World Journal of Vaccines
Vol. 2 No. 2 (2012) , Article ID: 19310 , 12 pages DOI:10.4236/wjv.2012.22009
Evaluation of the Kinetic Change of the Immunogenicity of Dengue-2 DNA Vaccine in Mice Administered by Different Administration Routes
![]()
1The Institute of Cellular and Organismic Biology, Academia Sinica, Taipei, Taiwan; 2Graduate Institute of Microbiology and Public Health, College of Veterinary Medicine, National Chung-Hsing university, Taichung, Taiwan; 3Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, USA; 4Institute of Medical Technology, School of Medicine, National Taiwan University, Taipei, Taiwan.
Email: *dychao@nchu.edu.tw
Received December 23rd, 2011; revised January 26th, 2012; accepted February 28th, 2012
Keywords: Dengue; DNA vaccine; immunological response; gene gun delivery
ABSTRACT
A plasmid DNA vaccine is able to induce both humoral and cellular immune responses; however, the kinetic change of the Th1/Th2 response, antibody avidity, cytokine secretion, and neutralization activity after different priming and boosting strategies have not been evaluated. A plasmid DNA, designated pCBD2 and previously shown to efficiently induce an immune response very similar to that by a wild type virus, was evaluated kinetically in this study. Our results suggest that a DNA vaccine delivered by the gene gun (gg) route produced higher and longer DENV-2-specific antibody titers than those induced through the intramuscular (im) route. Although the gg group induced a Th2 response and im delivery induced a Th1 response, priming by gg delivery, followed by a boosting by im delivery, did not shift the immune response from a Th2 to Th1 response. Furthermore, the antibody avidity (AI) measured by ELISA demonstrated a gradual increase of AI from low (AI range from 6.8% - 9.6%) on day 42 to high (AI value > 30) on day 119 in all but the gene-gun immunization group, in which an AI value of 23 was observed. Although there was lower avidity in the gg group, the mice sera from all three groups of mice demonstrated significant neutralization activity. This is the first report about the kinetics of immunogenicity of a DNA vaccine through different administration strategies, which suggests that gene gun delivery of a DNA vaccine can induce an immune response containing both neutralizing and nonneutralizing antibodies at high titers important for neutralization.
1. Introduction
Dengue is a mosquito-borne viral disease in humans and is a significant public health threat in the tropics and subtropics, where billions of people are at risk and an estimated 100 million new infections occur each year [1]. There are four dengue virus serotypes, named dengue virus serotype 1 (DENV-1) through dengue virus serotype 4 (DENV-4). These viruses form an antigenically distinct subgroup within the flavivirus family [2]. One infected by any of these viruses may be either asymptomatic or inflicted with a self-limited febrile illness known as dengue fever (DF). In a small percentage of cases, however, infection results in a life-threatening dengue hemorrhagic fever or dengue shock syndrome (DHF/DSS) [3]. Despite the magnitude of public health implications DF and DHF have, vector control, which has proven difficult and costly to sustain over time, is currently the only available control measure [4]. Vaccines are one of the most promising strategies for preventing DF and DHF, although they are currently unavailable for dengue viruses [5].
DENV are enveloped, RNA viruses that encode 10 proteins; three are structural proteins: capsid (C), membrane (M) with its precursor (pr), and envelope (E), and seven are nonstructural (NS) proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 [6]. In flaviviruses, the E protein is involved in a number of important functions related to viral infection, such as receptor binding and membrane fusion [7]. E protein antibodies neutralize virus activity in vitro and in vivo [8,9]. Furthermore, subviral particles consisting only of prM and E proteins are highly effective in generating a protective immune response against DENV in mice [10]. Recent studies suggested that most of the virus-neutralizing epitopes, important in immunity, are located on the virion surface of the structural E glycoprotein and are the target for which the vaccine is designed [11].
There are currently no licensed vaccines available for dengue infection, although several vaccine candidates are in pre-clinical and clinical trials [5,12,13]. The leading candidates are live-attenuated dengue virus vaccines (LAV) in Phase 2 clinical trials in the United States and Thailand [5,14]. Attenuated vaccines are inexpensive and produce long-term durations in immune responses; however, interference among the four dengue viruses during propagation in vaccinated hosts may cause an imbalanced immune response to four serotypes, which may cause an elevated disease severity following subsequent dengue virus infections. There are continued efforts for developing alternative vaccine candidates [15]. DNA vaccine technology is a novel approach for preventing infectious diseases [16]. Inoculating animals with purified plasmid vectors (DNA) via the intramuscular (im) or intradermal (i.d.) route leads to the expression of the recombinant vector-encoded protein in transfected cells, resulting in the stimulation of a protein-specific immune response. Such responses include induction of antibodies, generation of CD4 Thelper lymphocytes and CD8 cytotoxic lymphocytes (CTL), and protection against a range of viral infections [17]. Recent studies demonstrate that DNA vaccines containing full-length prM and E (prM-E) genes of dengue-1 and dengue-2 viruses are immunogenic in mice [18-21], and that a dengue-1 prM-E DNA vaccine is also immunogenic and partially protective in rhesus and Aotus monkeys [22]. DNA vaccines that encode antigenic proteins have immunized against dengue viruses, flaviviruses such as Murray Valley, encephalitis (MVE), the encephalitis virus (JEV), and tick-born encephalitis viruses (TBEV) successfully in experiments [23-27]. These vaccines are likely to be inexpensive, safe, and easy to produce.
Since DNA vaccines are able to induce both humoral and cellular immune responses, an important consideration in the application of DNA vaccines is the immunization routes employed. DNA vaccinations were performed predominantly using two different methods: i.d./im injection and gene gun delivery. Injection delivers purified DNA into the extracellular space for uptake by a limited numbers of muscle fibers, while particle bombardment using gene gun delivers DNA directly into the intraepidermal cells where the professional antigen presenting Langerhans cells (LCs) are situated [28]. Gene gun delivery requires a smaller amount of DNA compared to the injection method. Furthermore, a few studies have suggested that the two vaccination techniques differ in the immune response induced [29]. While injections tend to induce a Th1 response, the gene gun response is more of a Th2 response. A lot of efforts has been trying to reverse the predominantly Th2 immune response after gene gun delivery by co-delivery of the IL-2, IL-7 or IFN-γ genes [30-32]. It is unknown whether the Th1-type response can be enhanced through DNA vaccination priming by gene gun delivery and boosted by im injection.
In formal studies, we have identified a potential membrane retention region between E-397 and E-436 of the DENV-2 virus E protein [10]. A chimeric plasmid was formed by replacing the membrane-anchoring region of the E protein, E-397 to E450, with the corresponding region in the Japanese encephalitis virus (JEV). This DEN-2-JE virus chimeric plasmid, consisting of prM and 80% E (E-1 to E-397) of DENV-2, followed by 20% E from JEV (corresponding to DENV-2 E-397 to E450), secreted prM/M and E proteins efficiently in plasmid transformed COS-1 cells. The chimeric DENV-2 plasmid can form subviral particles (SVP), whose immunogenicity is very similar to that of a wild type virus. Also, the immune response after im immunization of DENV-2 chimeric plasmids correlated with the efficiency of prM/ M and E secretion. A similar chimeric DNA plasmid has also been constructed to produce SVP in the St. Louis encephalitis virus (SLEV) and the West Nile virus (WNV), which protects from wild type virus challenges in mice and horses after DNA immunization [33].
