World Journal of Condensed Matter Physics
Vol.4 No.2(2014), Article ID:45119,8 pages DOI:10.4236/wjcmp.2014.42009
Polariton Evaporation: The Blackbody Radiation Nature of the Low-Frequency Radiation Emitted by Radiative Polaritons to the Surrounding Space
Yosyp Schwab, Harkirat S. Mann, Brian N. Lang, Giovanna Scarel*
Department of Physics and Astronomy, James Madison University, Harrisonburg, USA
Email: *scarelgx@jmu.edu
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 19 February 2014; revised 23 March 2014; accepted 8 April 2014
ABSTRACT
Upon formation, radiative polaritons in thin oxide films or crystals emit radiation to the surrounding space. This radiation is confined in a small range of the microwave to far-infrared region of the electromagnetic spectrum, independently of the oxide chemistry. This work shows that the low-frequency radiation is blackbody radiation associated with a temperature directly related to the boson character of the radiative polaritons and to their amount. The proximity of this temperature to absolute zero Kelvin explains the confinement of the frequency. This phenomenon is named polariton evaporation.
Keywords:Polaritons, Dielectrics, Thin Films, Infrared Spectroscopy

1. Introduction
Radiative polaritons (RPs) were discovered in the late sixties [1] -[3] and recently gained attention due to their ability to explain optical and thermal properties of thin oxide films or crystals [4] -[11] . Radiative polaritons form upon the absorption of photons from infrared (IR) radiation by phonons in thin oxide films or crystals. The coupling occurs when photons and phonons oscillate at the same frequency. Unlike surface phonon-polaritons [12] , whose frequency is a real number, RPs are characterized by a complex angular frequency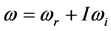 , where
, where  is the imaginary unit and the subscripts
is the imaginary unit and the subscripts  and
and  refer to the real and imaginary parts, respectively [3] . The real part,
refer to the real and imaginary parts, respectively [3] . The real part, 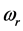 , is the resonant frequency, and is larger than the imaginary part,
, is the resonant frequency, and is larger than the imaginary part, 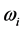 [13] . Additionally, through IR absorption spectra,
[13] . Additionally, through IR absorption spectra, 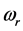 provides the central frequency of the absorption peak, while
provides the central frequency of the absorption peak, while 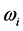 supplies the spread of the absorption peak around
supplies the spread of the absorption peak around 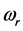 [2] [3] [13] , or half of the peak width. It was recently found that the frequency
[2] [3] [13] , or half of the peak width. It was recently found that the frequency 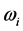 corresponds to the frequency of the experimentally observed low-frequency radiation emitted to the space surrounding the RP’s formation site [13] . Such radiation lasts as long as the exciting IR radiation illuminates the targeted thin oxide film or crystal. The existence of such low-frequency radiation, however, is so far only viewed as the consequence of the presence of
corresponds to the frequency of the experimentally observed low-frequency radiation emitted to the space surrounding the RP’s formation site [13] . Such radiation lasts as long as the exciting IR radiation illuminates the targeted thin oxide film or crystal. The existence of such low-frequency radiation, however, is so far only viewed as the consequence of the presence of 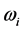 in one of the exponential terms in the expression for the polarization
in one of the exponential terms in the expression for the polarization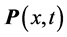 :
: 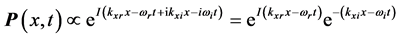 , (1) where
, (1) where 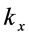 is a component of the complex wave-vector
is a component of the complex wave-vector 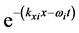 in Equation (1) generates a wave propagating away from the site of the thin oxide film or crystal that generated the RP without decay. This description gives merely a mathematical explanation without providing a physical mechanism of the origin of the low-frequency radiation with frequency
in Equation (1) generates a wave propagating away from the site of the thin oxide film or crystal that generated the RP without decay. This description gives merely a mathematical explanation without providing a physical mechanism of the origin of the low-frequency radiation with frequency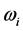 . Furthermore, no explanation is provided for the wide span of the
. Furthermore, no explanation is provided for the wide span of the 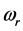 values, on one hand, and the confinement of the
values, on one hand, and the confinement of the 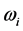 values in a small frequency range in the microwave to far-IR region for a large variety of thin oxide films in a broad thickness range. The goal of this work is to unveil the explanation (1) of the physical mechanism underpinning the formation of the low-frequency radiation with frequency
values in a small frequency range in the microwave to far-IR region for a large variety of thin oxide films in a broad thickness range. The goal of this work is to unveil the explanation (1) of the physical mechanism underpinning the formation of the low-frequency radiation with frequency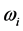 , and (2) for the wide span of the
, and (2) for the wide span of the 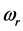 values, on one hand, and the confinement of the
values, on one hand, and the confinement of the 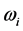 values. The importance of this effort is in its ability to elucidate whether a new source of radiation accompanies the formation of RPs, or if a connection can be found with already known phenomena.
