Soft Nanoscience Letters
Vol.3 No.4A(2013), Article ID:40406,3 pages DOI:10.4236/snl.2013.34A002
Comparative H2S Sensing Characteristics of Fe2O3: Thin Film vs. Bulk
![]()
1Technical Physics Division, Bhabha Atomic Research Center, Mumbai, India; 2Material Science Laboratory, Department of Physics, Guru Nanak Dev University, Amritsar, India.
Email: vishalbalouria@yahoo.com
Copyright © 2013 Vishal Balouria et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 1st, 2013; revised November 2nd, 2013; accepted November 9th, 2013
Keywords: Fe2O3; Thin Film; Pellets; H2S Sensor
ABSTRACT
Comparative investigations of gas sensing characteristics of Fe2O3 in both thin film as well as bulk forms have been performed. Thin film sensors were realized by first depositing Fe films using electron-beam evaporation followed by thermal oxidation. Bulk sensors in the form of pellets were prepared by cold pressing commercial Fe2O3 powder with subsequent sintering. Both thin film and bulk Fe2O3 sensors exhibited a selective and reversible response characteristics towards H2S with maximum response at an operating temperature of 250˚C and 200˚C, respectively. A negligible response towards other interfering gases was observed. Thin film sensors exhibited an enhanced response in comparison to that of pellets.
1. Introduction
H2S is a colorless, highly flammable and toxic gas, which in low concentration has an offensive odor similar to that of rotten eggs. As the Threshold Limit Value (TLV) for H2S is 10 ppm, there is a need for its detection in either ppm or sub ppm level [1-3]. α-Fe2O3, an intrinsically n-type semiconductor has been widely exploited as a gas sensitive material owing to its good structural and thermodynamical stability along with resistance to photocorrosion. In the present work, H2S sensing characteristics of Fe2O3 in both thin film and conventional forms have been investigated and compared. Our results indicate that thin film based sensors exhibited better response towards H2S in comparison to that of pellets.
