Journal of Diabetes Mellitus
Vol.07 No.03(2017), Article ID:77837,15 pages
10.4236/jdm.2017.73006
Lycopene Ameliorates Diabetic-Induced Changes in Erythrocyte Osmotic Fragility and Lipid Peroxidation in Wistar Rats
Ejike Daniel Eze1*, Yusuf Tanko2, Ahmed Abubakar3, Sheu Oluwadare Sulaiman4, Karimah Mohammed Rabiu5, Aliyu Mohammed2
1Department of Physiology, Faculty of Biomedical Sciences, Kampala International University, Kampala, Uganda
2Department of Human Physiology, Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Pharmacognosy and Drug Development, Ahmadu Bello University, Zaria, Nigeria
4Department of Physiology, Faculty of Medicine, Kampala International University, Dar es Salaam, Tanzania
5Department of Biological Sciences, Faculty of Science, Yobe State University, Damaturu, Nigeria

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: May 1, 2017; Accepted: July 18, 2017; Published: July 21, 2017
ABSTRACT
Introduction: Diabetes mellitus has remained one of the serious health problems in the world; and oxidative stress has been reported to be a root cause for the progression and development of diabetes mellitus and its associated complications. Aim: This study investigated the possible ameliorative effects of lycopene on diabetic-induced changes in erythrocyte osmotic fragility and lipid peroxidation in Wistar rats. Methodology: The animals were made diabetic by single intraperitoneal injection of streptozotocin at 60 mg/kg b w. Diabetes was confirmed by the presence of high fasting blood glucose level ≥ 200 after 72 hours. Thereafter, diabetic rats were randomly assigned into six groups (1, 2, 3, 4, 5 and 6) comprising five animals each. Group 1 (Diabetic control) and group 2 (Normal control) rats received 0.5 ml of olive oil, groups 3, 4, 5 rats received 10, 20, 40 mg/kg bw of lycopene respectively, while those in group 6 received 2 mg/kg bw of glibenclamide orally once daily for a period of four weeks. At the end of the treatment, all animals were sacrificed; blood samples collected for determination of erythrocyte osmotic fragility (EOF) and lipid peroxidation (LPO). Results: The results obtained showed that there was a significantly (P < 0.05) lowered erythrocyte osmotic fragility in diabetic animals treated with lycopene when compared with diabetic control group. In addition, there was also a significantly (P < 0.05) reduced erythrocyte malondialdehyde concentration, an index of lipid peroxidation in the diabetic treated groups when compared with diabetic control group. Conclusion: From the available findings, it can be concluded that administration of lycopene to diabetic rats attenuated diabetic-induced changes in EOF and LPO and these observed effects may be attributed to anti-oxidative property of lycopene.
Keywords:
Diabetes Mellitus, Streptozotocin, Lycopene, Erythrocyte Osmotic Fragility, Lipid Peroxidation, Rats

1. Introduction
Diabetes mellitus is a complex metabolic disorder in the endocrine system characterized by abnormalities in insulin secretion and/or insulin action that leads to the progressive deterioration of glucose tolerance, which causes hyperglycaemia [1] [2] . There are two main categories of the disease, type 1 diabetes mellitus also called insulin-dependent diabetes mellitus (IDDM) and type 2, the non-in- sulin dependent diabetes mellitus (NIDDM) [3] . Several mechanisms are involved in the pathogenesis of diabetes and its complications but the most commonly accepted cause of diabetes is the oxidative damage that is caused by free radicals [4] . It has been shown that people who have diabetes have higher levels of free radicals, which can cause diabetic complications [5] .
Hyperglycaemia is mediated in large part, by a state of enhanced oxidative stress, which results in the excessive production of reactive oxygen species. These reactive oxygen species then cause both adverse structural and functional changes in tissues [6] including red blood cells. Oxidative stress, mediated mainly by hyperglycemia-induced generation of free radicals, contributes to the development and progression of diabetes mellitus and its related complications. The exact mechanism that leads to the hyperglycaemia-induced membrane lipid peroxidation in RBC of diabetics is not known. However, previous studies in a cell-free system have suggested that in hyperglycaemia, glucose can enolize and thereby reduce molecular oxygen yielding a-keto aldehydes and free radical intermediates [7] .