Our present work deals with the characterization of the antibody responses induced in mice immunized with a recombinant plasmid encoding the prM and E genes of dengue virus type 2, administered either i.m or i.d with different priming and boosting strategies. The effects of the immune response and antibody avidity elicited by different immunization regimens and/or inoculation routes were analyzed and compared. This is the first report concerning the simultaneous comparison of different administration routes for delivering a DNA vaccine, and adds further support for the utility of dengue DNA vaccines.
2. Materials and Methods
2.1. Cells and Viruses
The Hawaii strain of DENV-1, the 16681 strain of DENV-2, the H87 strain of DENV-3, and the H241 strain of DENV-4 were used. Baby hamster kidney (BHK)-21 cells were grown at 37˚C in Dubelco’s medium (D-MEM) with 10% FBS, and were used to determine the wild-type viruses in tissue culture supernatants for the plaque assay and the 50% plaque reduction neutralization test (PRNT- 50), as described previously [34]. C6/36 cells derived from mosquito cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown at 28˚C in MEM with 10% FBS and amino acids, to grow wild-type DEN viruses. Culture fluids harvested from C6/36 mosquito cells infected with each of these viruses were used for neutralization tests and ELISA experiments. For T-cell stimulation assays, the culture fluids were further purified by centrifugation at 20,000 rpm for two hours at 4˚C, and the equivalent amount of purified viral antigens and VLPs determined at OD 1.2 was used for stimulation.
2.2. Construction of the Plasmid and Virus-Like Particle (VLP) Expression
The pcDNA3-based vaccine plasmids encoding the prM and E genes of the DENV-2 16681 strain, afterwards named pCBD2, have been constructed as described before [10]. A mixture of 100 µg of DNA was used as the DNA vaccine. The DNA was purified using a plasmid DNA purification kit and was kept in sterile distilled water at a concentration of 1 mg/mL for transfection. The VLPs of DENV-2 were expressed by electroporating pCBD2 into COS-1 cells and then partially purifying from clarified tissue culture medium by ultracentrifugation at 19,000 rpm for 8 to 16 hours, as previously described [35]. The purified DENV-2 VLPs were used as antigens in ELISA and the T-cell stimulation assay.
2.3. Preparation of Cartridges for Genetic Immunization
For the pCBD2 plasmid DNA to be tested, 25 mg of 1.6-micron gold powder was weighed into a microcentrifuge tube. One hundred microliters of 50 mM spermidine (Sigma-Aldrich, St. Louis, MO) was added to the tube, and the gold was resuspended by vortexing and brief centrifugation. Twenty-five micrograms of pCBD2 plasmid DNA was added to the tube, followed by the addition of 100 µL of 10% CaCl2 (Sigma-Aldrich, St. Louis, MO) while gently vortexing to effective precipitation of the DNA onto the gold beads. The precipitation reaction was allowed to proceed for 10 minutes on the bench top, after which the gold beads were collected by brief microcentrifugation and washed three times with absolute ethanol (J.T. Bakers Inc., Gardena, CA) to remove excess precipitation reagents. The washed goldDNA complex was then resuspended in a solution of 0.05 mg/mL of polyvinylpyrrolidone (PVP) (360 kD; Sigma Chemicals, Inc.) in absolute ethanol to a volume of 3.6 mL (for mice). This slurry was injected into a TefzelR tube (McMaster-Carr, Chicago, IL) that was positioned in a tube turner to coat the inside of the TefzelR tube with the gold-DNA complex. After the tube turning procedure was completed and the ethanol dried, the tubes were cut into 0.5-inch shots of vaccine, which were stored at 4˚C in the presence of desiccators. Each shot contained 0.25 µg of DNA (a function of the amount of gold per shot and the DNA:gold ratio), a parameter that was previously established to be nearly optimal for genetic immunizations [36]. At least one hour before use, the shots were moved to room temperature and loaded into the XR1 gene gun device (Bio-Rad, Hercules, CA.) for delivery.
2.4. Animals and Immunization
Twelve female BALB/c mice 4 - 6 weeks old, purchased from the Laboratory Animal Center of National Taiwan University, College of Medicine, will be accommodated to the environment seven days before study. The mice will be divided into three groups to evaluate the induction of antibody and protective immunity: group gg will be primed and boosted with DNA through gene gun delivery, group im will be primed and boosted with DNA through intramuscular delivery, and group gg + im will have gene gun delivery first followed by one shot of intramuscular delivery 3 weeks later. The use of animals has been reviewed and fully complied with the Committee of Animal Review of the institute. For gene gun immunization with a pCBD2 DNA vaccine, a hand-held, helium-driven Helios gene delivery system (Bio-Rad, Hercules, CA) will be used. Gene gun injection into the abdominal epidermis will be performed using a helium pressure setting of 400 lb/inch2. All animals from each group were primary immunized by pCBD2 with the designated delivery routes. For higher immunization efficiency, a booster immunization will be given three to four weeks after the primary immunization. The booster doses contain the same amount of DNA as the primary dose. For intramuscular immunization, 100 µg of plasmid at a concentration of 1 µg/µL in PBS will be administered, and another booster immunization with the same concentration as the primary will be given 3 weeks later. For gene gun delivery, each mouse will be given 4 shots for each immunization. Mice were bled from the retroorbital sinus 2 and 4 weeks after primary and before booster immunization, and also at 2, 3, 2, 4 week intervals after booster immunization. The serum samples were evaluated for DENV-2-specific antibodies by ELISA and PRNT as described below.
2.5. Dengue Virus Plaque-Reduction Neutralizing Antibody Assay (PRNT)
Various dilutions of serum samples from the immunized mice were prepared in EMEM not containing FCS and antibiotics. Diluted serum was incubated at 56˚C for 30 min. to inactivate the complement. The serum sample (100 mL) was then mixed with an equal volume of DENV-2 culture supernatant containing 30 pfu of the virus. The virus-antibody mixture was incubated at 4˚C for 1 h before it was added to a 24-well plate containing a 70% confluent monolayer of BHK-21 cells. The plates were incubated at 37˚ for 1 h with gentle rocking every 15 min. The wells were then overlaid with 0.5 mL of 0.6% methylcellulose prepared in E-MEM supplemented with 1% FCS and incubated at 37˚ in 5% CO2 for 5 days. Plaques were stained with 1% crystal violet and counted. The 50% PRNT titers (PRNT50) were calculated as the reciprocal of the highest dilution resulting in a 50% reduction in plaques compared to that of a control virus with no added antibodies as determined by probit analysis. A pool of the sera of animals that were collected before they were primed was used as the negative control for PRNT.
