Soft Nanoscience Letters
Vol. 2 No. 4 (2012) , Article ID: 23422 , 4 pages DOI:10.4236/snl.2012.24012
TiO2—Polysulfone Beads for Use in Photo Oxidation of Rhodamine B
![]()
1Applied Physics Division, Bhabha Atomic Research Centre, Mumbai, India; 2Chemistry Division, Bhabha Atomic Research Centre, Mumbai, India; 3Desalination Division, Bhabha Atomic Research Centre, Mumbai, India.
Email: svingale@barc.gov.in
Received July 24th, 2012; revised August 27th, 2012; accepted September 6th, 2012
Keywords: Photo Oxidation; Rhodamine B; TiO2 Beads; Polysulphone; Nanocrystallite
ABSTRACT
The nano sized TiO2 has been synthesized by sol gel process. The titaniumisopropaxide diluted in propanol hydrolyzed under acidic condition to form a gel. The solvent from gel pores has been extracted at ambient pressure resulting in nano sized TiO2 crystallites. The crystalline phase of TiO2 could be assigned to anatase structure. An average crystallite size is about 12 nm. The surface area of TiO2 found to be 235 m2/g. The TiO2 nanocrystallites thus produced were blended with polysulphone to form its beads for ease of operation. These beads of TiO2 were used as photo catalyst in conjunction with H2O2 oxidizer in presence of UV light (254 nm) for treating the 50 ppm Rhodamine B aqueous solution. The solution decolorized within 10 minutes resulting in disappearance of absorption peak at around 600 nm in UV spectrometry. The organic entities degrade in about 60 minutes. The beads of nano sized TiO2 could be easily recovered from the treated effluent for further use.
1. Introduction
The semiconductor photo catalysis using titania (TiO2) powdered material is recognized as one of the promising techniques for treating the effluents contaminated with dye materials [1]. It is known that to enhance TiO2 photo activity, particles should be small enough to offer a high specific surface area for efficient catalytic oxidation. To synthesize high surface area TiO2 for use in photooxidation of organic contaminants, various processes such as hydrothermal methods using amorphous TiO2, TiCl4 or TiOCl2 aqueous solutions, and sol-gel methods using titanium alkoxides, have been investigated and reported [2,3]. In spite of good photo catalytic activity, use of TiO2 in effluent treatment has certain limitations. Use of nano sized TiO2 is proved to be effective in degradation of organic contaminants [4] but the separation of the TiO2 powder material from the treated effluent is difficult. This issue has been addressed in the present paper.
We synthesized nano sized TiO2 from alkoxide precursor of Titanium using sol-gel method. The high surface area TiO2 powder thus obtained was blended with polysulphone to form TiO2 beads. The use of these beads provides large TiO2 surface area for effective photo oxidation of contaminant and avoids mixing of TiO2 particles with the treated effluent. The TiO2 beads have been used for treating the aqueous solution containing Rhodamine B dye, a known contaminant in textile industries effluents. The Rhodamine B, used in textiles and food stuffs is known to be harmful due to its carcinogenicity and the effluents containing this waste need to be treated effectively [2]. We developed a photo oxidation process using TiO2 xerogel beads as catalyst for successful removal of Rhodamine from aqueous solution. The advantage of using beads of TiO2 catalyst is that it can be separated easily from the treated effluent.
2. Experimental
The nano sized TiO2 has been synthesized by sol gel process [4] using titanium isopropoxide (TIP) as a precursor for TiO2. The Titanium (IV) isopropoxide (97% Aldrich) diluted in propanol (AR grade, Thomas and Bakers) was hydrolyzed under acidic condition to form a gel. The molar ratio of TIP: propanol: hydrofluoric acid (0.1 M) was kept at 1:12:4, respectively. The solvent from gel pores was extracted at ambient pressure resulting in nano sized TiO2 xerogel. The crystalline data for the nano sized TiO2 prepared by sol-gel process was obtained on a Philips X-ray diffractometer using a PW 1710 goniometer (CuKα, 30 kV, 20 mA). Commercially available anatase TiO2 (98%, Aldrich) is used as reference for comparison. The diffracted X-rays were collected by scanning between 10.01 to 79.99˚ in a scan step size of 0.02. UV-Vis spectra for the samples were recorded on a Jasco model V-670 spectrophotometer and the spectra were recorded in 200 - 800 nm wavelength range. The specific surface area and pore size distribution has been determined by nitrogen physisorption at 77 K using a Sorptomatic 1990 analyzer from CE Instruments, Italy.