Red blood cells are distinctive, highly specialized and the most abundant cells in humans and contain high levels of both enzymatic and non-enzymatic cytoplasmic antioxidants [8] . They are the first cells in the body to be exposed to stressful stimuli and hence, prone to oxidative stress [8] . More so, because of its role as O2 and CO2 transporter, the erythrocytes are under constant exposure to reactive oxygen species (ROS) and oxidative stress [9] . As such, the erythrocytes have been used for the evaluation of the impact of free-radical induced oxidative stress in humans and animal models because of several reasons. For example, 1) these cells are continually exposed to high oxygen tensions, 2) unable to replace damaged components, 3) their membrane lipid bilayers are rich in polyunsaturated fatty acids side chains which make them vulnerable to peroxidation and 4) they have enzymatic and non-enzymatic antioxidant systems [10] . Management of diabetes without any side effects is still a challenge to the medical system. This leads to increasing demand for natural products with potent anti-diabetic activity and fewer side effects [11] . It has been reported that ameliorating oxidative stress using antioxidants might be an effective strategy for the treatment of diabetes mellitus and also reducing diabetic complications [12] . The philosophy that food can be health promoting beyond its nutritional value is gaining acceptance within the public arena and among the scientific community as mounting research link diet or food supplements to disease prevention and treatment such as in diabetes mellitus. Chemoprevention by dietary means continues to attract major attention in the management of chronic diseases such as of diabetes mellitus [13] .
Lycopene being an antioxidant has been suggested to protect critical biomolecules including lipids, protein and DNA from free radicals [14] . However, there is paucity of information on the benefit of lycopene in the management of diabetes mellitus. There has been increased interest in finding naturally occurring antioxidants for use in pharmaceutical applications, which can protect the human body from free radicals and retard the progress of many diseases such as diabetes mellitus and lycopene is one of such. This compound has great importance for their ability to prevent oxidation and is usually used as major ingredients in foods [15] . Oxidative stress is itself known to be a root cause for the progression and development of many diseases such as diabetes mellitus and its associated complications [16] [17] .
Glibenclamide is one of the members of the sulfonylurea class of drugs whose therapeutic benefits as oral hypoglycemic agents date back to the 1960s [18] . The drug exerts its effect by binding to and inhibiting the ATP-sensitive potassium channels (KATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1) in pancreatic beta cells. This inhibition results to cell membrane depolarization and opening voltage-dependent calcium channels thus resulting to an increase in intracellular calcium in the beta cell and subsequent stimulation of insulin release [19] [20] [21] [22] .Therefore, the present investigation explored the possible ameliorative effects of lycopene administration in diabetic-induced changes in erythrocyte osmotic fragility and lipid peroxidation in Wistar rats.
2. Materials and Methods
2.1. Materials
2.1.1. Animal Care
Adult Wistar rats of both sexes that weighed between 150 and 200 g were purchased from the Animal House of the Department of Human Physiology, Ahmadu Bello University, Zaria, Kaduna State, Nigeria. The animals were kept and maintained under the optimal laboratory conditions of temperature, humidity and light; and in clean Aluminum cages, where they were fed on standard commercial rat pellets (Vital Feeds) with free access to drinking water.
2.1.2. Chemicals and Lycopene
Streptozotocin was purchased from Sigma chemicals (St Louis U.S.A), while Lycopene (30 mg capsule, General Nutrition Corporation, Pittsburgh, U.S.A.) was procured from live well Pharmacy, Ceddi Plaza Central Area, Abuja, Federal Capital Territory, Nigeria. It was reconstituted in olive oil (Goya en espana, S.A.U., Savilla, Spain) to appropriate working dosage. All other chemicals and solvents used were of analytical grade.
2.2. Methods
2.2.1. Preparation of Lycopene Solution
30 mg of lycopene in a gelatinous capsule (General Nutrition Corporation, Pittsburgh, U.S.A) was reconstituted in olive oil (Goya en espana, S.A.U., Sevilla, Spain) to appropriate working doses as described by [23] with little modifications to obtain the required doses that were eventually used in the study.