2.6. ELISA
The ELISA used to study the antibody response was performed in a 96-well plate. DENV-2 infected C6/36 cells, or purified DENV-2 E-containing virus-like particles (VLP), were used as the antigens. The DENV-2 VLP antigens were independently titrated against a positive control serum sample with a twofold dilution series and standardized by selecting a dilution that yielded an absorbance of 0.8 to 1.2 at 450 nm (A450). The C6/36 cell-based antigen preparation was performed as follows. 1 × 104 C6/36 cells were seeded into each well of a 96-well plate. The next day, the 16681 strain of DENV-2 with multiplicity of infection (m.o.i) 0.1 was used to infect the cells at 37˚C for 1 h. The wells were then washed with PBS and cultured in MEM with 10% FBS at 28˚C for 7 days before being fixed with 10% acetone in PBS at 4˚C for 10 minutes. The plates were then blocked by 1% bovine serum albumin (BSA) at room temperature for 1 hour and stored at −20˚C until needed for use. Also, the DENV-2 rabbit antiserum (kindly provided by Dr. GJ Chang at US-CDC) with 1:3500 dilution in carbonate-bicarbonate buffer (45.3 mM NaHCO3, 18.2 mM Na2CO3 [pH 9.6]) was added to each well. The coating was done overnight at 4˚C. The following day, the wells were blocked by 1% BSA in the wash buffer (PBS containing 0.1% Tween 20) at room temperature for 1 hour, and then the VLPs or virus culture supernatants of DENV-2 were added to each well, which allowed for saturated binding of antigens to the wells of ELISA plate. The immunized mice serums (50 µL/well) were added to the plates and incubated for 2 hours at room temperature. The plates were later washed six times with washing buffer, and incubated with horseradish-peroxidase (HRP)- conjugated goat anti-mouse IgG (50 µL/well at 1:5000 dilution) (Jackson ImmunoResearch, Pennsylvania, USA) in blocking buffer for 1 hour. Afterward, the plates were washed six times with washing buffer, and the colorimetric reaction will be developed with the substrate ortho-phenylene-diamine (OPD), prepared according to manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO) for 4 - 5 minutes and stopped by the addition of 50 µL/well of 3N HCl. Absorbance at 490 nm was measured using a microplate reader. Each control and test group consisted of three replicate wells, and each experiment was repeated at least three times. Antibody isotyping ELISAs were done in a similar fashion, however, antimouse IgG-HRP, anti-mouse IgG1-HRP, or anti-mouse IgG2a-HRP conjugates (PharMingen, San Diego, California), were added.
2.7. Antibody Avidity Analyses
Antibody avidity measurements were performed by comparing antibody binding to antigens in the presence and absence of 6 M urea in a standard ELISA [37,38]. Briefly, sera were diluted so as to obtain a standard ELISA absorbance of between 0.8 and 1.5. ELISA was performed in duplicate microtiter plates. After antibody binding, both plates were washed three times with wash buffer. 200 µL of wash buffer was added per well to one plate, and 200 µL of wash buffer per well containing 6 M urea was added to the other plate. Both plates were incubated for 5 min at room temperature. The buffers were removed and the plates were washed three more times with wash buffer. Incubations with conjugate and substrate were carried out according to the standard ELISA protocol. The avidity index (AI) was calculated as:
100 × {(A450D2-VLP[+urea] − A450NC[+urea])/(A450D2-VLP[−urea] − A450NC[−urea])}.
2.8. Spleen T-Cell Stimulation Cytokine ELISA
Two weeks after the last immunization, spleen cells were isolated and stimulated in vitro by incubating with the dengue antigens in RPMI medium at a concentration of 2 × 106 cells/mL. Two types of DENV-2 antigens were used here as the stimulant, including DENV-2 culture supernatants and VLPs. These two types of antigens were adjusted to the equivalent amount by using ELISA with serial dilution to 1:10 with OD > 2.0 (at 10 mg/mL). Fixed amounts of DENV-2 E antigens were incubated with 107 splenocytes/well in a 24-well plate in 2 mL of RPMI 1640 medium supplemented with 10% FCS and antibiotics at 37˚C in 5% CO2. Negative controls included untransfected COS-1 cell supernatants and virus-free C6/36 cell supernatants. As the positive control for viability, spleen cells were stimulated with ConA (purchased from Sigma-Aldrich and used as 1 µg/mL) or anti-CD28 antibodies (purchased from Biolegend, San Diego, CA and used as final concentration of 1 µg/ml). Spleen cells were incubated with the stimulating antigens overnight in a cell culture incubator, and 200 µL of each of the reactions were transferred in triplicate to ELISA plates. Aliquots of the culture supernatant were collected 24 and 48 hours later and assayed for interferon (IFN)-γ and interleukin (IL)-4 by the ELISA kit purchased from eBioscience (San Diego, California) by following the manufacturer’s protocol. In brief, 96-well plates were coated with 0.4 mg/well purified rat anti-mouse IL-4 or IFN-γ antibodies. Wells were blocked with 1% BSA in wash buffer. We added 100 µL of antigen-stimulated spleen-cell suspensions to the wells, and plates were incubated at 4˚C overnight. The plates were washed before the addition of diluted biotinylated anti-mouse IL-4 or IFN-γ (100 µL/well) and incubated for 1 h at 37˚C. The plates were washed again before the addition of diluted anti-mouse streptavidin-HRP conjugate (100 μL/ well) and incubated for 1 h at 37˚C. Color was developed by adding 100 μL/well of TMB substrate solution. The reaction was stopped by adding 50 μL of 5 N H2SO4. Absorbance was read at 450 nm in a microplate reader. As little as 15 pg/mL of both IFN-γ and IL-4 could be detected with these ELISAs.
2.9. Statistical Analysis
Differences in responses across multiple groups and between two groups were analyzed for significance using ANOVA and t tests, respectively. Logarithmic transformations of the reciprocal PRNT50 titers of the mice in each immunization group were made to stabilize variance.
3. Results
3.1. Serum Antibody Response to Immunization in Mice
Three groups of 4 BALB/c mice 4 weeks old were immunized with DENV-2 plasmid pCBD2 by direct im injection of DNA, gene gun delivery to the skin, or both as described in materials and methods section. The antibody responses of these mice were compared among these groups. Serum samples were obtained from mice at various times, and anti-DENV-2 specific antibody responses were determined by ELISA. Figure 1 shows that anti-DENV-2 IgG antibodies were detectable in all mouse groups 28 days after immunization. Levels of these antibodies increased five weeks after the second booster dose (on day 63) and were particularly enhanced when the immunization was primed and boosted by gene gun. At these points in time, the anti-E antibody response in mice immunized by im DNA inoculation was comparable to that in mice injected by gene gun followed by im. However, there is a statistically significant higher antibody response both in the level and duration for the
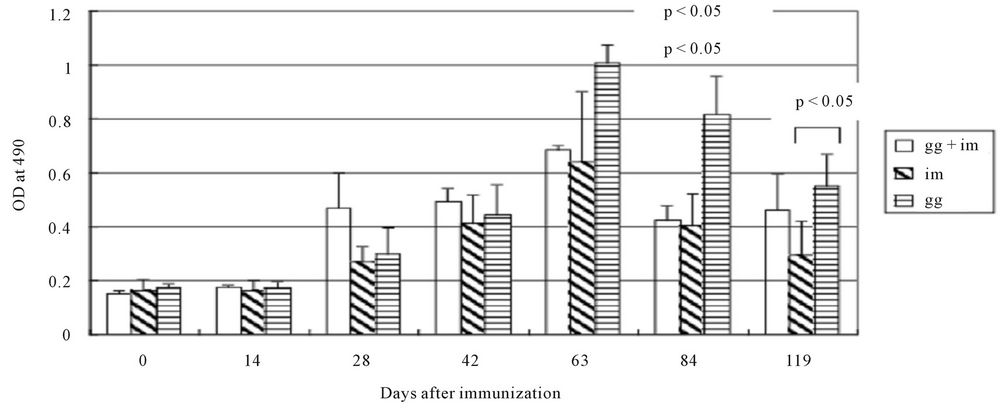
Figure 1. Kinetics of antibody response of 4-week-old BALB/c mice immunized with pCBD2 plasmid DNA expressing the secretory forms of the E protein by different methods of immunization. Booster injections were given on postimmunization days 28. Serum samples were obtained from mice on the indicated days, before booster immunization and the samples were stored at −20˚C. Serum samples were diluted 1:2400 and assayed for anti-DENV-2 antibody response by ELISA. Each column shows the mean antibody response for a group as ELISA optical density (OD): left diagonal stripes, mice immunized by intramuscular (im) inoculation; horizontal stripes, mice immunized by gene gun (gg); blank, mice immunized by gene gun + im. The t-test was performed by comparing the averages of OD value between the gg and im groups and p-values were indicated.