The beads of TiO2 have been made using polysulfone (PS). The PS was dissolved in N-Methyl Pyrrolidone (NMP) along with polyvinyl pyrrolidone (PVP) of molecular weight 40,000 and to this solution, nano sized TiO2 prepared by sol-gel process was added. The weight % of TiO2 is 20% compared to PS. The resulting viscous solution was injected to water using 1 mm diameter syringe needle to obtain TiO2-PS beads. These beads have been used for photo oxidation of Rhodamine B.
The aqueous solution of Rhodmine B (50 ppm) was treated with H2O2 oxidizer in presence of TiO2 beads. In the photo oxidation experiments, 0.5 g of TiO2-PS beads added to 500 ml Rhodamine solution and the solution was irradiated using ultraviolet (UV) light (253.7 nm) in an UV reactor. H2O2 was added at a dose rate of 0.03 ml/minute to the Rhodamine solution being treated in the reactor. The treated solution of Rhodamine was filtered to separate TiO2 beads and was analyzed by photo absorption measurement in UV-Vis region using UV 3000+ spectrometer, LABINDIA, India.
3. Results and Discussion
The XRD pattern of nano sized TiO2 prepared by sol-gel process and commercially available TiO2 is shown in Figure 1. From the XRD studies, the crystalline phase of TiO2 could be assigned to anatase structure [5]. The average size of the TiO2 crystallites, as derived using Scherer formula and full-width—at-half maximum (FWHM) for the (101) diffraction line is 12 nm. It enhanced the surface area of TiO2 material multifold.