2.2.2. Experimental Induction of Diabetes Mellitus
Diabetes was induced by single intraperitoneal injection of 60 mg/kg body weight dose of streptozotocin (STZ) dissolved in freshly prepared 0.1 M cold citrate buffer of pH 4.5 into the animals. The animals were starved of feeds for 18 hrs prior to diabetes induction but were allowed access to drinking water. Seventy-two hours after streptozotocin injection, blood was drawn from tail vein of the rats. Animals having fasting blood glucose levels ≥ 200 mg/dL were considered diabetic and used in the study. Thereafter, diabetic animals were randomly assigned into different groups according to the design of the study.
2.2.3. Experimental Design
A total of thirty (30) Wistar rats of both sexes were used in the study. They comprised of twenty-five (25) diabetic and five (5) normal (control) rats. The animals were randomized into six (6) groups of five (5) rats each.
Group 1: Normal control (NC) rats that were administered with 0.5 ml/kg body weight of olive oil.
Groups 2: Diabetic control (DC) group that received 0.5 ml/kg body weight of olive oil.
Group 3: Diabetic rats that received 10 mg/kg body weight of lycopene.
Group 4: Diabetic rats that received 20 mg/kg body weight of lycopene.
Group 5: Diabetic rats that received 40 mg/kg body weight of lycopene.
Group 6: Diabetic rats that received 2 mg/kg body weight of Glibenclamide.
30 mg lycopene in a gelatinous capsule was reconstituted in olive oil to appropriate working doses as described by Ogundeji et al. [23] with little modifications. All treatments were administered orally once daily for a period four (4) weeks.
2.2.4. Determination of Erythrocyte Osmotic Fragility
Determination of erythrocyte osmotic fragility was based on the method described by Faulkner and King [24] and modified by Oyewale [25] . It is an indirect method of determination of oxidative stress status. Briefly, 1 mL of freshly obtained blood which was collected into heparinized sample bottles from each rat was pipette into a set of test tubes containing 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 g/dL of sodium chloride stock solution (pH 7.4). This was followed by careful mixing and incubation for 30 min at room temperature (18˚C - 25˚C). Thereafter, the test tubes were centrifuged at 800 g for 10 minutes using a centrifuge IEC HNSH (Damon/IEC Division, UK). The supernatant was transferred into a glass cuvette. The concentration of haemoglobin in the supernatant was measured colorimetrically by reading the absorbance at a wavelength of 540 nm using a spectrophotometer (Jenwey, 6405, Japan). The percentage haemolysis was calculated using the following formula:

2.2.5. Determination of Erythrocyte Malondialdehyde Concentration
To evaluate the erythrocyte malondialdehyde concentration, which is an index of lipid peroxidation, heparinzed blood samples (2 ml) obtained from each rat was centrifuged at 3000 g for 5 minutes, and the plasma discarded. By washing erythrocytes three times in cold isotonic saline (0.9%, w/v), erythrocyte packets were prepared and used to assay for MDA concentrations using the double- heating method of Draper and Hadley [26] , as modified by [27] . The principle of the method was based on spectrophotometric measurement of the colour produced during the reaction to thiobarbituric acid (TBA) with MDA. The concentration of MDA was calculated by the absorbance coefficient of MDA?TBA complex, 1.56 × 105 cm−1∙M−1, and expressed in nanomoles per gram of haemoglobin. The method of Dacie and Lewis [28] was used to evaluate the haemoglobin concentration in the washed erythrocytes.
2.3. Statistical Analysis
Data obtained from each group were expressed as mean ± SEM of five determinations. The data were analyzed statistically using ANOVA with Tukey’s Post hoc test to compare the levels of significant between the control and experimental groups. All statistical analysis was evaluated using SPSS version 17.0 software and Microsoft Excel (2007). The values of p ≤ 0.05 were considered as significant.