values. The importance of this effort is in its ability to elucidate whether a new source of radiation accompanies the formation of RPs, or if a connection can be found with already known phenomena.2. Experimental Data and Simulation Method
The experimental data consist of the IR spectroscopic information on thin oxide films reported by previous research. From IR absorption spectra, the frequency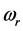 can be derived from the centroid of the absorption peak, while the frequency
can be derived from the centroid of the absorption peak, while the frequency 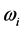 is derived from half of the width of the absorption peak [2] [3] [13] , as illustrated in Figure 1. Table 1 summarizes the findings obtained for the 0TH type RP [2] [3] of various oxides, such as TiO2, La2O3, Al2O3, and Lu2O3 [13] -[17] . It is found that, for the 0TH type RP, the
is derived from half of the width of the absorption peak [2] [3] [13] , as illustrated in Figure 1. Table 1 summarizes the findings obtained for the 0TH type RP [2] [3] of various oxides, such as TiO2, La2O3, Al2O3, and Lu2O3 [13] -[17] . It is found that, for the 0TH type RP, the 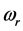 values span in
values span in 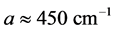 wide range, while the corresponding
wide range, while the corresponding 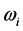 values span in a range
values span in a range 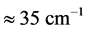 wide. The range of the
wide. The range of the 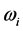 values is
values is 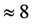 % that of the
% that of the 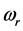 values. In addition, the
values. In addition, the 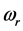 values are very characteristic of a peculiar crystal structure
values are very characteristic of a peculiar crystal structure 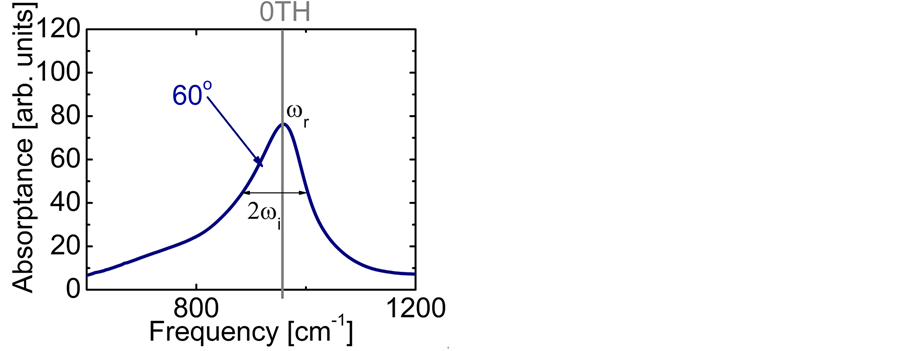
Figure 1. Experimental absorptance spectra at a 60˚ incidence angle for a 250 nm thick Al2O3 film on Si(100) measured in reflection mode [13] . The 0TH type RP is labeled with the frequency 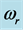 at the centroid of the absorption peak, and the frequency
at the centroid of the absorption peak, and the frequency 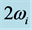 indicating the width of the absorption peak [13] .
indicating the width of the absorption peak [13] .
Table 1. Values of the real part, 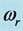 , and of the imaginary part,
, and of the imaginary part, 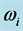 , of the complex angular frequency
, of the complex angular frequency 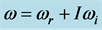 of the 0TH type RP, where
of the 0TH type RP, where 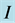 is the imaginary unit, for various oxide thin films at various thicknesses. The chemical potential
is the imaginary unit, for various oxide thin films at various thicknesses. The chemical potential 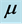 is estimated in
is estimated in ![]() for
for 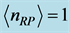 mole, and room temperature (
mole, and room temperature ( , or
, or  ˚C).
˚C).