Figure 2 shows the UV-VIS spectra for (a) silica (b) commercial anatase TiO2 and (c) TiO2 xerogel. The blue shift in the wavelength threshold is observed for TiO2 xerogel (at λ—390 nm) as compared to that of commercial anatase TiO2 (at λ—410 nm) which indicated the increase in band gap energy for TiO2 xerogel. The increase in band gap energy for TiO2 xerogel to 3.18 eV from 3.03 eV derived for commercial anatase TiO2 is attributed to reduced particle size of TiO2 in the xerogel [6].
The nano sized TiO2 xerogel then were blended with polusulfone and N-Methyl Pyrrolidone viscous solution and the resultant slurry was injected in water to obtain TiO2 beads. The specific surface area derived from BET analysis [7] for commercial anatase TiO2 was found to be 40 m2/g whereas the surface area for TiO2 xerogel was found to be 235 m2/g. The increase in specific surface area is an important factor in photo catalytic oxidation
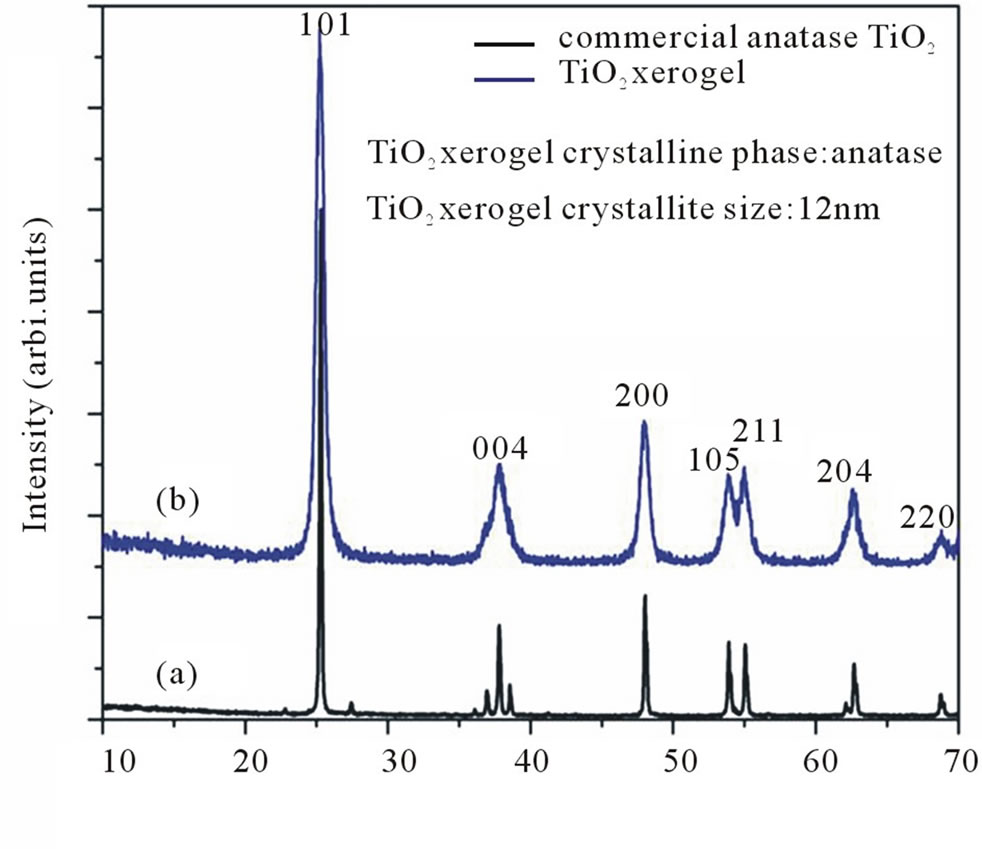
Figure 1. XRD pattern for (a) Commercially available anatase TiO2 and (b) TiO2 xerogel.
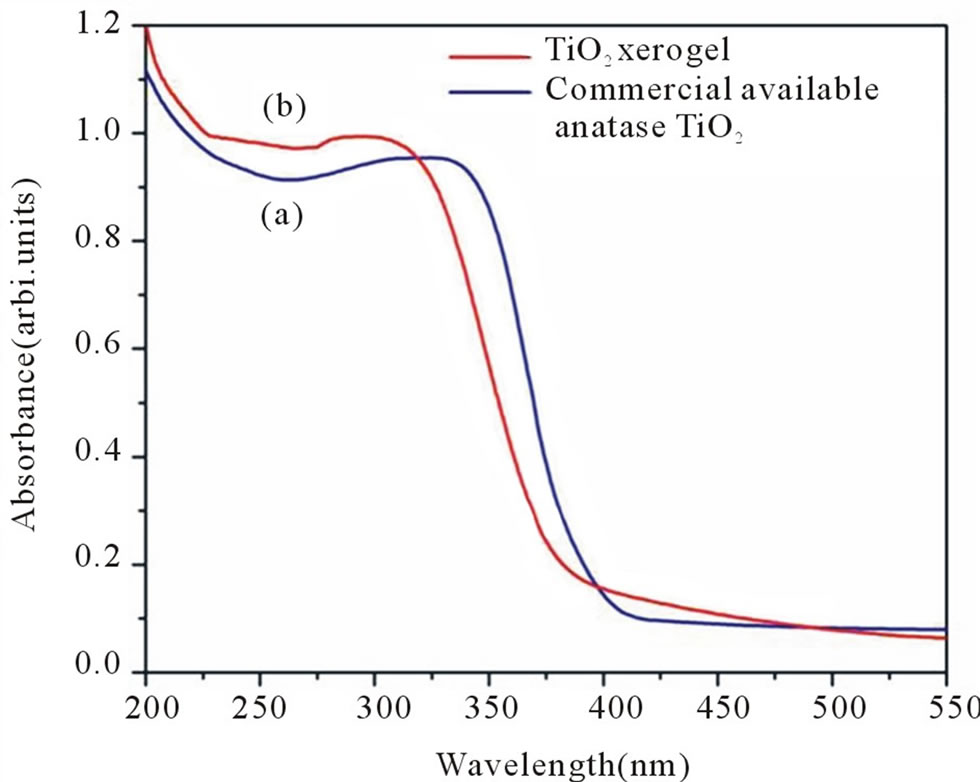
Figure 2. UV-VIS spectra for (a) commercial anatase TiO2 and (b) TiO2 xerogel.
reactions [8]. The specific surface area for TiO2-PS beads made from nano sized TiO2 xerogel powder was found to be 108 m2/g To demonstrate the photo oxidation potential capacity of the TiO2 beads, 50 ppm aqueous solution of Rhodamine was treated by using the TiO2 beads as photo catalyst along with H2O2 oxidizer. The TiO2-polysulphone beads and the Rhodamine solution before and after treatment are shown in Figure 3.