3. Results
3.1. Effect of Lycopene Treatment on Erythrocyte Osmotic Fragility
The percentage erythrocyte osmotic fragility decreased significantly with increasing NaCl concentration. There was complete (100%) haemolysis at 0.0 and 0.1% of NaCl. And there was also no significant changes in erythrocyte osmotic fragility were observed at 0.0% and 0.1% of Na Cl (distilled water) in all control and experimental groups when compared. However, significant (P < 0.05) changes in percentage erythrocyte fragility were recorded at 0.3%, 0.5%, 0.7% and 0.9% of Nacl concentrations, when compared with the corresponding diabetic control group (Figure 1). At 0.3% NaCl concentration, the erythrocyte osmotic fragility of the diabetic control animals was significantly (P < 0.05)
Figure 1. Effects of lycopene treatment on erythrocyte osmotic fragility. Each bar represent mean of five animals. Bars with different superscript letters (a, b, c, d) differ significantly (P < 0.05) compared with the control groups, while bars with the same superscript letters are not significantly different (P > 0.05) compared with the control groups. DC + OL = Diabetic rats treated with olive oil, NC + OL = Normal (Non-diabet- ic) rats treated with olive oil, D+LYC10 mg/kg = Diabetic rats treated with 10 mg/kg of lycopene, D + LYC 20 mg/kg = Diabetic rats treated with 20 mg/kg of lycopene, D+LYC 40 mg/kg = Diabetic rats treated with 40 mg/kg of lycopene and D + GLB 2 mg/kg = Diabetic rats treated with glibenclamide 2 mg/kg.
higher (98.40% ± 0.51%) than those obtained in the normal control group (89.60% ± 1.17%). Following oral treatment with graded doses of lycopene and glibenclamide, the study recorded a significantly (P < 0.05) dose dependent decrease in erythrocyte osmotic fragility of (97.60% ± 1.17%, 94.00% ± 1.82%, 86.20% ± 1.49%) and (80.40% ± 6.52%) at 0.3% NaCl concentration, when compared with the diabetic control group. However, glibenclamide appeared to have a better effect than the lycopene treated groups especially at 0.3% NaCl concentration. Similarly, at 0.5%, 0.7% and 0.9% NaCl concentrations, a significant (P < 0.05) increase in the erythrocyte osmotic fragility of (32.00% ± 1.00%, 11.80% ± 0.66% and 7.60% ± 0.51%) was recorded in the diabetic control animals when compared with those obtained in the normal control rats (8.20% ± 0.74%, 2.00% ± 0.32% and 1.20% ± 0.20%). Treatment of diabetic animals with lycopene and glibencalmide produced a significantly (P < 0.05) lower erythrocyte osmotic fragility of (12.20% ± 0.66%, 8.80% ± 0.3%7, 8.40% ± 0.75% and 10.6% ± 1.25%), (3.00% ± 0.45%, 2.00% ± 0.32%, 1.60% ± 0.25% and 3.40% ± 0.75%) and (1.60% ± 0.25%, 1.80% ± 0.49%, 1.40% ± 0.25% and 1.40% ± 0.25%), when compared with the diabetic untreated group (Figure 1).
3.2. Effect of Lycopene Treatment on Erythrocyte MDA Concentration
The erythrocyte MDA concentrations an index of lipd peroxidation was significantly increased (P < 0.05) in the diabetic untreated group (2.10 ± 0.14 µmol/g Hb), when compared to those obtained in the normal control animals (1.10 ± 0.09 µmol/g Hb). The erythrocytes MDA concentration of all lycopene and glibenclamide treated diabetic animals was significantly (P < 0.01) decreased (1.48 ± 0.07, 1.26 ± 0.08, 1.02 ± 0.09 µmol/g Hb) and (1.44 ± 0.11 µmol/g Hb), when compared with diabetic control group that recorded (2.10 ± 0.14 µmol/g Hb) (Figure 2).
4. Discussion
In the present study, single intra-peritoneal injection of streptozotocin (STZ) effectively induced diabetes mellitus in rats, which was confirmed by the presence of sustained elevated fasting blood glucose levels 72 hrs after STZ administration in the animals. This result agrees with the findings of previous researchers [29] [30] [31] [32] , who demonstrated that fasting blood glucose levels increased significantly 72 hrs following STZ injection to experimental animals. STZ induces diabetes which resembles human hyperglycaemic non-ketotic diabetes mellitus in animal models [33] . Also, STZ selectively destroys the insulin producing β- cells of the Islet of Langerhans which is accompanied by characteristic alterations in blood insulin and glucose concentrations [34] .