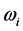 values, instead, are confined in the microwave to far-IR region of the electromagnetic frequency spectrum [13] , as shown in Table 1, for a large variety of oxides and in a broad thickness range. In this research, the spectroscopic data collected in Table 1 are used and elaborated according to the hypothesis formulated in the following section (Section 3).
values, instead, are confined in the microwave to far-IR region of the electromagnetic frequency spectrum [13] , as shown in Table 1, for a large variety of oxides and in a broad thickness range. In this research, the spectroscopic data collected in Table 1 are used and elaborated according to the hypothesis formulated in the following section (Section 3).3. Hypothesis
For a large variety of thin oxide films in a broad thickness range, the understanding of the wide span of the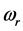 values, on one hand, and of the confinement, on the other, of the
values, on one hand, and of the confinement, on the other, of the 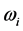 values requires a new approach beyond the analysis of the polarization
values requires a new approach beyond the analysis of the polarization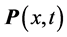 , as done in previous research [2] [13] . The alternative path chosen here is of statistical mechanics nature. It involves photons and phonons, which are mass-less bosons obeying the general Bose-Einstein statistics:
, as done in previous research [2] [13] . The alternative path chosen here is of statistical mechanics nature. It involves photons and phonons, which are mass-less bosons obeying the general Bose-Einstein statistics: 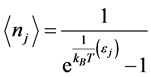 . (2)
. (2) Here, 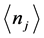 is the occupation number of bosons with energy
is the occupation number of bosons with energy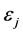 ,
, 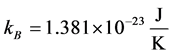 the Boltzmann constant, and
the Boltzmann constant, and ![]() the temperature. Stemming from the coupling between photons and the phonons, RPs must be bosons. The formation of a RP can be described as the result of an annihilation operator
the temperature. Stemming from the coupling between photons and the phonons, RPs must be bosons. The formation of a RP can be described as the result of an annihilation operator ![]() applied to both the Hamiltonians of the photon and phonon, and contemporarily a creation operator
applied to both the Hamiltonians of the photon and phonon, and contemporarily a creation operator 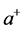 applied to the Hamiltonian of the RP. Alternatively, the effort made by a thin oxide film or crystal to couple photons and phonons and generate a RP can be expressed in terms of the chemical potential
applied to the Hamiltonian of the RP. Alternatively, the effort made by a thin oxide film or crystal to couple photons and phonons and generate a RP can be expressed in terms of the chemical potential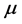 . In this context,
. In this context, 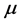 is thus defined as the free energy needed to rise or lower the number or moles of RPs in a thin oxide film or crystal. Assuming that
is thus defined as the free energy needed to rise or lower the number or moles of RPs in a thin oxide film or crystal. Assuming that 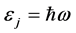 is the energy of a RP, and considering
is the energy of a RP, and considering 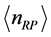 as the number of moles of RPs, the Bose-Einstein statistics for RPs is:
as the number of moles of RPs, the Bose-Einstein statistics for RPs is:
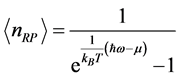 , (3) where
, (3) where 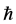 is the reduced Planck’s constant (
is the reduced Planck’s constant (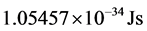 ). It follows that the temperature
). It follows that the temperature 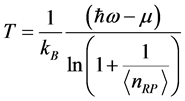 , (4) where
, (4) where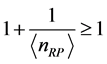 ,
, 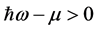 , and
, and 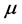 is real and positive. Furthermore, since
is real and positive. Furthermore, since 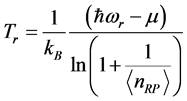 , (5) is related to the frequency of the radiation absorbed at
, (5) is related to the frequency of the radiation absorbed at  and to the chemical potential
and to the chemical potential , whereas the imaginary part:
, whereas the imaginary part: 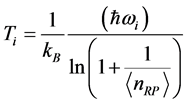 , (6) is associated with the low-frequency radiation emitted at frequency
, (6) is associated with the low-frequency radiation emitted at frequency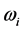 . For both the real and the imaginary parts, Equation (3) links
. For both the real and the imaginary parts, Equation (3) links 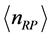 , the number of moles of RPs, and to the magnitude of the characteristic RPs frequencies,
, the number of moles of RPs, and to the magnitude of the characteristic RPs frequencies, 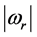 and
and 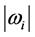 respectively. The real and an imaginary temperatures
respectively. The real and an imaginary temperatures 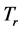 and
and 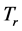 and
and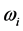 .