The mechanism of photo catalysis process for TiO2 is well described in literature. However, in brief, hit is mentioned here. The illumination of an aqueous TiO2 suspension with irradiation energy greater than the band gap energy (Eg) of the semiconductor TiO2 (hv ˃ Eg) generates valence band holes (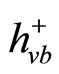 ) and conduction band electrons (
) and conduction band electrons (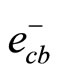 ) as follows:
) as follows:
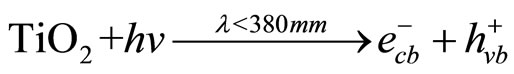 (1)
(1)
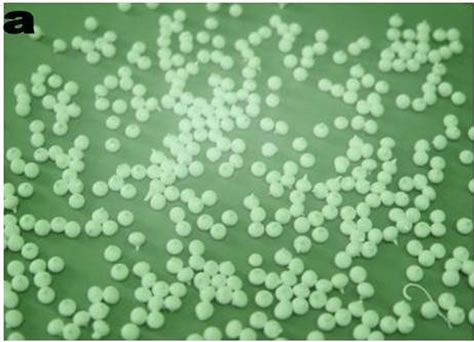


Figure 3. (a) TiO2 beads (b) Untreated Rhodamine solution (c) Rhodamine solution treated for 90 min.
In aqueous media, the photo generated charge carriers undergo redox processes with adsorbed species to form oxidation products.
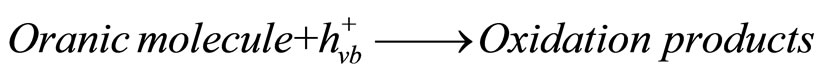 (2)
(2)
 (3)
(3)
The Rhodamine solution (50 pm) that was treated by using TiO2 beads as photo catalyst was analyzed by the photo absorption measurements and the results have been shown in Figure 4. The spetrophotometric analysis of treated solution that was sampled out at various time intervals shows a decrease in peak at about 550 nm. The absorbance becomes significantly low within 10 minutes of treatment and comes down to virtually zero level after 60 minutes. It indicates removal of dye from the solution and is attributed to breaking of conjugated chains or rings in Rhodamine that causes absorption in visible region [9]. The absorption peak at around 210 nm corresponds to absorption due to organic entities in the solution. It is observed that the peak at 210 nm decreases significantly down to 15% (Figure 4d) as compared to untreated solution (Figure 4a). It may be due to the degradation of Rhodamine into products like carbon dioxide and water which result in decrease in organic content in the solution. It indicates that about 90% mineralization efficiency for the dyes could be achieved by using TiO2 -PS beads. The data obtained for the Rhodamine solution treated by using TiO2 xerogel material as catalyst is shown here in Figure 5, for comparison. It is found that though the surface area of the TiO2-PS beads is less as compared to TiO2 xerogel powder the photo catalytic efficiency is almost same. The advantage of polysulfone to get adsorb the contaminant might have compensated the reduced surface area. The spectrometric measurement showed that the beads of nano sized TiO2 blended in polymer are effective catalyst in degradation of Rhodamine. The advantage of using beads is that the beads of nano sized TiO2 could be easily separated out from the treated effluent and could be reused.