gene-gun group compared to the gg + im and im group on day 84 and 119 post-immunization (p < 0.05). At no time were any statistically significant differences seen between groups in the antibody responses of mice when DENV-2 infected cells or when a VLP was used as the antigen for ELISA. The results suggested that gene-gun delivery of a DNA vaccine induced a higher and longer duration of DENV-2-specific antibody responses compared to im delivery.
3.2. Isotype Analysis of Anti-E Antibodies
To further analyze the immune response to the E protein among three groups of mice, end-point titers of anti-E IgG1 and IgG2a antibodies were determined (figure 2). Delivery of DNA to the skin by gene gun preferentially induced IgG1-type immunoglobulins with a mean IgG1/ IgG2a ratio of 1.32 ± 0.51. However, the im inoculation of plasmid DNA preferentially induced IgG2a antibodies with a mean IgG1/IgG2a ratio of 0.59 ± 0.17. The gg + im group induced a IgG1-type immunoglobulin similar to that by the gene gun group, with a mean IgG1/IgG2a ratio of 1.36 ± 0.36. The IgG antibody isotypes were followed post-immunization for all three groups on days 63, 84, and 119, and the results were similar to those seen on day 42. These data suggest that im inoculation of DNA produced a Th1 immune response and that gene gunbased delivery led to a Th2 immune response. Furthermore, the immunoglobulin isotypes were determined by a procedure during primary immunization, and the switched procedure during booster immunization did not shift the immunoglobulin isotype presentation.
3.3. Cytokine Production by Spleen Cells from Immunized Mice
Spleen cells from different groups of immunized mice were cultured in vitro in the presence or absence of viral antigens or VLP proteins of DENV-2. Levels of IFN-γ and IL-4 were measured in these cultures 48 h later. Figure 3 shows that spleen cells from mice immunized with the DNA vaccine produced moderately lower levels of IL-4 and IFN-γ in the presence of the DENV-2 VLP protein compared to those in the presence of DENV-2 viral antigens. High levels of IL-4 and very low levels of IFN-γ were seen in mice immunized with pCBD2 by gene gun. Similarly, delivery of pCBD2 by gene gun followed by im induced high levels of IL-4 and low levels of IFN-γ as seen in the gene gun group. Levels of IL-4 in spleens from gg + im immunized mice were lower than those in spleens from mice immunized by gene gun. In mice immunized im with pCBD2, IFN-γ levels were very high and IL-4 levels were very low. The presence or absence of CD28, the co-stimulatory molecules of the CD4 T cell, didn’t influence the cytokine expression during the co-culture process. These results suggest that gene gun-based delivery of plasmid DNA induced Th2 immune responses. These responses were not considerably affected by whether the pCBD2 DNA plasmid was delivered later on by im for boosting which indicated that the primary immunization determined the immune memory and the booster dose did not shift the memory from Th2 to Th1 response. On the contrary, im inoculation of pCBD2 gave a Th1-dominated immune response with high levels of IFN-γ and low levels of IL-4.

Figure 2. IgG1 and IgG2a isotype analysis of anti-E antibodies of 4-week-old BALB/c mice immunized with pCBD2 plasmid DNA by different methods of immunization. Each column shows the mean IgG1/IgG2a ratios: left diagonal stripes, mice immunized by intramuscular (im) inoculation; horizontal stripes, mice immunized by gene gun (gg); blank, mice immunized by gene gun + im.
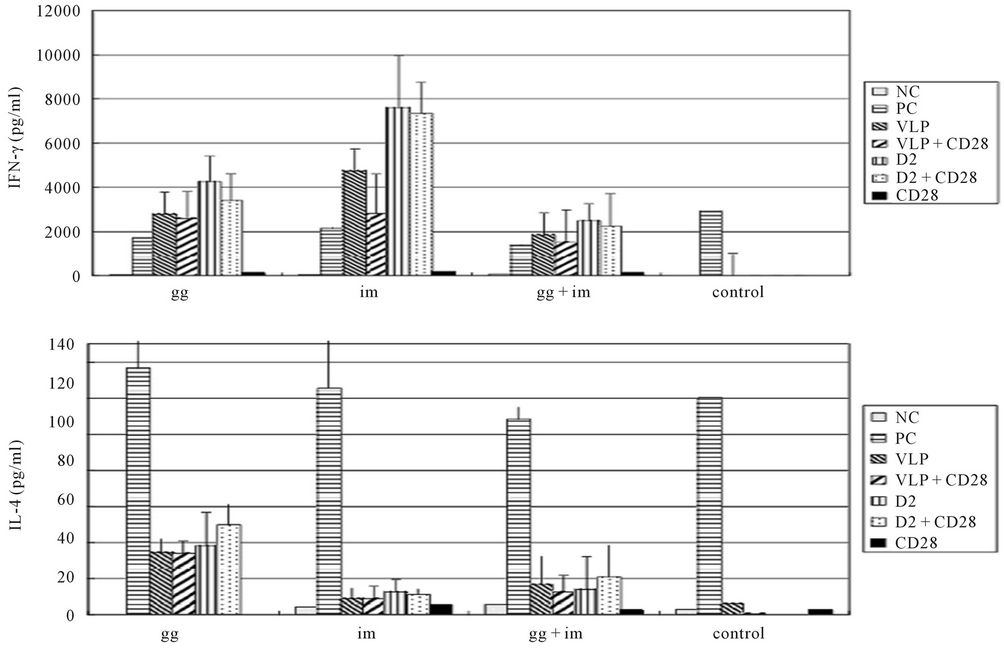
Figure 3. Cytokine response of 4-week-old BALB/c mice immunized with pCBD2 plasmid DNA generated by different methods of immunization. Different groups indicated different types of antigens were used for stimulation of splenocytes for cytokine release. NC: no antigen, PC: ConA, VLP: DENV-2 VLP, D2: partially purified DENV-2 viruses from C6/36 culture supernatant. Anti-CD28 antibody was also added in case CD28 was needed as a co-stimulatory molecule.