. 4. Results and Discussion
Equation (5) provides an expression for the real temperature,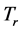 , whose nature needs to be disclosed alongside the value of the chemical potential
, whose nature needs to be disclosed alongside the value of the chemical potential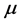 . Two possibilities can be considered. One is that the value of the chemical potential
. Two possibilities can be considered. One is that the value of the chemical potential 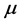 is the same for the RPs in all types of a thin oxide film or crystal that can support them. In this case, according to Equation (5),
is the same for the RPs in all types of a thin oxide film or crystal that can support them. In this case, according to Equation (5), 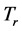 depends on the oxide chemistry, thus on
depends on the oxide chemistry, thus on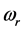 . This picture rules out the influence on the results due to the laboratory temperature, which determines the number of moles for RPs
. This picture rules out the influence on the results due to the laboratory temperature, which determines the number of moles for RPs 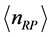 at a given frequency [19] , and will therefore be neglected. The alternative possibility is that the value of the chemical potential
at a given frequency [19] , and will therefore be neglected. The alternative possibility is that the value of the chemical potential 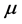 varies for different types of thin oxide films or crystals. In this case,
varies for different types of thin oxide films or crystals. In this case, 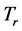 can be constant, and taken as the laboratory temperature at which the RPs were generated. The data in Table 1 were collected at
can be constant, and taken as the laboratory temperature at which the RPs were generated. The data in Table 1 were collected at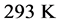 , or 20˚C, which is thus assumed as the laboratory’s temperature. In this case, the chemical potential
, or 20˚C, which is thus assumed as the laboratory’s temperature. In this case, the chemical potential 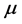 values vary depending upon the thin oxide films considered, and their values in
values vary depending upon the thin oxide films considered, and their values in 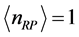 . Low
. Low 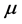 values characterize a crystal structure and chemistry which require a small amount of energy to promote RPs formation. The span in the
values characterize a crystal structure and chemistry which require a small amount of energy to promote RPs formation. The span in the 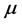 values explains the large span of the
values explains the large span of the 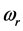 values measured for thin oxide films. The trend of the chemical potential
values measured for thin oxide films. The trend of the chemical potential 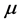 versus
versus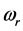 :
: 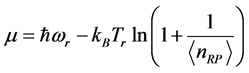 , (7) is illustrated in Figure 2. It can be observed that larger chemical potential
, (7) is illustrated in Figure 2. It can be observed that larger chemical potential 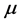 values are associated to larger absorption frequencies
values are associated to larger absorption frequencies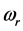 .The trends are similar for
.The trends are similar for 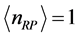 and
and 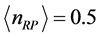 with
with 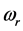 values ranging between
values ranging between 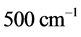 and
and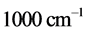 , as observed in experimental spectra. Equation (6) gives the expression for the imaginary temperature
, as observed in experimental spectra. Equation (6) gives the expression for the imaginary temperature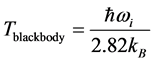 [20] , the temperature of blackbody radiation with maximum spectral radiance
[20] , the temperature of blackbody radiation with maximum spectral radiance 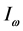 at
at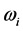 . The factor
. The factor 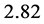 stems from the maximization of
stems from the maximization of 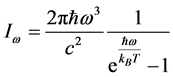 with respect to
with respect to 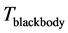 suggests two possible conse- quences. One is that that the radiation emitted to the space surrounding the RP’s formation site at frequency