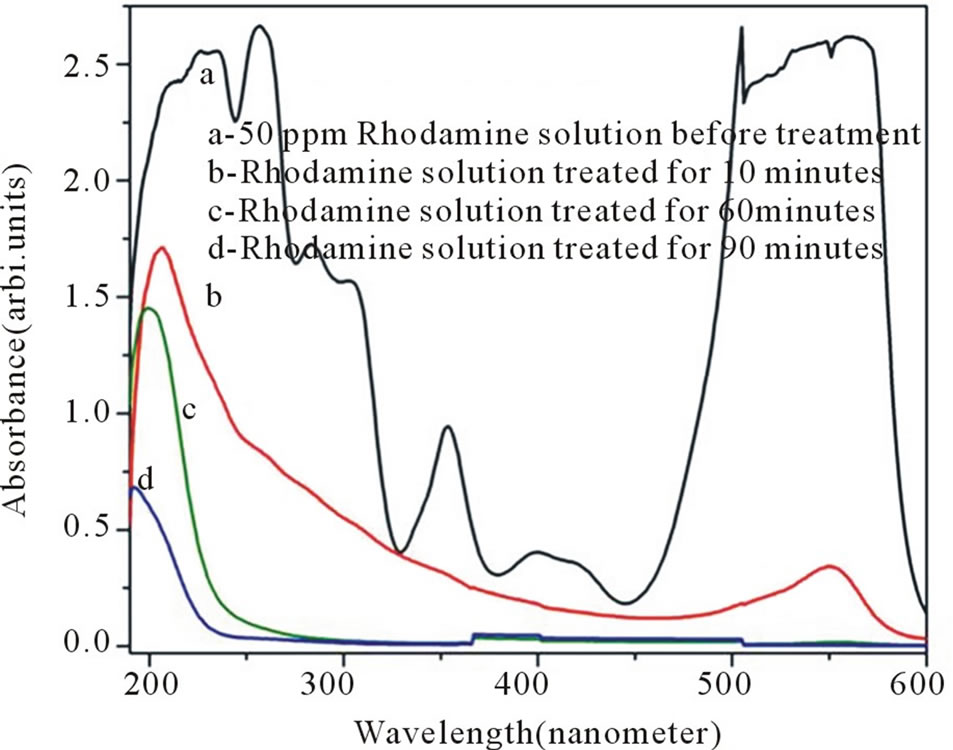
Figure 4. Spectrophotometric analysis of 50 ppm Rhodamine B solution treated by using H2O2 oxidizer and TiO2 beads as photo catalyst at different time intervals (a) 0 min (b) 10 min (c) 60 min (d) 90 min.
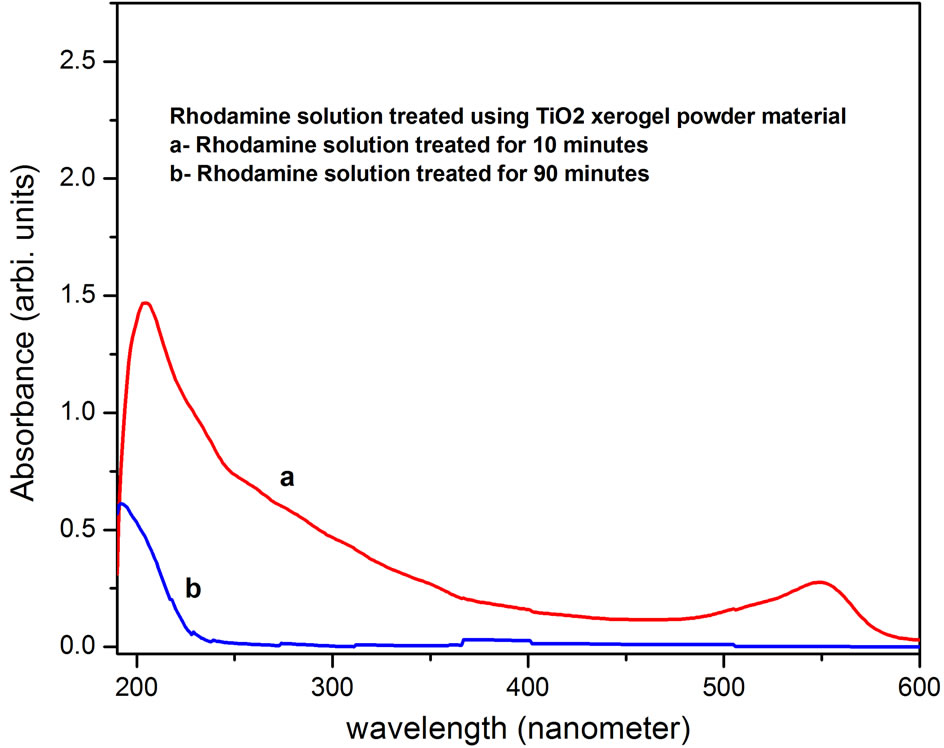
Figure 5. Spectrophotometric analysis of 50 ppm Rhodamine solution treated by using H2O2 oxidizer and TiO2 xerogel powder as photo catalyst at different time intervals (a) 10 min (b) 90 min.