2. Experimental
Fe2O3 thin films were prepared in two steps. In the first step, Fe films with ~100 nm thickness were deposited onto pre-cleaned (0001) Al2O3 substrates using electron-beam evaporation under a base vacuum of ~1.3 × 10−4 Pa. A high purity (99.99%) Fe powder was cold pressed in the form of a pellet and used as source material. These films were subsequently subjected to thermal oxidation at 800˚C in an oxygen environment (O2 flow: 50 sccm) for 2 h. Bulk sensors in the form of pellets were prepared by cold pressing commercial Fe2O3 powder followed by sintering at 800˚C for 2 h. The response characteristics were recorded as a function of temperature, gas (H2S, C2H5OH, NH3, CH4, CO, CO2, NO, and Cl2) and their concentrations using a static setup as described elsewhere [4]. For this purpose, the sensor was mounted on a Pt-100 based heater assembly in a leaktight glass chamber (net volume: 500 ml). Electrical contacts were made by thermally depositing two Au pads (120 nm thick). A desired concentration of the test gas in the chamber was achieved by injecting a known quantity of the test gas using a micro-syringe. The sensor response was calculated using the relations:
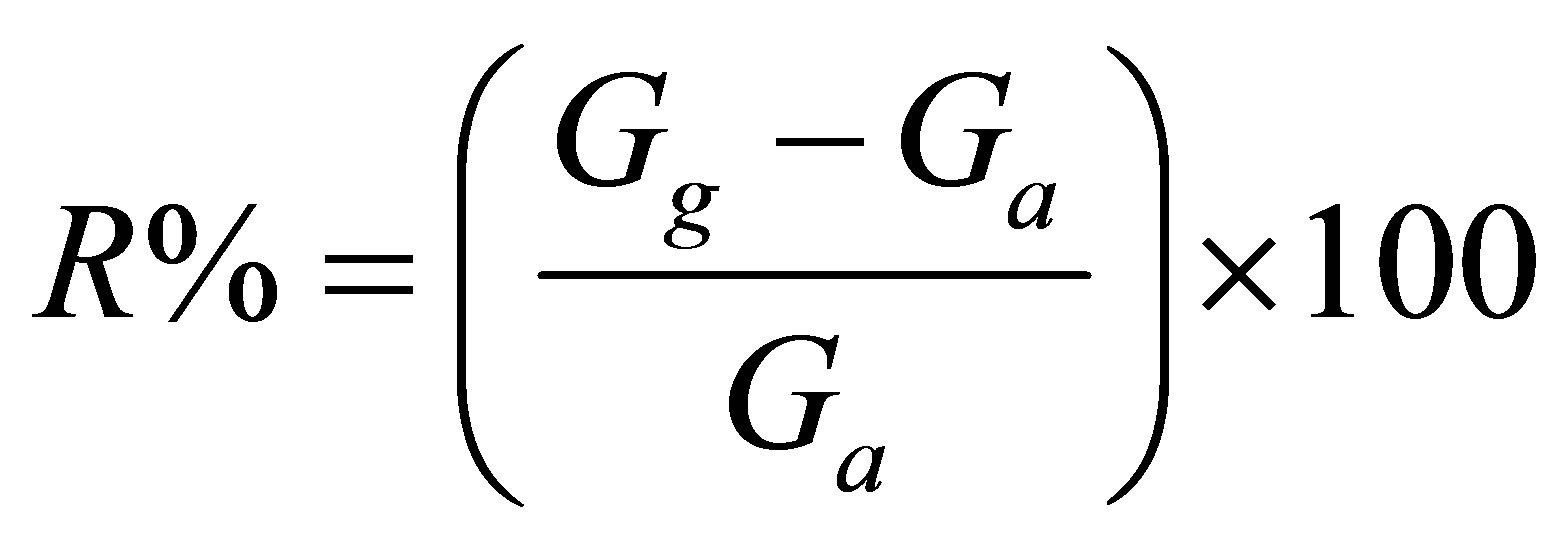 (1)
(1)
where Gg and Ga are conductance in the presence of test gas and air, respectively.
3. Results
Figure 1 shows the X-ray diffraction (XRD) pattern ob-
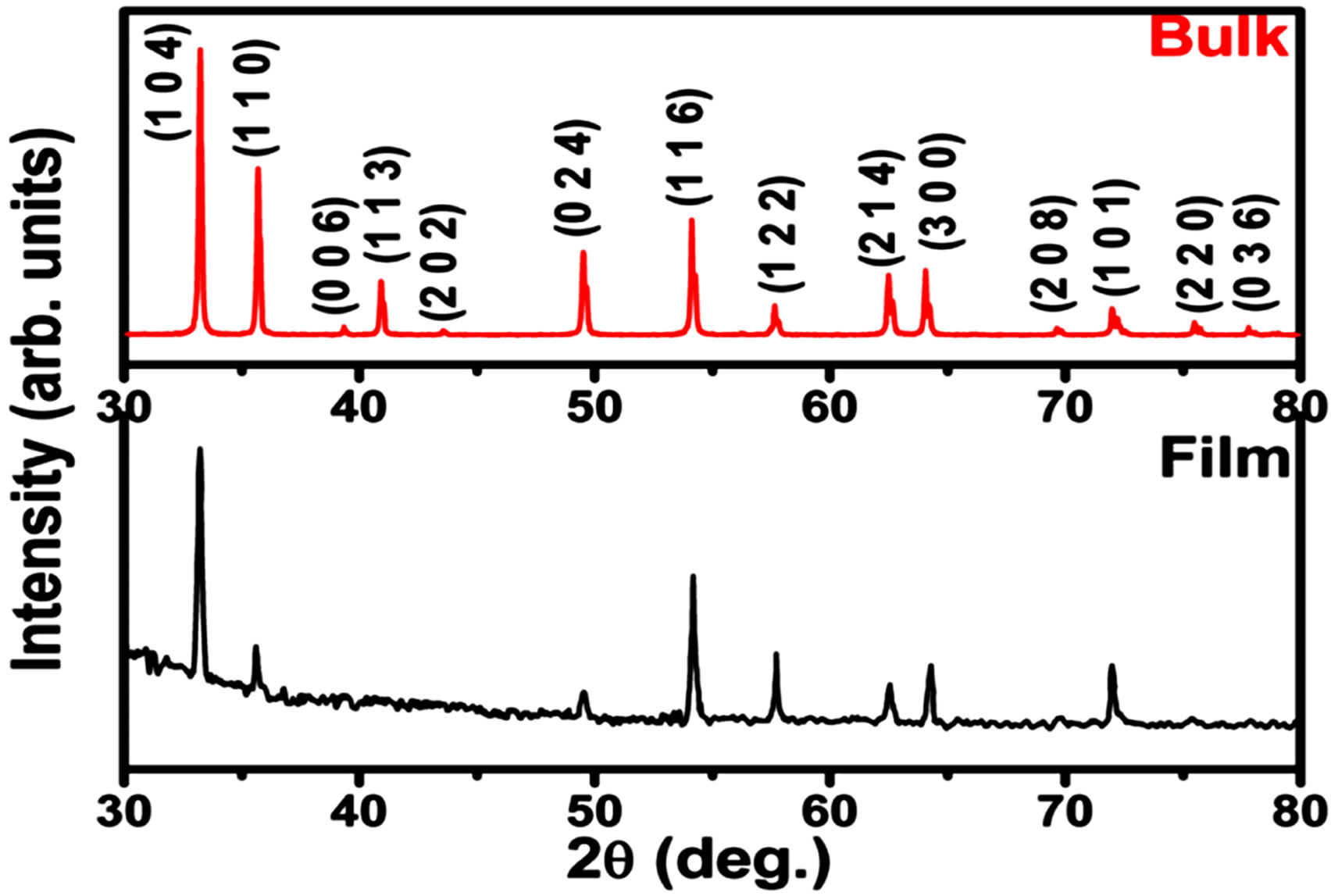
Figure 1. XRD diffraction pattern for Fe2O3 in bulk and thin film form.
