 2. Growth promoting effect of >30 and 10 - 30 kDa fractions of culture supernatant. Recombinant BCG were inoculated and cultured in 200 mL 7H9 medium or 190 mL 7H9 medium plus 10 mL of >30 kDa or 10 - 30 kDa fractions generated from culture supernatant.
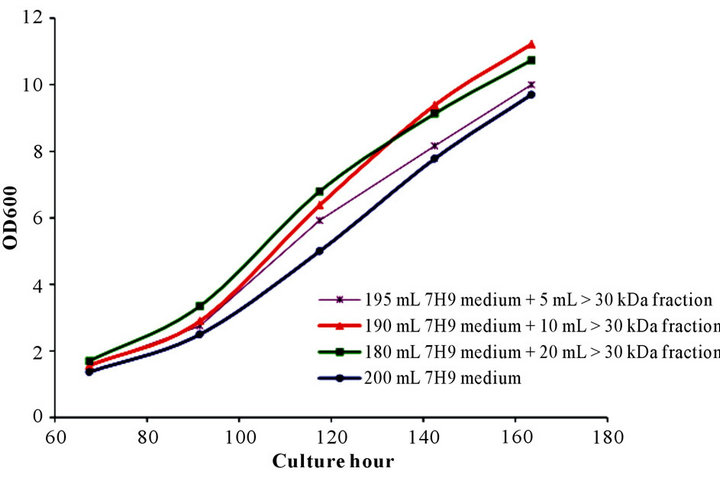
Figure 3. Dose/volume and growth response pattern induced by >30 kDa fraction of culture supernatant. Recombinant BCG was inoculated and cultured in 7H9 medium or 7H9 medium containing 5, 10 or 20 mL of >30 kDa fraction generated from culture supernatant.
than the control medium inoculated culture. A dose/ volume response pattern was observed, with increased volume of the >30 kDa fraction resulting in increased GP effect through around 130 hr. At 144 hr, growth of the culture inoculated with 20 mL of the >30 kDa fraction slowed and was overtaken by the culture inoculated with 10 mL of the >30 kDa fraction. The increased growth of the culture inoculated with 10 mL vs 20 mL of the >30 kDa fraction was maintained through the final time point at 164 hr. The optimum protoming volume of 10 mL of the >30 kDa fraction mixed with 190 mL 7H9 medium was selected for the rest of the Rpf studies.
3.2. Growth Promoting Test on 7H10 Agar Plate
When a diluted bacterial mixture containing the >30 kDa fraction was dropped or spread on 7H10 agar plates, the colonies were observed clearly on the 14th day, with colonies appearing one to two weeks earlier than the ones on the regular 7H10 plates (data not shown). After the bacteria mixed with the >30 kDa, or 10 - 30 kDa fraction, or control medium, were spread and incubated at 37˚C for 3 weeks, the countable colony number (CN) from the plate having the >30 kDa fraction was 326 (at serial dilution of E + 6; Figure 4(a)), which was 2 to 3 fold higher than the plate containing the 10 - 30 kDa fraction (CN of 112) or the regular 7H10 plate (CN of 121), or (Figures 4(b) and (c)).
3.3. Growth Inhibition by Anti-RpfB
The growth inhibition effect of anti-RpfA and anti-RpfB was investigated in rBCG plating samples containing the >30 kDa supernatant fraction. As expected, anti-RpfB showed significant inhibition of cell growth stimulated by the >30 kDa fraction. After the plates were incubated at 37˚C for 21 days, the colony count was 137 (at serial
 (a)
(a) (b)
(b) (c)
(c)
Figure 4. Growth promoting effect of different supernatant fractions detected on 7H10 plate. (a) (CN: 326): 100 µL diluted rBCG mixed with 50 µL of >30 kDa fraction and spread on 7H10 plate; (b) (CN: 112): 100 µL diluted rBCG mixed with 50 µL of 10 - 30 kDa fraction and spread on 7H10 plate; (c) (CN: 121): 100 µL diluted rBCG directly spread on regular 7H10 plate. Note: the plates were counted at the 21 day (dilution at E + 6); CN: countable colony number.
dilution of E + 7) for the plate without anti-RpfB, and 74 or 19 for the plate having 2 µL or 5 µL, respectively, of pre-mixed anti-RpfB. Adding anti-RpfB did not totally inhibit bacterial growth on the solid agar plates. In a further test, adding 5 µL anti-RpfA instead of anti-RpfB did not show a growth inhibition effect, with an average CFU of 71, which was similar to the control without antiRpfA and anti-RpfB (CFU = 70). However, adding 5 µL of both anti-RpfA and anti-RpfB resulted in reduction of the CFU count to 12.