suggests two possible conse- quences. One is that that the radiation emitted to the space surrounding the RP’s formation site at frequency 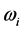 is blackbody radiation. Thus, the association between
is blackbody radiation. Thus, the association between 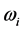 and the imaginary temperature
and the imaginary temperature 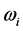 . Stated differently, the dissipated RP’s energy
. Stated differently, the dissipated RP’s energy 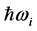 plus the energy from the IR radiation absorbed around frequency
plus the energy from the IR radiation absorbed around frequency 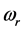 and transformed into heat [21] , give the energy of the IR
and transformed into heat [21] , give the energy of the IR 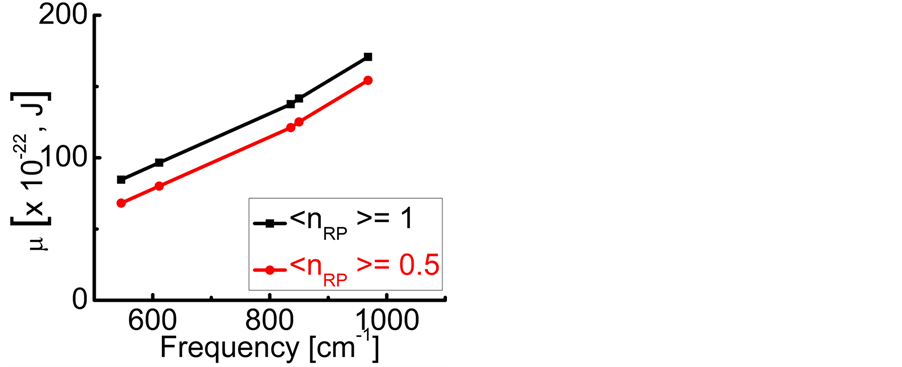
Figure 2. The chemical potential ![]() versus frequency
versus frequency 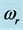 evaluated numerically for
evaluated numerically for 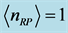 mole (filled squares), and for
mole (filled squares), and for 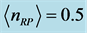 mole (filled circles). Room temperature (293 K, or 20˚C) is assumed in the evaluation. The symbols correspond to the experimentally determined frequencies
mole (filled circles). Room temperature (293 K, or 20˚C) is assumed in the evaluation. The symbols correspond to the experimentally determined frequencies 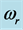 of the 0TH type RP for thin oxide films of TiO2, La2O3, Al2O3, and Lu2O3 at thicknesses specified in Table 1.
of the 0TH type RP for thin oxide films of TiO2, La2O3, Al2O3, and Lu2O3 at thicknesses specified in Table 1.
photons absorbed by the thin oxide film or crystal. The other consequence is that, for
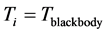 ,
, 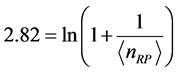 . (8) Equation (8) has a solution for
. (8) Equation (8) has a solution for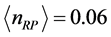 . Since here the magnitude of
. Since here the magnitude of 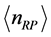 is a fractional value, this quantity should not be related to number of particles. Rather, defining
is a fractional value, this quantity should not be related to number of particles. Rather, defining 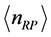 as the mole number of RPs seems more appropriate. With this assumption, the trend of
as the mole number of RPs seems more appropriate. With this assumption, the trend of 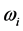 between
between 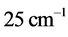 and
and 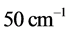 is illustrated in Figure 3 for
is illustrated in Figure 3 for 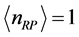 and
and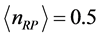 . The
. The 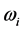 . The values of
. The values of 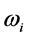 between
between 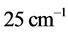 and
and 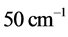 for
for 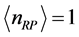 and
and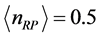 , respectively. Further more, for
, respectively. Further more, for 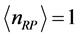 the slope is
the slope is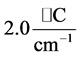 , while for
, while for  it is
it is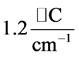 . The dependence of the
. The dependence of the  values to film thickness, or the amount of material in the thin oxide film, as discussed in previous research [13] . The proximity of the
values to film thickness, or the amount of material in the thin oxide film, as discussed in previous research [13] . The proximity of the 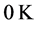 , or
, or 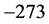 ˚C, combined with the effects of the number of moles of the thin oxide film, confines the
˚C, combined with the effects of the number of moles of the thin oxide film, confines the 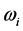 values in a small range in the microwave to far-IR region of the electromagnetic frequency spectrum [13] , as shown in Table 1. This observation also signifies that detecting Ti is not trivial: it would require a thermometer operating near
values in a small range in the microwave to far-IR region of the electromagnetic frequency spectrum [13] , as shown in Table 1. This observation also signifies that detecting Ti is not trivial: it would require a thermometer operating near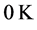 , and sensitive to very small variations of temperature.