Oral administration of graded doses of lycopene (10, 20 and 40 mg/kg b w) have been shown to significantly reduce the elevated fasting blood glucose levels in streptozotocin-induced diabetic rats, as demonstrated by previous researchers
Figure 2. Effects of lycopene treatment on erythrocyte malondialdehyde concentration. Each bar represents mean of five animals. Bars with different superscript letters (a, b, c, d, e) differ significantly (P < 0.05) compared with the control groups, while bars with the same superscript letters are not significantly different (P > 0.05) compared with the control groups. DC + OL = Diabetic rats treated with olive oil, NC + OL = Normal (Non- diabetic) rats treated with olive oil, D + LYC10 mg/kg = Diabetic rats treated with 10 mg/kg of lycopene, D + LYC 20 mg/kg = Diabetic rats treated with 20 mg/kg of lycopene, D + LYC 40 mg/kg = Diabetic rats treated with 40 mg/kg of lycopene and D + GLB 2 mg/kg = Diabetic rats treated with glibenclamide 2 mg/kg.
[35] [36] [37] [38] . Thus suggesting that lycopene may exhibit its antioxidant effect probably through scavenging of free radicals released from glucose auto- oxidation resulting from sustained hyperglycemia.
Erythrocyte is one of the main cells used as an oxidative stress marker in living animals, including humans, because their cell membranes are sensitive to the presence of free radicals in general [39] . Erythrocyte membranes are critical target in the lipid peroxidation process due to constant exposure to high oxygen tension and elevated polyunsaturated fatty acids (PUFA) in their membrane [40] , coupled with their inability to possess nucleus and other organelles [41] . The result obtained in the present investigation showed a significantly increased erythrocyte MDA concentration, an index of lipid peroxidation in the erythrocyte membrane of diabetic control animals, when compared to those obtained in the normal control group. This significant increase in erythrocyte MDA concentration in STZ-induced diabetic control rats indicates that hyperglycaemic increased free-radical induced lipid peroxidation in the erythrocytes and their membranes. These findings have been supported by data obtained from other researchers [42] [43] [44] who have demonstrated increased lipid peroxidation and protein oxidative damage as evidenced by higher MDA concentration in the erythrocytes of STZ-induced diabetic rats. Similarly, Sushi [7] has also reported hyperglycemia-induced membrane lipid peroxidation in human erythrocytes. The exact mechanism that leads to the hyperglycaemia-induced membrane lipid peroxidation in RBC of diabetics is not known. However, previous studies in a cell-free system have suggested that in hyperglycaemia, glucose can enolize and thereby reduce molecular oxygen yielding a-keto aldehydes and free radical intermediates [7] .
Red blood cells (RBCs) which are unique, highly specialized and the most abundant cells in the human organism have been reported to be highly susceptible to oxidative damage due to the high cell concentration of oxygen and hemoglobin, a powerful promoter of the oxidative process [8] . RBCs are one of the first cells to be affected by adverse conditions [45] . Circulating red blood cells act as a sink for free radicals since both superoxide radicals 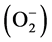 and hydrogen peroxide (H2O2) have the ability to penetrate membranes of the cells [46] . They are also subject to a continuous flux of O2 and H2O2, which results from auto-oxi- dation of haemoglobin [46] . However, lycopene treatment to diabetic rats attenuated the increased erythrocytes MDA concentration observed in the present study. This suggests that lycopene may be beneficial in ameliorating hyperglycaemia-induced oxidative damage to erythrocytes. This effect of lycopene may be attributed to its high oxygen-quenching capacity and singlet molecular oxygen (O2) and peroxyl radical-scavenging ability [47] , which consequently contribute to the defence against lipid peroxidation as reflected by significantly decreased erythrocytes MDA concentration observed in the present investigation.
and hydrogen peroxide (H2O2) have the ability to penetrate membranes of the cells [46] . They are also subject to a continuous flux of O2 and H2O2, which results from auto-oxi- dation of haemoglobin [46] . However, lycopene treatment to diabetic rats attenuated the increased erythrocytes MDA concentration observed in the present study. This suggests that lycopene may be beneficial in ameliorating hyperglycaemia-induced oxidative damage to erythrocytes. This effect of lycopene may be attributed to its high oxygen-quenching capacity and singlet molecular oxygen (O2) and peroxyl radical-scavenging ability [47] , which consequently contribute to the defence against lipid peroxidation as reflected by significantly decreased erythrocytes MDA concentration observed in the present investigation.