3.4. Down-Regulation Function of >30 kDa Fraction
As shown in Figure 5, during the culture period from day 4 to 11, the secretion of RpfC, RpfD and RpfE was down-regulated (samples in lane 2) when bacteria were grown in 7H9 medium containing the >30 kDa supernatant fraction. This down-regulation phenomenon was
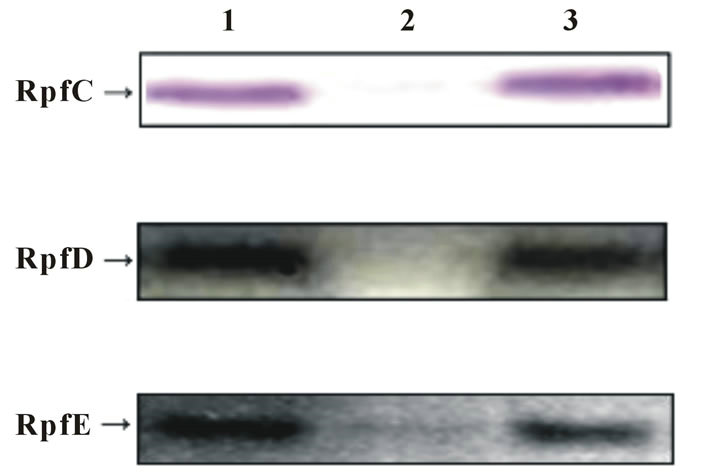
Figure 5. Growth inter-regulation function of different supernatant fractions. Lane 1: RpfC, D and E were examined in the culture grown in 7H9 medium; Lane 2: RpfC, D and E were examined in the culture grown in 7H9 medium containing > 30 kDa supernatant fraction; Lane 3: RpfC, D and E were examined in the culture grown in 7H9 medium containing 10 - 30 kDa fraction. Note: the inspection of each culture was executed from day 4 to 11, and the band patterns were similar.
not detected by Western Blotting in samples from the cultures of regular 7H9 medium (lane 1) and 7H9 medium containing the 10 - 30 kDa fraction (lane 3).
4. Discussion
Rpf is a secreted and muralytic enzyme that increases the culturability of dormant bacteria [5,18,19]. Recently, considerable progress has been made in understanding the structure, function and physiological role of Rpfs in different organisms, most notably the major human pathogen, Mycobacterium tuberculosis, which encodes multiple rpf-like genes [11,12]. A key unresolved question, however, concerns the relationship between the predicted biochemical activity of Rpfs—cleavage of the β-1,4 glycosidic bond in the glycan backbone of peptidoglycan—and the their effect on culturability. In M. tuberculosis, the interacting protein A (RipA) enables Rpfs to synergistically degrade peptidoglycan to facilitate growth. Furthermore, the combined action of Rpfs with RipA and other peptidoglycan hydrolases might produce muropeptides that could exert diverse biological effects through host and/or bacterial signaling, the latter involving serine/threonine protein kinases [20-25]. This suggests that the activation of latent Mtb requires peptidoglycan hydrolysis, which either alters the mechanical properties of the cell wall to facilitate cell division or releases lysis products that function as anti-dormancy signals.
Our data showed that only the high MW, but not the low MW fraction resulted in an apparent GP effect in bacterial growth and division. The data suggest that RpfA and/or RpfB were/was playing a key role in enhancing cell growth. Several studies have resulted in similar conclusions. Wu et al. demonstrated that RpfB could promote proliferation of M. tuberculosis in liquid and on solid media [26]. Tufariello et al. reported that of the five Rpf deletion mutants being tested, the RpfB deletion exhibited a delayed reactivation phenotype, manifested by delayed post-reactivation growth kinetics and prolonged median survival times among infected animals [14,15]. Russell-Goldman et al. showed that a double RpfA and RpfB deletion mutant exhibited reactivation deficiency in vivo. They found the reactivation deficiency of ∆rpfAB was more severe and persisted longer than that of a single RpfB or RpfA deletion in chronically Mtb-infected mice [27]. In M. tuberculosis, the role of rpfB appears to be non-redundant in pathogenesis [15]. However, it still needs to be clarified whether RpfB or RpfB plus RpfA in combination with RipA could fulfill complete GP function for bacilli to grow or reactivate. Anti-RpfB showed significant inhibition of cell growth stimulated by the >30 kDa fraction. Although adding anti-RpfB could not totally inhibit bacterial growth on solid agar plates, our data showed that the major GP function to stimulate BCG bacilli to multiply and divide was generated from RpfB.