, and sensitive to very small variations of temperature. 5. Comparison with Other “Evaporation” Phenomena
Evaporation phenomena and imaginary temperature in the available literature are now discussed. Evaporation phenomena in particular are not isolated, and one of the most popular is black hole evaporation [22] [23] . The emission of the so-called Hawking radiation was hypothesized after the discovery that black holes have entropy, and thus temperature. Because of the temperature, black holes must radiate blackbody radiation. Unlike the polariton evaporation case, the temperature of black holes was discovered before the radiation. The temperature of the black hole is a real, not an imaginary quantity [22] [23] . The evaporation phenomenon for black holes is of quantum-mechanical nature, and involves the “evaporation” of mass [23] in the form of particle production via 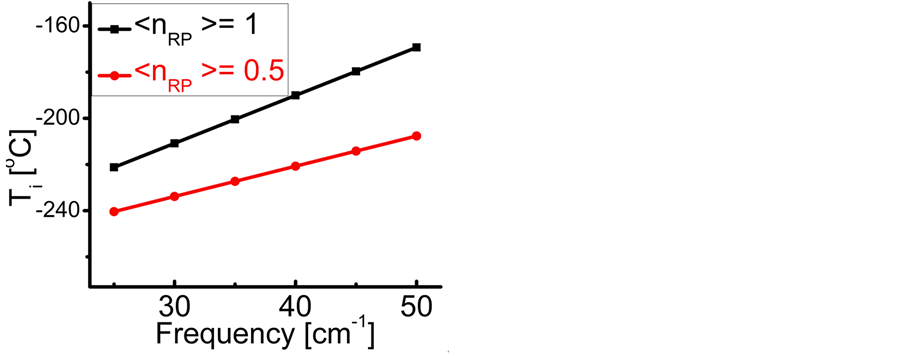
Figure 3. The imaginary temperature ![]() versus frequency
versus frequency 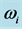 evaluated numerically for
evaluated numerically for 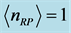 mole (filled squares), and for
mole (filled squares), and for 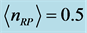 mole (filled circles). The frequency values cover the range in which
mole (filled circles). The frequency values cover the range in which 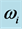 is found in experimental IR spectra of TiO2, La2O3, Al2O3, and Lu2O3 oxide films as specified in Table 1.
is found in experimental IR spectra of TiO2, La2O3, Al2O3, and Lu2O3 oxide films as specified in Table 1.
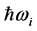 dissipated by the RPs plus the energy from the IR radiation absorbed around frequency
dissipated by the RPs plus the energy from the IR radiation absorbed around frequency 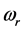 and transformed into heat [21] , are equal the energy of the IR photons irradiating the thin oxide film or crystal. Similarly to the case of the imaginary temperature
and transformed into heat [21] , are equal the energy of the IR photons irradiating the thin oxide film or crystal. Similarly to the case of the imaginary temperature 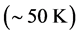 , the temperature of the black hole is predicted to be low (
, the temperature of the black hole is predicted to be low (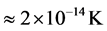 for a supermassive black hole) [26] . Investigations are under way to detect the existence of the Hawking radiation, whose signature is contained in gamma-ray bursts [23] . On the other hand, the radiation related to polariton evaporation was recently detected [13] . Finally, similarly to the temperature of the black holes which depends on
for a supermassive black hole) [26] . Investigations are under way to detect the existence of the Hawking radiation, whose signature is contained in gamma-ray bursts [23] . On the other hand, the radiation related to polariton evaporation was recently detected [13] . Finally, similarly to the temperature of the black holes which depends on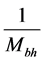 , where
, where 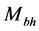 is the mass of the black hole [23] , the
is the mass of the black hole [23] , the 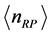 , as shown in Figure 3. Even with all the differences reported, polariton and black hole evaporation are both related to blackbody radiation, showing a deep unity in microscopic and macroscopic physical and natural phenomena. The concept of imaginary temperature is not as popular as that of “evaporation”. The literature is short of examples of imaginary temperatures deriving from mathematically complex quantities. On the other hand, the expression of imaginary temperature is used most notably in the biological field, where it is defined as the “statistical value for a thermostat made of particles with real mass” [27] . Mathematically imaginary quantities, however, are normally related to dissipation [13] [25] .