Furthermore, RBCs also contain high levels of both enzymatic and non-en- zymatic cytoplasmic antioxidants [8] . They are usually the first cells in the body to be exposed to stressful stimuli and hence, prone to oxidative stress [8] . In addition to its role as O2 and CO2 transporter, the erythrocytes are under constant exposure to reactive oxygen species (ROS) and oxidative stress [9] . Indeed, the erythrocytes have been used for the evaluation of the impact of free-radical induced oxidative stress in humans and animal models because of several reasons. For example, 1) these cells are continually exposed to high oxygen tensions, 2) unable to replace damaged components, 3) their membrane lipid bilayers are rich in polyunsaturated fatty acids side chains which make them vulnerable to peroxidation and 4) they have enzymatic and non-enzymatic antioxidant systems [10] .
The results of the present investigation showed that a significantly increased erythrocyte osmotic fragility were recorded at 0.3%, 0.5%, 0.7% and 0.9% of NaCl concentrations in streptozotocin-induced diabetic untreated animals when compared with those obtained in the normal control group. This finding corroborates the reports of other investigators who showed that erythrocyte osmotic fragility was significantly increased in streptozotocin-induced diabetic animals [48] [49] [50] . This current finding indicates the ability of streptozotocin-in- duced hyperglycaemia to compromise the integrity of the red blood cell membrane. The normal function of the RBC is largely hinged on the maintenance of the integrity of its membrane. The vulnerability of the red blood cell membrane integrity which resulted to increased erythrocyte fragility in the diabetic untreated group may have arisen from the increased lipoperoxidative (MDA) changes in the erythrocyte membranes as evidenced by increased MDA concentration in the erythrocytes of diabetic untreated animals observed in the present study. These results agree with findings of other researchers [51] [52] [53] that reported a significant positive correlation between the hyperglycaemia-induced membrane lipid peroxidation and the increased osmotic fragility of the erythrocyte membrane, which can cause changes in the properties of the RBC membrane and erythrocytes of STZ-induced diabetic rats. Erythrocyte is a convenient model to study oxidative damage of cell membranes by various pro-oxidants as well as the chemicals [54] . ROS-catalyzed oxidative damage to membrane lipids may impair the stability of erythrocytes and cause oxidative hemolysis or osmotic fragility [55] .
More so, the erythrocyte membrane has been shown to be highly sensitive to oxidative stress as it contains high amount of polyunsaturated fatty acids as well as higher concentration of oxygen and heme [56] . On the other hand, erythrocyte osmotic fragility test gives an in-vitro measure of the tensile strength of erythrocyte membrane and it is an indirect method of evaluating lipid peroxidation in animals [57] . It is a measure of erythrocyte strength and its ability to withstand varying osmotic gradients and it has being reported to be increased during oxidative stress [58] [59] . The greater the erythrocyte osmotic fragility, the weaker the tensile strength of erythrocyte membrane [23] . Similarly, hyperglycaemia associated with diabetes has been shown to increase the erythrocyte osmotic fragility and membrane lipid peroxidation in human erythrocytes [60] . Increased MDA level has been known to be associated with increased fragility of erythrocytes during oxidative stress [60] . The mechanisms for increased fragility of erythrocytes have been reported to be due to increased glycosylation of the erythrocyte membrane protein or/and alteration of the Na+/K+ ATPase on the erythrocyte membrane [50] . Administration of graded doses of lycopene and glibenclamide to diabetic animals recorded a significantly dose dependent decrease in percentage erythrocyte osmotic fragility, suggesting that lycopene and glibenclamide conferred some degree of stability to the erythrocytes of the diabetic rats when compared with the diabetic control group. The results are consistent with the observation of other researchers [61] [62] [63] [64] who showed that lycopene administration improved oxidative stress-induced lipid peroxidation in rats. Moreso, the increasing erythrocyte osmotic resistance (or less osmotically fragile) observed in the diabetic animals treated with lycopene shows the protective ability of lycopene against increased osmotic stress of the erythrocytes; that is, increasing hypotonicity. Hence, the observed effect in the current study may also be due to the ameliorative effects or/and anti-oxidative ac- tivities of lycopene on the erythrocyte oxidative damage associated with diabetes. This finding may be attributed to strong antioxidant property of lycopene [65] . Furthermore, another mechanism by which lycopene reduces the erythrocyte osmotic fragility in diabetic animals may probably be by mopping up free radicals that are usually involved in the destruction of membrane protein and lipid peroxidation. The finding in the present investigation which positively corroborates with same in erythrocyte MDA concentration, showed a significantly reduced lipid peroxidative (MDA) level in diabetic rats treated with lycopene.