The next important question is what is the relationship among Rpf proteins? The gene expression profiling of strains with a single rpf deletion was reported by Downing et al. [28]. They showed that there was a significant overlap or functional redundancy in the differential gene expression profile of rpfB to rpfE (but not rpfA) mutants. In addition, five cognate proteins could stimulate bacterial growth at picomolar concentrations [11]. Our study showed a different activation pattern among these Rpf proteins. Comparing the rBCG cultures with 7H9 medium, and 7H9 medium plus the 10 - 30 or >30 kDa supernatant fraction, only the culture with the >30 kDa fraction containing RpfA and RpfB could completely inhibit the secretions of RpfC to RpfE from day 4 to 11 (during exponential phase). This suggests that RpfB and/ or RpfA were/was involved in down-regulating the expression of rpfC to rpfE in order to permit their promoting effect to fully function. Gene expression of rpf may be regulated in a growth phase-dependent manner. This important regulation needs further tests to clarify.
The Rpfs identified in BCG/rBCG culture supernatants, especially RpfB and RpfA, have varied potential applications: 1) as tools to study the important role in controlling the growth, latency and reactivation of Mtb; 2) adding growth-promoting factor(s) in medium could reduce culture incubation time, increase the accuracy of the CFU potency test, and reduce the construction period of a rBCG candidate strain; 3) incorporating Rpfs into fermentation medium could improve the manufacturing productivity of live BCG/rBCG vaccine, if the culture is being harvested at growth phase with a relatively high proportion of viable cells; 4) the Rpf family might be employed in early diagnosis of Mtb infection or as vaccine components to provide interesting opportunities to treat and prevent mycobacterial infections.
In conclusion, the high MW and low MW fractions of BCG/rBCG culture supernatant were prepared by a stepultrafiltration process. Only the high MW fraction containing RpfA and RpfB showed an apparent promoting effect of bacterial growth. Adding the high MW fraction into cell culture resulted in inhibition of expression and down-regulation of low MW Rpfs. Further study of Rpfs could expand the understanding of latency and reactivation of M. tuberculosis, thereby permitting accelerated diagnosis of Mtb infection and future TB vaccine development.
5. Acknowledgements
The authors are grateful to Barbara Shepherd for her critical review of the manuscript. This work was supported by the Bill & Melinda Gates Foundation.
REFERENCES
- World Health Organization, “Global Tuberculosis Control Report 2011,” 2011.
- B. R. Bloom and C. J. Murray, “Tuberculosis: Commentary on a Reemergent Killer,” Science, Vol. 257, No. 5073, 1992, pp. 1055-1064. doi:10.1126/science.257.5073.1055
- S. M. Arend and J. T. van Dissel, “Evidence of Endogenous Reactivation of Tuberculosis after a Long Period of Latency,” The Journal of Infectious Diseases, Vol. 186, No. 6, 2002, pp. 876-877. doi:10.1086/342604
- J. Chan and J. Flynn, “The Immunological Aspects of Latency in Tuberculosis,” Clinical Immunology, Vol. 110, No. 1, 2004, pp. 2-12. doi:10.1016/S1521-6616(03)00210-9