tained for Fe2O3 thin film and bulk samples. All the peaks could be assigned to the single phase of Fe2O3 (hematite). The least-square fitting of the pattern indicated hexagonal rhombo-centred cubic unit cell structure with lattice parameters a = b = 5.028 Å, c =13.73 and α = β = 90˚, γ = 120˚, which are in agreement with the reported values [JCPDS card no. #79-0007].
Figure 2 is a plot of % response recorded for both the sensor films as a function of operating temperature towards 10 ppm of H2S. It is clearly evident that thin films and bulk sensors exhibit response maxima at 250˚C and 200˚C, respectively. At all the temperatures the response of thin films is higher than that of bulk samples. For instance, thin film sensors show, response maxima of 262% in comparison to 112%, exhibited by bulk samples towards 10 ppm H2S.
A Typical response curves for both the sensor films towards 10 ppm H2S is shown in Figure 3. The conductance of the sensors increases on exposure to H2S indicating an n-type response. Thin film sensors exhibited a maximum response towards H2S. A baseline drift was observed for bulk sensors.
Figure 4 shows the selectivity histogram recorded upon exposure to 10 ppm of various reducing and oxidizing test gases. It is clearly evident that both the sensors are highly selective towards H2S. A negligible response towards all the other interfering gases was observed.
4. Conclusion
Comparative gas sensing properties of Fe2O3 in thin film and bulk form have been investigated. Both the sensor types exhibited an n-type response characteristic with highly selective response towards H2S. An operating temperature for maximum response towards H2S was found to be 250˚C and 200˚C for thin film and bulk sensors, respectively. For H2S detection, thin film is a better sensor in comparison to bulk owing to the enhanced response and less baseline drift.