3.4. Kinetics of Antibody Avidity
Measurement of the kinetics of antibody avidity by ELISA demonstrated a low antibody avidity index (AI range from 6.8% - 9.6%) in mice of all three groups on day 42 (equivalent to 2 weeks after the last immunization) followed by gradual increase of antibody avidity (Figure 4). By day 119 (equivalent to 3 months after the last immunization), all mice in different vaccination groups presented high antibody avidity (AI value > 30) except the gene-gun immunization group, in which an AI value of only 23 was observed. Noticeably, the mice from im group showed a sharp increase in antibody avidity on day 63 one month after booster immunization and remained stable afterwards. Significant differences in antibody avidity indices among different vaccine groups on day 63, 84, or 119 were determined individually by ANOVA, which suggested a statistical significance (P < 0.001). The im group had the highest antibody avidity compared to the gg group on all days tested including day 63, 84 and 119; however, the significant difference was only seen between the im and gg + im group on day 63 and 84. The AIs for groups that received vaccines through gene gun were not significantly different from the AI for the gene gun followed by im group except on day 63. These data suggested that DNA immunization through the im route gave rise to higher antibody avidity compared to the gene gun immunization route. The im route would increase the antibody avidity 9.6% on day 119, even though the initial immunization was delivered through gene gun.
3.5. Virus-Neutralization Activity of Mouse Serums
The neutralizing antibody titers were measured against homologous (strain 16681) viruses using pooled sera obtained from mice on day 63, 84, and 119 post booster immunizations (Table 1). Serum from all immunization groups had considerable neutralizing activity kinetically against the DENV-2 parental strain 16681 up to four months after immunization. Of the routes which delivered the DNA plasmid, the gg group gave the highest neutralization activity with 77% plaque reduction at serum dilution of 400-fold collected on day 63; however, the im and gg + im group only gave about 50% of plaque
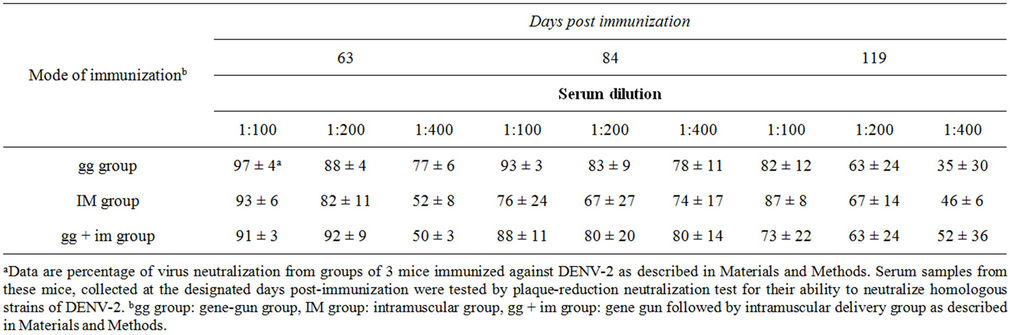
Table 1. Plaque-reduction neutralization activity of serum against DENV-2 from mice immunized by pCBD2.

Figure 4. Kinetics of antibody avidity indices (AI) of sera from 4-week-old BALB/c mice immunized with plasmid DNA by different immunization methods. Solid circle, mice immunized by intramuscular (im) inoculation; solid square, mice immunized by gene gun (gg); solid triangle, mice immunized by gene gun + im.
reduction under the same conditions with statistical significance. Interestingly, both im and gg + im groups increased their neutralization activity by yielding 74% and 80% plaque reduction at serum dilution of 400-fold collected on day 84, which were about the same as given by the gg group and then dropped to 46% and 52% on day 119 post immunization, respectively. The gg group gave to only 35% plaque reduction neutralization activity at serum dilution of 400-fold collected on day 118, which was lower than those yielded by the im or gg + im group. The neutralization titers from the sera obtained from three groups at day 84 and 119 were not statistically significant. These differences in neutralization activity were reproducible. The pooled pre-immune serum samples from the plasmid DNA immunization group and the serum samples from the control group (mice given vector DNA) had little DENV-neutralizing activity (data not shown).
4. Discussion
The leading candidates for a dengue vaccine are liveattenuated viruses (LAV), but it has proven difficult to produce LAV vaccines that are satisfactorily attenuated and, at the same time, sufficiently immunogenic [13,14]. Clearly, there is a reason for exploring alternatives. We previously reported that a DENV-2 DNA vaccine, consisting of prM and 80% E (E-1 to E-397) of DENV-2, followed by 20% E from JEV membrane-anchoring region, was able to generate high-titer antibodies in mice which persisted for more than 6 months [10]. Dengue virus-neutralizing antibodies directed against the virion E antigen are thought to play a key role in protection against disease, an idea supported directly by passive antibody transfer experiments in animal models and indirectly by epidemiological data from protective studies in areas where dengue is endemic [39,40]. However, the level and quality of antibody titers have not been established for dengue, and these might differ depending on the vaccine administration route. In the present study, we further evaluated different routes of immunizations in mice by measuring the kinetics of the DENV-2 typespecific antibody, antibody avidity, T cell-mediated cytokine responses, and neutralizing activity against wild type viruses. This is the first report that compares different administration routes for delivering a DNA vaccine, which adds further support for the utility of dengue DNA vaccines.
DNA vaccines have shown great potential in their ability to elicit potent humoral and cytotoxic cellular immune responses against the plasmid-encoded protein in a broad range of hosts [29,41,42]. In our study, the response observed following gene gun vaccination was more prominent, appearing earlier and resulting in higher and longer antibody titers than those seen with im injection. This result indicated that the gene gun technique is more efficient in eliciting a serum antibody response. This corresponds well with previous observations in mice, where particle bombardment of the epidermis gave rise to an increase in serum immunoglobulin by injecting DNA directly into the epidermal Langerhans cells, while im injection, targeting the muscle cells, is more efficient at inducing type 1 cellular immunity [26,43]. The efficient antibody response induced by gene gun vaccination, and the fact that im injection requires 100 times more DNA per vaccination than gene gun vaccinations, make the gene gun techniques appear to be a more effective way to deliver a DNA vaccine.
Various routes and doses of DNA vaccines have been used in mouse models previously. Intramuscular immunization by DNA plasmid expressing the secretory Japanese encephalitis virus (JEV) envelope protein induced higher anti-E antibody responses and end-point antibody titers than gene gun immunization [27]. However, in the result of the pig model, obtained by using the nucleoprotein of the porcine reproductive and respiratory syndrome virus as an antigen, suggested that gene gun delivery elicits a higher and more efficient immune response than im injection [43]. Similar results can also be seen in a DNA immunization mouse model when using plasmids expressing prM and E of the tick-borne encephalitis virus [26]. In our study, both vaccination techniques were efficient in eliciting an immune response, giving rise to specific serum antibody responses. However, the response observed following gene gun vaccination was more prominent, appearing earlier and resulting in higher and longer antibody titers than those seen with im injection. The explanation for the longevity of the antibody response after gene gun delivery could be due to the persistence of functional antigen-presenting dendritic cells for up to one month [28]. Compared to gene gun delivery, the ability to raise antibodies had been lost in im-immunized mice, in which the antigen-presenting dendritic cells were not detectable in lymphoid nodes after being immunized for one month [28].
DNA immunization is thought to elicit immune responses that closely resemble those seen though natural infections with intracellular pathogens. An important feature of the DNA vaccine is that antigen synthesis occurs intracellularly, allowing the processed antigen to enter the MHC class I pathway [44]. The previous study indicated that DNA vaccination favors memory rather than effector B cell responses [29]. In our experiments, priming with a DNA vaccine by gene gun delivery followed by a boosting after a second immunization by im delivery did not shift the immune response from a Th2 to Th1 response. This suggested that the DNA vaccine was effective in priming a specific anti-DENV-2 response, and apparently generated a broad range of both T-helper and B-cell memories. In both intramuscular and gene gun DNA immunization routes, the dominated Th1 or Th2 type immune responses could be efficiently activated after boosting. Boosting by a different administration route did not alter the isotypic nature of the response because of the T-helper memory.