- G. V. Mukamolova, A. S. Kaprelyants, D. I. Young, M. Young, D. B. Kell, “A Bacterial Cytokine,” Proceeding National Academy Science, Vol. 95, No. 15, 1998, pp. 8916-8921. doi:10.1073/pnas.95.15.8916
- G. V. Mukamolova, S. S. Kormer, D. B. Kell and A. S. Kaprelyants, “Stimulation of the Multiplication of Micrococcus Luteus by an Autocrine Growth Factor,” Archives of Microbiology, Vol. 172, No. 1, 1999, pp. 9-14. doi:10.1007/s002030050733
- G. V. Mukamolova, A. G. Murzin, E. G. Salina, G. R. Demina, D. B. Kell, A. S. Kaprelyants and M. Young, “Muralytic Activity of Micrococcus Luteus Rpf and Its Relationship to Physiological Activity in Promoting Bacterial Growth and Resuscitation,” Molecular Microbiology, Vol. 59, No. 1, 2006, pp. 84-98. doi:10.1111/j.1365-2958.2005.04930.x
- G. V. Mukamolova, O. A. Turapov, K. Kazarian, M. Telkov, A. S. Kaprelyants, D. B. Kell and M. Young, “The Rpf Gene of Micrococcus Luteus Encodes an Essential Secreted Growth Factor,” Molecular Microbiology, Vol. 46, No. 3, 2002, pp. 611-621. doi:10.1046/j.1365-2958.2002.03183.x
- G. V. Mukamolova, N. D. Yanopolskaya, D. B. Kell and A. S. Kaprelyants, “On Resuscitation from the Dormant State of Micrococcus Luteus,” Antonie Van Leeuwenhoek, Vol. 73, No. 3, 1998, pp. 237-243. doi:10.1023/A:1000881918216
- S. Biketov, G. V. Mukamolova, V. Potapov, E. Gilenkov, G. Vostroknutova, D. B. Kell, M. Young and A. S. Kaprelyants, “Culturability of Mycobacterium tuberculosis Cells Isolated from Murine Macrophages: A Bacterial Growth Factor Promotes Recovery,” FEMS Immunology Medical Microbiology, Vol. 29, No. 4, 2000, pp. 233-240. doi:10.1111/j.1574-695X.2000.tb01528.x
- G. V. Mukamolova, O. A. Turapov, D. I. Young, A. S. Kaprelyants, D. B. Kell and M. Young, “A Family of Autocrine Growth Factors in Mycobacterium Tuberculosis,” Molecular Microbiology, Vol. 46, No. 3, 2002, pp. 623-635. doi:10.1046/j.1365-2958.2002.03184.x
- V. V. Yeremeev, T. K. Kondratieva, E. I. Rubakova, S. N. Petrovskaya, K. A. Kazarian, M. V. Telkov, S. F. Biketov, A. S. Kaprelyants and A. S. Apt, “Proteins of the Rpf Family: Immune Cell Reactivity and Vaccination Efficacy against Tuberculosis in Mice,” Infection and Immunity, Vol. 71, No. 8, 2003, pp. 4789-4794. doi:10.1128/IAI.71.8.4789-4794.2003
- R. K. Gupta, B. S. Srivastava and R. Srivastava, “Comparative Expression Analysis of Rpf-Like Genes of Mycobacterium tuberculosis H37Rv Under Different Physiological Stress and Growth Conditions,” Microbiology, Vol. 156, No. Pt9, 2010, pp. 2714-2722. doi:10.1099/mic.0.037622-0
- J. M. Tufariello, W. R. Jacobs Jr. and J. Chan, “Individual Mycobacterium tuberculosis Resuscitation-Promoting Factor Homologues are Dispensable for Growth in Vitro and in Vivo,” Infection and Immunity, Vol. 72, No. 1, 2004, pp. 515-526. doi:10.1128/IAI.72.1.515-526.2004
- J. M. Tufariello, K. Mi, J. Xu, Y. C. Manabe, A. K. Kesavan, J. Drumm, K. Tanaka, W. R. Jacobs Jr. and J. Chan, “Deletion of the Mycobacterium tuberculosis Resuscitation-Promoting Factor Rv1009 Gene Results in Delayed Reactivation from Chronic Tuberculosis,” Infection and Immunity, Vol. 74, No. 5, 2006, pp. 2985-2995.