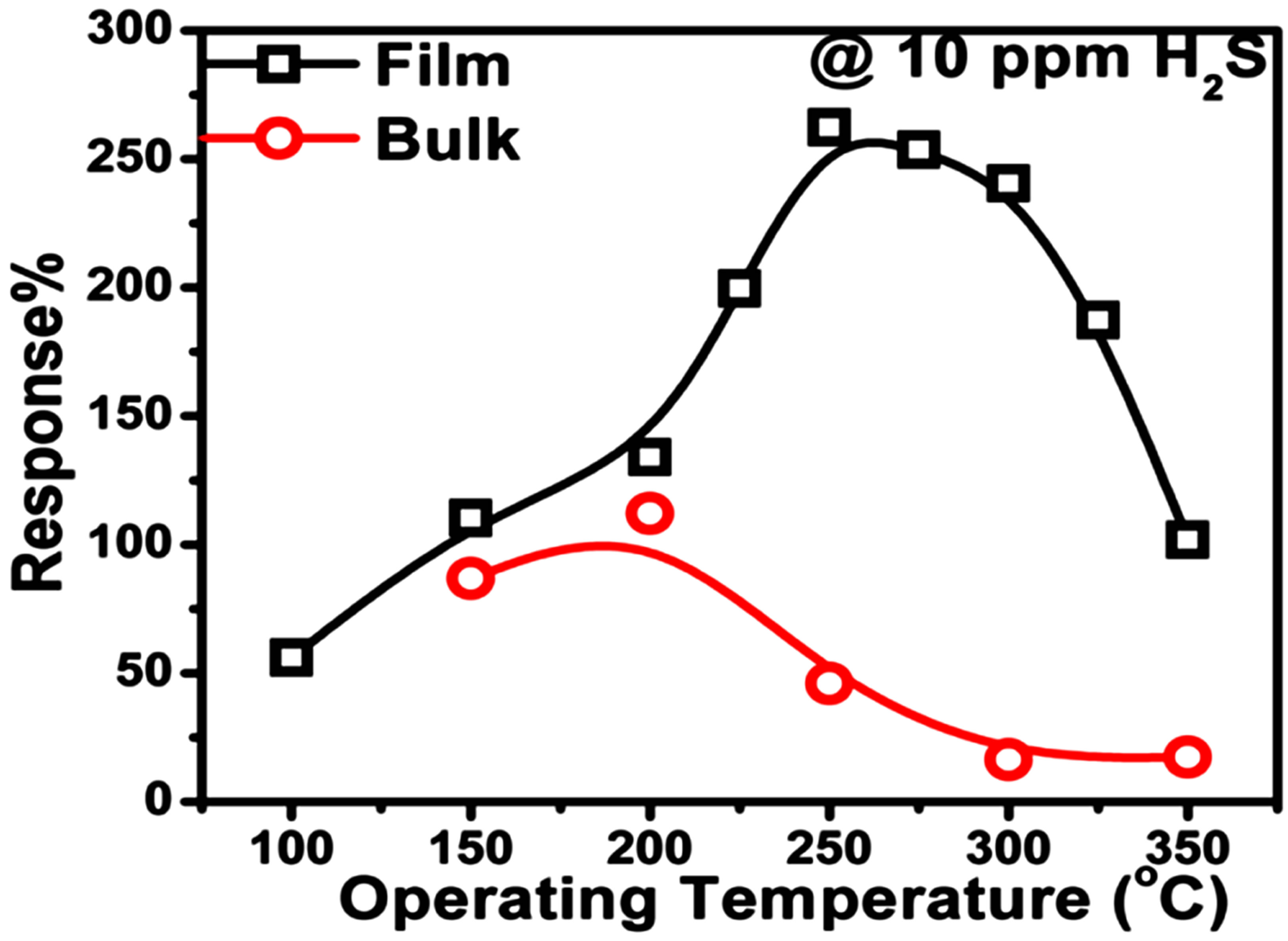
Figure 2. Sensor responses as a function of operating temperature.
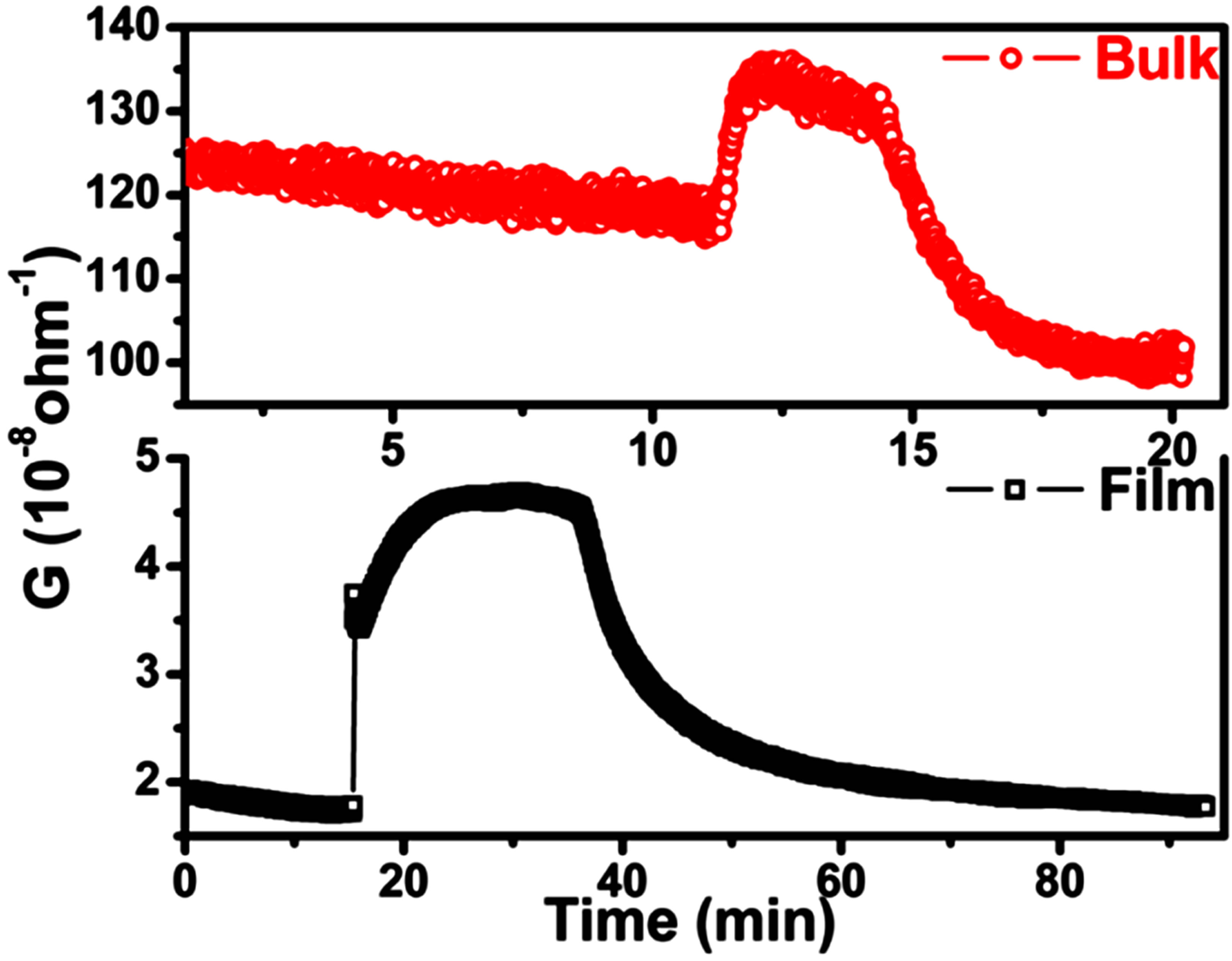
Figure 3. Sensor responses @ 10 ppm H2S for bulk and thin film sensor.
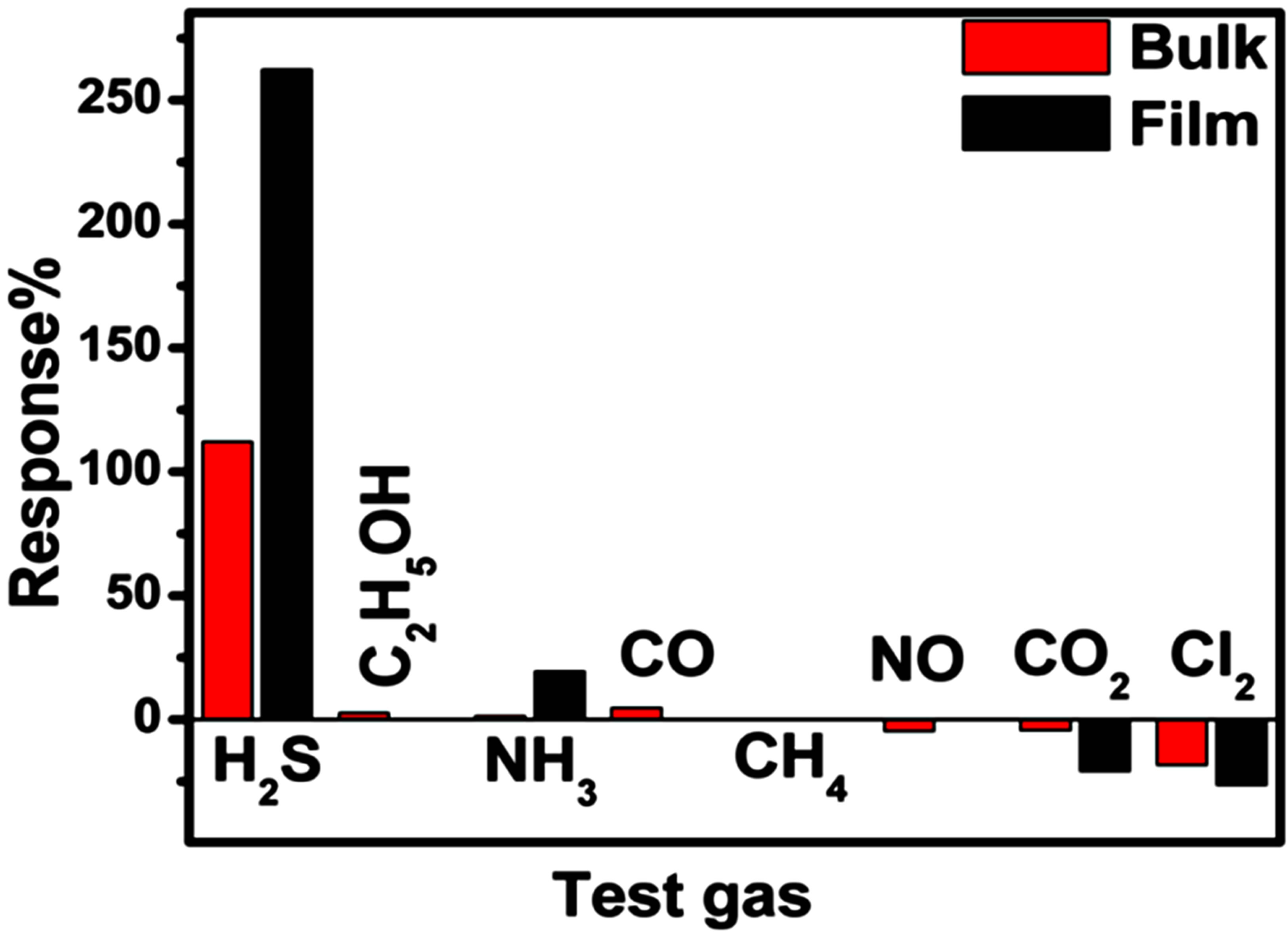
Figure 4. Selectivity data for bulk and thin films.
5. Acknowledgements
V.B. thanks CSIR, New Delhi for the senior research fellowship. The authors are thankful to BRNS-DAE for providing financial assistance vide Sanction No. 2008/ 37/4/BRNS.
REFERENCES