5. Conclusion
The present study confirmed the involvement of oxidative stress in the progression of diabetes; and the findings obtained demonstrated that lycopene ameliorated the diabetic-induced alterations in erythrocyte osmotic fragility and lipid peroxidation in Wistar rats.
Acknowledgements
The authors of this work wish to acknowledge the technical assistance of Mr. O. Ayegbusi of the Department of Chemical Pathology, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria and Mallam Bala Mohammed, Mr Yusuf Inua, Mallam Yau Bello and Mallam Nura Mohammed of the Department of Human Physiology, Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria towards the successful completion of this research work.
Conflict of Interest
Authors of this manuscript declare that no competing interests exist.
Cite this paper
Eze, E.D., Tanko, Y., Abubakar, A., Sulaiman, S.O., Rabiu, K.M. and Mohammed, A. (2017) Lycopene Ameliorates Diabetic-Induced Changes in Erythrocyte Osmotic Fragility and Lipid Peroxidation in Wistar Rats. Journal of Diabetes Mellitus, 7, 71-85. https://doi.org/10.4236/jdm.2017.73006
References
- 1. Granner, D.K. (2000) Hormones of the Pancreas and Gastrointestinal Tract. In: Meyes, P.A., Ed., Harpers Biochemistry, McGraw Hill, New York, 610-626.
- 2. Eteng, M.U., Bassey, B.J., Atangwho, I.J., Egbung, G.E., Eyong, E.U., Ebong, P.E. and Abolaji, A.O. (2008) Biochemical Indices of Macrovascular Complication in Diabetic Rat Model: Compared Effects of Vernonia amygdalina, Catharantus roseus and Chlorpropamide. Asian Journal of Biochemistry, 3, 228-234.
https://doi.org/10.3923/ajb.2008.228.234 - 3. Raubenheimer, P. (2010) What Type of Diabetes Does My Patient Have and Is It Relevant? Continuing Medical Education, 28, 474-478.
- 4. Maritim, A.C., Sanders, R.A. and Watkins, J.B. (2003) Diabetes, Oxidative Stress, and Antioxidants: A Review. Journal of Biochemical and Molecular Toxicology, 17, 24-38.
https://doi.org/10.1002/jbt.10058 - 5. Memisogullari, R. and Bakan, E. (2004). Levels of Ceruloplasmin, Transferrin, and Lipid Peroxidation in the Serum of Patients with Type 2 Diabetes Mellitus. Journal of Diabetes Complications, 18, 193-197.
https://doi.org/10.1016/S1056-8727(03)00032-1 - 6. Mehta, J.L., Rasouli, N., Sinha, A.K. and Molavi, B. (2006) Oxidative Stress in Diabetes: A Mechanistic Overview of Its Effects on Atherogenesis and Myocardial Dysfunction. The international Journal of Biochemistry and Cell Biology, 38, 794-803.
https://doi.org/10.1016/j.biocel.2005.12.008 - 7. Sushil, K.J. (1989) Hyperglycemia Can Cause Membrane Lipid Peroxidation and Osmotic Fragility in Human Red Blood Cells. The Journal of Biological Chemistry, 264, 21340-21345.