Cytokines play a pivotal role in immune responses against viruses, not only through direct antiviral activity, but also by orchestrating a wide range of immune responses designed to limit infection. We examined the responses to immunizations by using routes delivering plasmids expressing the prM/E protein to study the predominant anti-E antibody isotype and the cytokine secretion profile of spleen cells from immunized mice. Results indicated that im inoculation of plasmid DNA encoding DENV-2 E protein induced Th1 immune responses, whereas the gene-gun-based delivery induced Th2 responses. The differences in the lymphokine patterns of the raised T cells response appeared to be rooted in the differences in antigen-presenting cells (APCs) that were targeted. After im immunization, gene expression can take place in more than one lymphoid tissue and in more than one cell type, although only the muscle cell has been reported [45]. For gene gun immunization, dendritic cells are the major APCs, and approximately 5% of the cells are epidermal Langerhans cells [46,47]. After im immunization, large amounts of extracellular vaccine DNA is then taken up by resident and migrant cells, which induce a Th1 response through activation of the Toll-like receptor 9 (TLR9) [48]. In contrast, gene gun immunization delivers DNA coated onto gold beads directly into cells, largely bypassing TLR9 and inducing a Th2 response [49]. Our findings regarding the type of T helper cell immune response generated by im or gene gun-based delivery of DNA are consistent with the general pattern observed by others using various plasmid DNA constructs [26-28,41].
DENV infection produces a mild acute DF, and a life threatening DHF and DSS. The precise mechanism of DHF/DSS is not yet fully known. The important hypotheses put forward regarding the role of host factors are antibody-dependent enhancement (ADE) of DENV replication, shift of Th1 to Th2-type cytokine response and other T cell responses resulting into cytokine storm [4,50]. It is possible that the gene gun-based delivery system shown in this study will exacerbate the immune response by re-infection after DNA vaccination [51]. Since boosting by a different administration route such as im did not alter the response from Th2 to Th1 as shown in this study, future efforts should focus on other alternatives for DNA delivery to improve the efficiency of DNA vaccination, such as liposome encapsidation, electroporation or high pressure gene gun apparatus [52-54].
We also determined the antibody avidity index, which is an approximate measure of the strength or stability of an antibody-antigen interaction. High-avidity antibodies might be conductive for maintaining dengue viruses in a neutralized state. For most animals, the antibody avidity index increased since the last booster immunization, from low affinity of two weeks after the booster to 3 months after vaccination, possibly as a result of affinity maturation. The highest average antibody avidity index on day 63 was seen in the im vaccine group followed by the gg + im and gg group. However, contrasting with the higher neutralizing antibody titers observed in the gg group on the same day, higher antibody avidity in the im vaccine group didn’t correlate with higher neutralizing antibody titers. Likewise, Simmons et al reported that the DNA vaccination group by gene gun delivery produced a significantly high avidity index (>30), which did not correlate with the reduction of the duration of viremia [25]. In our study, the mice from the gg group produced the lowest antibody avidity among the three groups, probably due to the antibody subclass IgG1; however, the neutralizing activity was more correlated with the total antibodies produced by the gene gun method in the gg group. Therefore, our results suggested that total antibodies containing both neutralizing and nonneutralizing antibodies present at high titers are important for neutralization in vitro in this model.
In summary, our results suggest that a DNA vaccine delivered by the gene gun route produced higher and longer total antibody titers as measured by ELISA. The high quantity of antibody titers correlates with the observed higher neutralizing activity against homologous wild type viruses but not with antibody avidity or antibody subclasses. This vaccine may provide an alternative for LAV vaccines in dengue virus immunizations, possibly having a lower risk for immediate reactogenicity.
5. Acknowledgements
We would like to thank Lydia Wang for the English editing. This project was supported by the grant from the National Science Council in Taiwan (Grant number 96- 3111-B-005-001) and Academia Sinica. The authors have declared that no conflict of interests exists regarding the submitted manuscript.
REFERENCES
- J. Kyle and E. Harris, “Global spread and persistence of dengue,” Annual Review of Microbiology, Vol. 62, 2008, pp. 71-92. doi:10.1146/annurev.micro.62.081307.163005
- S. B. Halstead, “Dengue,” Lancet, Vol. 370, No. 9599, 2007, pp. 1644-1652. doi:10.1016/S0140-6736(07)61687-0
- I. Kautner, M. J. Robinson and U. Kuhnle, “Dengue virus infection: Epidemiology, Pathogenesis, Clinical Presentation, Diagnosis, and prevention,” Journal of Pediatrics, Vol. 131, No. 4, 1997, pp. 516-524. doi:10.1016/S0022-3476(97)70054-4
- W. J. H. McBride and H. Bielefeldr-Ohmann, “Dengue viral infections; pathogenesis and epidemiology,” Microbes and Infection, Vol. 2, No. 9, 2000, pp. 1041-1050. doi:10.1016/S1286-4579(00)01258-2
- S. S. Whitehead, J. E. Blaney, A. P. Durbin and B. R. Murphy, “Prospects for a Dengue Virus Vaccine,” Nature Reviews Microbiology, Vol. 5, No. 7, 2007, pp. 518-528. doi:10.1038/nrmicro1690
- B. D. Lindenbach and C. M. Rice, “Flaviviridae: The viruses and Their Replication,” In: D. M. Knipe and P. M. Howley, eds., Fields’ Virology, 4th edidion, Lippincott Williams & Wilkins, Philadelphia, 2001, pp. 991-1110.