- M. Kaur, D. K. Aswal and J. V. Yakhmi, “Chemiresistor Gas Sensors: Materials, Mechanisms and Fabrication,” In: D. K. Aswal and S. K. Gupta, Eds., Science and Technology of Chemiresistor Gas Sensors, Nova Science Publisher, New York, 2007, pp. 33-93.
- V. Balouria, A. Kumar, A. Singh, S. Samanta, A. K. Debnath, A. Mahajan, R. K. Bedi, D. K. Aswal, S. K. Gupta and J. V. Yakhmi, “Temperature Dependent H2S and Cl2 Sensing Selectivity of Cr2O3 Thin Films,” Sensors and Actuators B: Chemical, Vol. 157, No. 2, 2011, pp. 466-472. http://dx.doi.org/10.1016/j.snb.2011.05.002
- V. Balouria, S. Samanta, A. Singh, A. K. Debnath, A. Mahajan, R. K. Bedi, D. K. Aswal, S. K. Gupta, “Chemiresistive Gas Sensing Properties of Nanocrystalline Co3O4 Thin Films,” Sensors and Actuators B: Chemical, Vol. 176, 2013, pp. 38-45. http://dx.doi.org/10.1016/j.snb.2012.08.064
- V. Balouria, A. Kumar, S. Samanta, A. Singh, A. K. Debnath, A. Mahajan, R. K. Bedi, D. K. Aswal, S. K. Gupta, “Nano-Crystalline Fe2O3 Thin Films for ppm Level Detection of H2S,” Sensors and Actuators B: Chemical, Vol. 181, 2013, pp. 471-478. http://dx.doi.org/10.1016/j.snb.2013.02.013