4. Conclusion
The high surface area nano sized TiO2 material has been synthesized using sol-gel method. The nano sized TiO2 material was blended with polysulfone to form beads that can be easily used in photo catalytic oxidation of dyes. The TiO2-PS beads decolorize the 50 ppm Rhodamine solution within 10 minutes and found very effective in oxidative mineralization of Rhodamine B. The TiO2 beads are advantageous over the TiO2 powder that it avoid mixing of TiO2 particles with the effluent and could be easily recovered from the treated effluent for reuse.
5. Acknowledgements
The authors thank to Ratanesh Kumar, R. P. Patel, Sonu Gavit and Sandip Virnak, APD, BARC for their assistance in experimental work.
REFERENCES
- J. Wang, R. Li, Z. Zhang, W. Sun, X. Wang, R. Xu, Z. Xing and X. Zhang, “Degradation of Hazardous Dyes in Wastewater Using Nanometer Mixed Crystal TiO2 Powders under Visible Light Irradiation,” Water Air Soil Pollution, Vol. 189, No. 1-4, 2008, pp. 225-237. doi:10.1007/s11270-007-9570-2
- R. Jaina, M. Mathura, S. Sikarwara and A. Mittal, “Removal of the Hazardous Dye Rhodamine B through Photocatalytic and Adsorption Treatments,” Journal of Environmental Management, Vol. 85, No. 4, 2007, pp. 956-964. doi:10.1016/j.jenvman.2006.11.002
- H. B. Yin, Y. J. Wada, T. Kitamura, S. Kambe, S. Murasawa, H. Mori, T. Sakata and S. Yanagida, “Hydrothermal Synthesis of Nanosize Anatase and Rutile TiO2 Using Amorphous Phase TiO2,” Journal of Materials Chemistry, Vol. 11, No. 6, 2001, pp. 1694-1703. doi:10.1246/cl.2001.334
- S. V. Ingale, P. B. Wagh, A. K. Tripathi, A. S. Dudwadkar, S. S. Gamre, P. T. Rao, I. K. Singh and S. C. Gupta, “Photo Catalytic Oxidation of TNT Using TiO2-SiO2 Nano-Composite Aerogel Catalyst Prepared Using Solgel Process,” Journal of Sol-Gel Science & Technology, Vol. 58, No. 3, 2011, pp. 682-688. doi:10.1007/s10971-011-2445-4
- Y. Zhao, C. Li, X, Liu, F. Gu, H. Jiang, W. Shao, L. Zhang and Y. He, “Synthesis and Optical Properties of TiO2 Nanoparticles,” Materials Letters, Vol. 61, No. 1, 2007, pp. 79-83. doi:10.1016/j.matlet.2006.04.010
- G. Liu, C. Sun, H. Yang, S. Smith, L. Wang, G. Lu (Max) and H. Cheng, “Nanosized Anatase TiO2 Single Crystals for Enhanced Photocatalytic Activity,” Chemical Communications, Vol. 46, No. 5, 2010, pp. 755-757. doi:10.1039/b919895d
- D. C. M. Dutoit, M. Schneiderand and A. Baiker, “Titania-Silica Mixed Oxides: I. Influence of Sol-Gel and Drying Conditions on Structural Properties,” Journal of Catalysis, Vol. 153, No. 1, 1995, pp. 165-176. doi:10.1006/jcat.1995.1118
- Y.-H. Tseng, H.-Y. Lin, C.-S. Kuo, Y.-Y. Li and C.-P. Huang, “Thermostability of Nano TiO2 and Its Photo Catalytic Activity,” Reaction Kinetics and Catalysis Letters, Vol. 89, No. 1, 2006, pp. 63-69. doi:10.1007/s11144-006-0087-2
- C. W. Ma and W. Chu, “Photodegradation Mechanism and Rate Improvement of Chlorinated Aromatic Dye in Non-Ionic Surfactant Solutions,” Water Research, Vol. 35, No. 10, 2001, pp. 2453-2459. doi:10.1016/S0043-1354(00)00522-4