- S. Mukhopadhyay, R. J. Kuhn and M. G. Rossmann, “A Structural Perspective of the Flavivirus Life Cycle,” Nature Reviews Microbiology, Vol. 3, No. 1, 2005, pp. 13- 22. doi:10.1038/nrmicro1067
- S. J. Seligman and D. J. Bucher, “The importance of being Outer: Consequences of the distinction between the outer and Inner Surfaces of Flavivirus Glycoprotein E,” Trends in Microbiology, Vol. 11, No. 3, 2003, pp. 108- 110. doi:10.1016/S0966-842X(03)00005-2
- J. T. Roehrig, “Antigenic structure of Flavivirus Proteins,” Advances in Virus Research, Vol. 59, 2003, pp. 141-175. doi:10.1016/S0065-3527(03)59005-4
- G. J. Chang, A. R. Hunt, D. A. Holmes, T. Springfield, T.-S. Chiueh, J. T. Roehrig and D. J. Gubler, “Enhancing biosynthesis and secretion of premembrane and envelope Proteins by the Chimeric Plasmid of Dengue Type 2 and Japanese Encephalitis Virus,” Virology, Vol. 306, No. 1, 2003, pp. 170-180. doi:10.1016/S0042-6822(02)00028-4
- W. D. Crill and J. T. Roehrig, “Monoclonal antibodies that bind to domain III of Dengue Virus E Glycoprotein Are the Most Efficient Blockers of Virus Adsorption to Vero cells,” Journal of Virology, Vol. 75, No. 16, 2001, pp. 7769-7773. doi:10.1128/JVI.75.16.7769-7773.2001
- J. Schmitz, J. Roehrig, A. Barrett and J. Hombach, “Next Generation Dengue Vaccines: A Review of candidates in Preclinical Development,” Vaccine, Vol. 29, No. 42, 2011, pp. 7276-7284. doi:10.1016/j.vaccine.2011.07.017
- D. P. Webster, J. Farrar and S. Rowland-Jones, “Progress towards a Dengue Vaccine,” Lancet Infectious Diseases, Vol. 9, No. 11, 2009, pp. 678-687. doi:10.1016/S1473-3099(09)70254-3
- B. Guy and J. W. Almond, “Towards a Dengue Vaccine: Progress to date and Remaining Challenges,” Comparative Immunology, Microbiology and Infectious Diseases, Vol. 31, No. 2-3, 2008, pp. 239-252. doi:10.1016/j.cimid.2007.07.011
- K. V. Pugachev, F. Guirakhoo and T. P. Monath, “New developments in Flavivirus Vaccines with Special Attention to Yellow Fever,” Current Opinion in Infectious Diseases, Vol. 18, No. 5, 2005, pp. 387-394. doi:10.1097/01.qco.0000178823.28585.ad
- R. Putnak, K. Porter and C. Schmaljohn, “DNA vaccines for flaviviruses,” Advances in Virus Research, Vol. 61, 2003, pp. 445-468. doi:10.1016/S0065-3527(03)61012-2
- S. Koyama, C. Coban, T. Aoshi, T. Horii, S. Akira and K. J. Ishii, “Innate Immune Control of Nucleic Acid-Based Vaccine Immunogenicity,” Expert Review of Vaccines, Vol. 8, No. 8, 2009, pp. 1099-1107. doi:10.1586/erv.09.57
- T. Kochel, S. J. Wu, K. Raviprakash, P. Hobart, S. Hoffman, K. Porter and C. Hayes, “Inoculation of plasmids expressing the dengue-2 Envelope Gene Elicit Neutralizing Antibodies in mice,” Vaccine, Vol. 15, No. 5, 1997, pp. 547-552. doi:10.1016/S0264-410X(97)00215-6
- K. R. Porter, T. J. Kochel, S. J. Wu, K. Raviprakash, I. Phillips and C. G. Hayes, “Protective efficacy of a denngue 2 DNA vaccine in mice and the effect of CpG immuno-Stimulatory Motifs on Antibody Responses,” Archives of Virology, Vol. 143, No. 5, 1998, pp. 997-1003. doi:10.1007/s007050050348
- K. Raviprakash, T. J. Kochel, D. Ewing, M. Simmons, I. Phillips, C. G. Hayes and K. R. Porter, “Immunogenicity of dengue Virus Type 1 DNA Vaccines Expressing Truncated and Full Length Envelope Protein,” Vaccine, Vol. 18, No. 22, 2000, pp. 2426-2434. doi:10.1016/S0264-410X(99)00570-8
- E. Konishi, M. Yamaoka, I. Kurane and P. W. Mason, “A DNA Vaccine Expressing Dengue Type 2 Virus Premembrane and Envelope Genes Induces Neutralizing Antibody and Memory B Cells in mice,” Vaccine, Vol. 18, No. 11- 12, 2000, pp. 1133-1139. doi:10.1016/S0264-410X(99)00376-X
- T. J. Kochel, K. Raviprakash, C. G. Hayes, D. M. Watts, K. L. Russell, A. S Gozalo, I. A. Phillips, D. F. Ewing, G. S. Murphy and K. R. Porter, “A Dengue Virus Serotype-1 DNA Vaccine Induces Virus Neutralizing Antibodies and Provides Protection from Viral Challenge in Aotus monkeys,” Vaccine, Vol. 18, No. 27, 2000, pp. 3166-3173. doi:10.1016/S0264-410X(00)00105-5
- G. J. Chang, B. S. Davis, A. R. Hunt, D. A. Holmes and G. Kuno, “Flavivirus DNA vaccines: Current Status and potential,” Annals of the New York Academy of Sciences, Vol. 951, 2001, pp. 272-285. doi:10.1111/j.1749-6632.2001.tb02703.x
- G. J. Chang, A. R. Hunt and B. S. Davis, “A Single Intramuscular Injection of Recombinant Plasmid DNA Induces Protective Immunity and prevents Japanese encephalitis in mice,” Journal of Virology, Vol. 74, No. 9, 2000, pp. 4244-4252. doi:10.1128/JVI.74.9.4244-4252.2000
- M. Simmons, K. R. Porter, C. G. Hayes, D. W. Vaughn and R. Putnak, “Characterization of Antibody Responses to combinations of a Dengue Virus Type 2 DNA vaccine and Two Dengue Virus Type 2 Protein Vaccines in rhesus macaques,” Journal of Virology, Vol. 80, No. 19, 2006, pp. 9577-9585. doi:10.1128/JVI.00284-06
- J. H. Aberle, S. W. Aberle, S. L. Allison, K. Stiasny, M. Ecker, C. W. Mandl, R. Berger and F. X. Heinz, “A DNA immunization Model Study with Constructs Expressing the Tick-Borne Encephalitis Virus Envelope Protein E in Different Physical Forms,” Journal of Immunology, Vol. 163, No. 12, 1999, pp. 6756-6761.
- R. Kaur, G. Sachdeva and S. Vrati, “Plasmid DNA immunization against Japanese Encephalitis Virus: Immunogenicity of Membrane-Anchored and Secretory Envelope Protein,” Journal of Infectious Diseases, Vol. 185, No. 1, 2002, pp. 1-12. doi:10.1086/338015
- C. M. Boyle and H. L. Robinson, “Basic mechanisms of DNA-Raised Antibody Responses to intramuscular and Gene Gun Immunizations,” DNA and Cell Biology, Vol. 19, No. 3, 2000, pp. 157-165. doi:10.1089/104454900314546
- A. E. Oran and H. L. Robinson, “DNA Vaccines, Combining Form of antigen and method of delivery to raise a spectrum of IFN-r an dIL-4-producing CD4+ and CD8+ T cells,” Journal of Immunology, Vol. 171, 1999, pp. 1999-2005.
- Y. Chow, W. Huang, W. Chi, Y. Chu and M. Tao, “Improvement of hepatitis B virus DNA vaccines by plasmids Coexpressing Hepatitis B Surface Antigen and interleukin-2,” Journal of Virology, Vol. 71, No. 1, 1997, pp. 169-178.
- Y. Chow, B. Chiang, Y. Lee, W. Chi, W. Lin, Y. Chen and M. Tao, “Development of Th1 and Th2 populations and the nature of Immune Responses to hepatitis B virus DNA vaccines Can Be Modulated by codelivery of various Cytokine Genes,” Journal of Immunology, Vol. 160, No. 3, 1998, pp. 1320-1329.
- J. Sin, J. Kim, R. Arnold, K. Shroff, D. McCallus, C. Pachuk, S. McElhiney, M. Wolf, S. Pompa-de Bruin, T. Higgins, et al., “IL-12 gene as a DNA Vaccine Adjuvant in a Herpes Mouse Model: IL-12 enhances Th1-type CD4+ T Cell-Mediated Protective Immunity against Herpes Simplex Virus-2 Challenge,” Journal of Immunology, Vol. 162, No. 5, 1999, pp. 2912-2921.