, as shown in Figure 3. Even with all the differences reported, polariton and black hole evaporation are both related to blackbody radiation, showing a deep unity in microscopic and macroscopic physical and natural phenomena. The concept of imaginary temperature is not as popular as that of “evaporation”. The literature is short of examples of imaginary temperatures deriving from mathematically complex quantities. On the other hand, the expression of imaginary temperature is used most notably in the biological field, where it is defined as the “statistical value for a thermostat made of particles with real mass” [27] . Mathematically imaginary quantities, however, are normally related to dissipation [13] [25] .6. Summary and Significance
Because of their bosonic nature, radiative polaritons have a temperature associated with the low frequency radiation they emit to the space surrounding their formation site. The radiation is due to blackbody radiation associated with a temperature which stems from the imaginary part of the complex frequency of radiative polaritons and is related to their amount. The relationship with blackbody radiation aids in explaining the confinement of the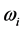 values in a narrow frequency interval in the microwave to far-infrared region. The
values in a narrow frequency interval in the microwave to far-infrared region. The 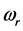 values span in a wide frequency range because they are related to the oxide film and crystal properties, as illustrated here specifically for the 0TH type radiative polariton in Table 1. Finally, polariton evaporation resembles black hole evaporation, giving insight on the profound unity among physical phenomena in the nanoand the macro-scale.
values span in a wide frequency range because they are related to the oxide film and crystal properties, as illustrated here specifically for the 0TH type radiative polariton in Table 1. Finally, polariton evaporation resembles black hole evaporation, giving insight on the profound unity among physical phenomena in the nanoand the macro-scale.Acknowledgements
This work was supported by the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (grant # J-1053), the James Madison University (JMU) Center for Materials Science, the JMU Department of Physics and Astronomy, and the NSF-REU and Department of Defense ASSURE program (grant #0851367). The authors thank Profs. K. Fukumura, A. Constantin, C. S. Whisnant, and J.C. Zimmerman (JMU) for fruitful discussions.
References
- D. W. Berreman (1963) Infrared Absorption at Longitudinal Optic Frequency in Cubic Crystal Films. Physical Review, 130, 2193-2198. http://dx.doi.org/10.1103/PhysRev.130.2193
- K. L. Kliewer and R. Fuchs (1966) Optical Modes of Vibration in an Ionic Crystal Slab including Retardation. II. Radiative Region. Physical Review, 150, 573-588. http://dx.doi.org/10.1103/PhysRev.150.573
- R. Fuchs, K. L. Kliewer, and W. J. Pardee (1966) Optical Properties of an Ionic Crystal Slab. Physical Review, 150, 589-596. http://dx.doi.org/10.1103/PhysRev.150.589
- G. Scarel, J.-S. Na, B. Gong, and G. N. Parsons (2010) Phonon Response in the Infrared Region to Thickness of Oxide Films Formed by Atomic Layer Deposition. Applied Spectroscopy, 64, 120-126. http://dx.doi.org/10.1366/000370210790571954
- G. Scarel, J.-S. Na, and G. N. Parsons (2010) Angular Behavior of the Berreman Effect Investigated in Uniform Al2O3 Layers Formed by Atomic Layer Deposition. Journal of Physics: Condensed Matter, 22, Article ID: 155401. http://dx.doi.org/10.1088/0953-8984/22/15/155401
- M. Załuzny and W. Zietkowski (2013) Semiclassical Study of Intersubband Cavity Polaritons: Role of Plasmonic and Radiative Coupling Effects. Physical Review, B88, Article ID: 195408. http://dx.doi.org/10.1103/PhysRevB.88.195408
- M. Fancoeur, M. P. Mengü̧, and R. Vaillon (2010) Local Density of Electromagnetic States within a Nanometric Gap Formed between Two Thin Films Supporting Surface Phonon Polaritons. Journal of Applied Physics, 107, Article ID: 034313. http://dx.doi.org/10.1063/1.3294606
- F. Neubrech and A. Pucci (2012) Nanoantenna: Plasmon Enhanced Spectroscopies for Biotechnological Applications. Pan Stanford Publishing Pte. Ltd., Singapore City, 297-312.