- H. J. Jin, L. Nguyen, T. Qu and E. Tsao, “Improved Formulation and Lyophilization Cycle for rBCG Vaccine,” Vaccine, 2011, Vol. 29, No. 29-30, pp. 4848-4852. doi:10.1016/j.vaccine.2011.04.056
- R. Sun, Y. A. Skeiky, A. Izzo, V. Dheenadhayalan, Z. Imam, E. Penn, et al., “Novel Recombinant BCG Expressing Perfringolysin O and the Over-expression of Key Immunodominant Antigen; Pre-Clinical Characterization, Safety and Protection against Challenge with Mycobacterium tuberculosis,” Vaccine, Vol. 27, No. 33, 2009, pp. 4412-4423. doi:10.1016/j.vaccine.2009.05.048
- M. O. Shleeva, K. Bagramyan, M. V. Telkov, G. V. Mukamolova, M. Young, D. B. Kell and A. S. Kaprelyants, “Formation and Resuscitation of ‘Non-Culturable’ Cells of Rhodococcus Rhodochrous and Mycobacterium tuberculosis in Prolonged Stationary Phase,” Microbiology, Vol. 148, No. Pt5, 2002, pp. 1581-1591. doi:10.1016/j.tube.2010.12.006
- M. O. Shleeva, G. V. Mukamolova, M. Young, H. D. Williams and A. S. Kaprelyants, “Formation of ‘NonCulturable’ Cells of Mycobacterium Smegmatis in Stationary Phase in Response to Growth under Suboptimal Conditions and Their Rpf-mediated Resuscitation,” Microbiology, Vol. 150, No. Pt6, pp. 1687-1697. doi:10.1099/mic.0.26893-0
- M. Cohen-Gonsaud, P. Barthe, C. Bagnéris, B. Henderson, J. Ward, C. Roumestand and N. H. Keep, “The Structure of a Resuscitation-promoting Factor Domain from Mycobacterium tuberculosis Shows Homology to Lysozymes,” Nature Structural & Molecular Biology, Vol. 12, No. 3, 2005, pp. 270-273. doi:10.1038/nsmb905
- E. C. Hett, M. C. Chao, L. L. Deng and E. J. Rubin, “A Mycobacterial Enzyme Essential for Cell Division Synergizes with Resuscitation-Promoting Factor,” PLoS Pathogens, Vol. 4, No. 2, 2008, pp. 1-8. doi:10.1371/journal.ppat.1000001
- E. C. Hett, M. C. Chao, A. J. Steyn, S. M. Fortune, L. L. Deng and E. J. Rubin, “A Partner for the ResuscitationPromoting Factors of Mycobacterium tuberculosis,” Molecular Microbiology, Vol. 66, No. 3, 2007, pp. 658-668. doi:10.1111/j.1365-2958.2007.05945.x
- A. Ruggiero, D. Marasco, F. Squeglia, S. Soldini, E. Pedone and C. Pedone, “Structure and Functional Regulation of RipA, a Mycobacterial Enzyme Essential for Daughter Cell Separation,” Structure, Vol. 18, No. 9, 2010, pp. 1184-1190. doi:10.1016/j.str.2010.06.007
- A. Ruggiero, F. Squeglia, C. Esposito, D. Marasco, E. Pedone, C. Pedone and R. Berisio, “Expression, Purification, Crystallization and Preliminary X-Ray Crystallographic Analysis of the Resuscitation Promoting Factor Interacting Protein RipA from M. tuberculosis,” Protein & Peptide Letters, Vol. 17, No. 1, 2010, pp. 70-73. doi:10.2174/092986610789909557
- A. Ruggiero, B. Tizzano, E. Pedone, C. Pedone, M. Wilmanns and R. Berisio, “Crystal Structure of the Resuscitation-Promoting Factor (DeltaDUF) RpfB from M. tuberculosis,” Journal of Molecular Biology, Vol. 385, No. 1, 2009, pp. 153-162. doi:10.1016/j.jmb.2008.10.042
- X. Wu, Y. Yang, Y. Han, J. Zhang, Y. Liang, H. Li, B. Li and L. Wang, “Effect of Recombinant Rv1009 Protein on Promoting the Growth of Mycobacterium tuberculosis,” Journal of Applied Microbiology, Vol. 205, No. 4, 2008, pp. 1121-1127. doi:10.1111/j.1365-2672.2008.03850.x
- E. Russell-Goldman, J. Xu, X. Wang, J. Chan and J. M. Tufariello, “A Mycobacterium tuberculosis Rpf DoubleKnockout Strain Exhibits Profound Defects in Reactivation from Chronic Tuberculosis and Innate Immunity Phenotypes,” Infection and Immunity, Vol. 76, No. 9, 2008, pp. 4269-4281. doi:10.1128/IAI.01735-07
- K. J. Downing, J. C. Betts, D. I. Young, R. A. McAdam, F. Kelly, M. Young and V. Mizrahi, “Global Expression Profiling of Strains Harbouring Null Mutations Reveals That the Five Rpf-Like Genes of Mycobacterium tuberculosis Show Functional Redundancy,” Tuberculosis, Vol. 84, No. 3-4, 2004, pp. 167-179. doi:10.1016/j.tube.2003.12.004
NOTES
*Corresponding author.