- J. E. Martin, T. C. Pierson, S. Hubka, S. Rucker, I. J. Gordon, M. E. Enama, C. A. Andrews, Q. Xu, B. S. Davis, M. Nason, et al., “A West Nile virus DNA vaccine Induces Neutralizing Antibody in Healthy Adults During a phase 1 Clinical Trial,” Journal of Infectious Diseases, Vol. 196, No. 12, 2007, pp. 1732-1740. doi:10.1086/523650
- P. Russell, A Nisalak, P. Sukhavachana and S. Vivona, “A Plaque Reduction Test for Dengue Virus Neutralizing Antibodies,” Journal of Immunology, Vol. 99, No. 2, 1967, pp. 285-290.
- D. E. Purdy, A. J. Noga and G. J. Chang, “Noninfectious Recombinant Antigen for detection of St. Louis Encephalitis Virus-Specific Antibodies in serum by EnzymeLinked Immunosorbent Assay,” Journal of Clinical Microbiology, Vol. 42, No. 10, 2004, pp. 4709-4717. doi:10.1128/JCM.42.10.4709-4717.2004
- J. H. Aberle, S. W. Aberle, S. L. Allison, K. Stiasny, M. Ecker, C. W. Mandl, R. Berger and F. Heinz, “A DNA Immunization Model Study with Constructs Expressing the Tick-Borne Encephalitis Virus Envelope Protein E in Different Physical Forms,” Journal of Immunology, Vol. 163, No. 12, 1999, pp. 6756-6761.
- M. Narita, S. Yamada, Y. Matsuzono, O. Itakura, T. Togashi and H. Kikuta, “Immunoglobulin G Avidity Testing in serum and Cerebrospinal Fluid for analysis of Measles Virus Infection,” Clinical and Diagnostic Laboratory Immunology, Vol. 3, No. 2, 1996, pp. 211-215.
- M. Narita, Y. Matsuzono, Y. Takekoshi, S. Yamada, O. Itakura and M. Kubota, “Analysis of Mumps Vaccine Failure by means of Avidity Testing for Mumps VirusSpecific Immunoglobulin-G,” Clinical and Diagnostic Laboratory Immunology, Vol. 5, No. 6, 1998, pp. 799- 803.
- J. Kyle, S. Balsitis, L. Zhang, P. Beatty and E. Harris, “Antibodies play a Greater role than Immune Cells in Heterologous Protection against Secondary Dengue Virus Infection in a mouse model,” Virology, Vol. 380, No. 2, 2008, pp. 296-303. doi:10.1016/j.virol.2008.08.008
- T. Endy, A. Nisalak, S. Chunsuttitwat, D. W. Vaughn, S. Green and F. A. Ennis, “Relationship of Preexisting Dengue Virus (DV) Neutralizing Antibody Levels to viremia and severity of disease in a Prospective Cohort Study of DV infection in Thailand,” Journal of Infectious Diseases, Vol. 189, No. 6, 2004, pp. 990-1000. doi:10.1086/382280
- W. Zhu, C. Thomas and P. Sparling, “DNA immunization of mice with a Plasmid Encoding Neisseria Gonorrhea PorB protein by Intramuscular Injection and epidermal Particle Bombardment,” Vaccine, Vol. 22, No. 5-6, 2004, pp. 660-669. doi:10.1016/j.vaccine.2003.08.036
- C. H. Pan, H. W. Chen, H. W. Huang and M. H. Tao, “Protective Mechanisms Induced by a Japanese Encephalitis virus DNA vaccine: Requirement for antibody but not CD8+ cytotoxic T-Cell Response,” Journal of Virology, Vol. 75, No. 23, 2001, pp. 11457-11463. doi:10.1128/JVI.75.23.11457-11463.2001
- A. M. Barfoed, B. Kristensen, T. Dannemann-Jensen, B. Viuff, A. Botner, S. Kamstrup and M. B. Moller, “Influence of routes and Administration Parameters on antibody response of Pigs Following DNA Vaccination,” Vaccine, Vol. 22, No. 11-22, 2004, pp. 1395-1405. doi:10.1016/j.vaccine.2003.10.032
- H. L. Robinson and C. Torres, “DNA vaccines,” Seminars in Immunology, Vol. 9, No. 6, 1997, pp. 271-283. doi:10.1006/smim.1997.0083
- M. Chattergoon, T. Robinson, J. Boyer and D. Weiner, “Specific Immune Induction Following DNA-Based Immunization through in Vivo Transfection and activation of Macrophage/Antigen-Presenting Cells,” Journal of Immunology, Vol. 160, No. 12, 1998, pp. 5707-5718.
- A. Porgador, K. Irvine, A. Iwasaki, B. Barber, N. Restifo and R. Germain, “Predominant role for Directly Transfected Dendritic Cells in Antigen Presentation to CD8+ T cells after Gene Gun Immunization,” Journal of Experimental Medicine, Vol. 188, No. 6, 1998, pp. 1075-1082. doi:10.1084/jem.188.6.1075
- J. Williman, E. Lockhart, L. Slobbe, G. Buchan and M. Baird, “The use of Th1 cytokines, IL12 and IL-23, to Modulate The Immune Response Raised to a DNA Vaccine Delivered by Gene Gun,” Vaccine, Vol. 24, No. 21, 2006, pp. 4471-4474. doi:10.1016/j.vaccine.2005.08.011
- H. Hemmi, O. Takeuchi, T. Kawai, T. Kaisho, S. Sato and H. Sanjo, “A Toll-Like Receptor Recognizes Bacterial DNA,” Nature, Vol. 408, No. 6813, 2000, pp. 740- 745. doi:10.1038/35047123
- D. Feltquate, S. Heaney, R. Webster and H. Robinson, “Different T Helper Cell Types and Antibody Isotypes Generated by saline and Gene Gun DNA immunization,” Journal of Immunology, Vol. 158, No. 5, 1997, pp. 2278- 2284.
- A. Rothman and F. A. Francis, “Immunopathogenesis of Dengue Hemorrhagic Fever,” Virology, Vol. 257, No. 1, 1999, pp. 1-6. doi:10.1006/viro.1999.9656
- G. C. Perng, H.-Y. Lei, Y.-S. Lin and K. Chokephaibulkit, “Dengue Vaccines: Challenge and Confrontation,” World Journal of Vaccines, Vol. 1, No. 4, 2011, pp. 109-130. doi:10.4236/wjv.2011.14012
- G. Gregoriadis, A. Bacon, W. Caparros-Wanderley and B. McCormack, “A role for liposomes in Genetic Vaccination,” Vaccine, Vol. 20, No. S5, 2002, pp. B1-B9. doi:10.1016/S0264-410X(02)00514-5
- C.-C. Lin, M.-C. Yen, C.-M. Lin, S.-S. Huang, H.-J. Yang, N.-H. Chow and M.-D. Lai, “Delivery of noncarrier naked DNA vaccine into the skin by supersonic Flow Induces a polarized T helper type 1 Immune Response to cancer,” Journal of Gene Medicine, Vol. 10, No. 6, 2008, pp. 679-689. doi:10.1002/jgm.1183
- N. Y. Sardesai and D. B. Weiner, “Electroporation delivery of DNA Vaccines: Prospects for success,” Current Opinion in Immunology, Vol. 23, No. 3, 2011, pp. 421- 429. doi:10.1016/j.coi.2011.03.008
NOTES
*Corresponding author.