- S. Vassant, J.-P. Hugonin, F. Marquier, and J.-J. Greffet (2012) Berreman Mode and Epsilon Near Zero Mode. Optics Express, 20, 23971-23977. http://dx.doi.org/10.1364/OE.20.023971
- J. Le Gall, M. Olivier, and J.-J. Greffet (1997) Experimental and Theoretical Study of Reflection and Coherent Thermal emission by a SiC Grating Supporting a Surface-Phonon Polariton. Physical Review B, 55, Article ID: 10105. http://dx.doi.org/10.1103/PhysRevB.55.10105
- Y. Chalopin, M. Hayoun, S. Volz, and H. Dammak (2014) Surface Enhanced Infrared Absorption in Dielectric Thin Films with Ultra-Strong Confinement Effects. Applied Physics Letters, 104, Article ID: 011905. http://dx.doi.org/10.1063/1.4860989
- K. L. Kliewer and R. Fuchs (1966) Optical Modes of Vibration in an Ionic Crystal Slab including Retardation. I. Nonradiative Region. Physical Review, 144, 495-503. http://dx.doi.org/10.1103/PhysRev.144.495
- A. J. Vincent-Johnson, Y. Schwab, H. S. Mann, M. Francoeur, J. S. Hammonds, and G. Scarel (2013) Origin of the Low Frequency Radiation Emitted by Radiative Polaritons Excited by Infrared Radiation in Planar LA2O3 Films. Journal of Physics: Condensed Matter, 25, Article ID: 035901. http://dx.doi.org/10.1088/0953-8984/25/3/035901
- G. Scarel, C. R. Aita, and A. V. Sklyarov (2003) Effect of Substrate Conductivity on Infrared Reflection Spectra of Thin TiO2 Films. Journal of Non-Crystalline Solids, 318, 168-174. http://dx.doi.org/10.1016/S0022-3093(02)01875-6
- G. Scarel, A. Debernardi, D. Tsoutsou, S. Spiga, S. C. Capelli, L. Lamagna, S. N. Volkos, M. Alia, and M. Fanciulli (2007) Vibrational and Electrical Properties of Hexagonal La2O3 Films. Applied Physics Letters, 91, Article ID: 102901. http://dx.doi.org/10.1063/1.2779108
- Vincent-Johnson, A.J., Vasquez, K.A., Bridstrup, J.E., Masters, A.E., Hu, X. and Scarel, G. (2011) Heat Recovery Mechanism in the Excitation of Radiative Polaritons by Broadband Infrared Radiation in Thin Oxide Films. Applied Physics Letters, 99, 131901. http://dx.doi.org/10.1063/1.3643464
- Bonera, E., Scarel, G., Fanciulli, M., Delugas, P. and Fiorentini, V. (2005) Dielectric Properties of High-k Oxides: Theory and Experiment for Lu2O3. Physical Review Letters, 94, 027602. http://dx.doi.org/10.1103/PhysRevLett.94.027602
- Zhang, X.J., Liu, B.Q., Xu, X.J., Wu, X. and Yuan, R.M. (2014) A Study of the Enhancement in Near-Field Radiative Heat Transfer by Surface Polaritons. Applied Mechanics and Materials, 448-453, 3211-3216.
- Ashcroft, N.W. and Mermin, N.D. (1976) Solid State Physics. Saunders College, Philadelphia, 454.
- Fowles, G.R. (1975) Modern Optics. Dover Publications, Inc., New York, 212-217.
- Leonhardt, U. (2013) Cloaking of Heat. Nature, 498, 440-441. http://dx.doi.org/10.1038/498440a
- Rindler, W. (2006) Relativity. Oxford University Press Inc., New York, 279-281.
- Hawking, S.W. (1992) Evaporation of Two-Dimensional Black Holes. Physical Review Letters, 69, 406-409. http://dx.doi.org/10.1103/PhysRevLett.69.406
- Braunstein, S.L. and Patra, M.K. (2011) Black Hole Evaporation Rates without Spacetime. Physical Review Letters, 107, 071302. http://dx.doi.org/10.1103/PhysRevLett.107.071302
- Łach, G., DeKieviet, M. and Jentschura, U.D. (2012) Enhancement of Blackbody Friction Due to the Finite Lifetime of Atomic Levels. Physical Review Letters, 108, 043005. http://dx.doi.org/10.1103/PhysRevLett.108.043005
- Unruh, W.G. (1981) Experimental Black-Hole Evaporation? Physical Review Letters, 46, 1351-1353. http://dx.doi.org/10.1103/PhysRevLett.46.1351
NOTES

*Corresponding author.