- 8. Pandey, K.B. and Rizvi, S.I. (2011) Biomarkers of Oxidative Stress in Red Blood Cells. Biomedical Papers of Medical Faculty University Palacky Olomouc Czech Republic, 155, 131-136.
https://doi.org/10.5507/bp.2011.027 - 9. Matough, F.A., Budin, S.B., Hamid, Z.A., Louis, S.R., Alwahaibi, N. and Mohamed, J. (2012) Palm Vitamin E Reduces Oxidative Stress, and Physical and Morphological Alterations of Erythrocyte Membranes in Streptozotocin-Induced Diabetic Rats. Oxidants and Antioxidants in Medical Science, 1, 59-68.
https://doi.org/10.5455/oams.300412.or.006 - 10. Konyalioglu, S. and Karamenderes, C. (2005) The Protective Effects of Achillea L. Species Native in Turkey Against H2O2-Induced Oxidative Damage in Human Erythrocytes and Leucocytes. Journal of Ethnopharmacology, 102, 221-227.
https://doi.org/10.1016/j.jep.2005.06.018 - 11. Grover, J.K., Vats, V. and Yadav, S. (2002) Effect of Feeding Aqueous Extract of Petrocarpus marsupiun on Glycogen Content of Tissues and the Key Enzymes of Carbohydrate Metabolism. Molecular and Celular Biochemistry, 241, 53-59.
https://doi.org/10.1023/A:1020870526014 - 12. Mohammed, A., Adelaiye, A.B., Bakari, A.G. and Mabrouk, M.A. (2009) Anti-Diabetic and Some Haematological Effects of Ethylacetate and N-Butanol Fractions of Ganoderma lucidum Aqueous Extract in Alloxan-Induced Diabetic Wistar Rats. International Journal of Medicine and Medical Science, 1, 530-535.
- 13. Aruoma, O.I., Neergheen, V., Bahorun, T. and Jen, L.S. (2007) Free Radicals, Antioxidants and Diabetes: Embryopathy, Retinopathy, Neuropathy, Nephropathy and Cardiovascular Complications. Neuroembryology and Ageing, 4, 117-137.
https://doi.org/10.1159/000109344 - 14. Ashwani, K.S. and Prachi, G. (2013) Phytomodulatory Potential of Lycopene from Lycopersicum esculentum against Doxorubicin-Induced Nephrotoxicity. Indian Journal of Experimental Biology, 51, 635-645.
- 15. Slusarczyk, S., Hajones, M., Wozniak, K.S. and Mathowski, A. (2009) Antioxidant Activity of Polyphenols from Lycopus lucidus. Turcz, 113, 134-138.
- 16. Tiwari, A.K. (2004) Antioxidants: New Generation Therapeutic Base for Treatment of Polygenic Disorders. Current Science, 86, 1092-1102.
- 17. Kar, A. and Panda, S. (2005) Plant Extracts in the Regulation of Hypothyroidism. In: Sharma, S.K., Govil, J.N. and Singh, V.K., Eds., Recent Progress in Medicinal Plants, Vol. 10, Phytotherapeutics, Studium Press LLC, Houston, 419-426.
- 18. Marble, A. (1971) Glibenclamide, a New Sulphonylurea: Whither Oral Hypoglycaemic Agents? Drugs, 1, 109-115.
https://doi.org/10.2165/00003495-197101020-00001 - 19. Feldman, J.M. (1985) Glyburide: A Second-Generation Sulfonylurea Hypoglycemic Agent. History, Chemistry, Metabolism, Pharmacokinetics, Clinical Use and Adverse Effects. Pharmacotherapy, 5, 43-62.
https://doi.org/10.1002/j.1875-9114.1985.tb03404.x - 20. Kramer, W., Muller, G., Girbig, F., Gutjahr, U., Kowalewski, S., Hartz, D. and Summ, H.D. (1995) The Molecular Interaction of Sulfonylureas with β-Cell ATP-Sensitive K+ Channels. Diabetes Research and Clinical Practice, 28, S67-S80.
- 21. Ashcroft, F.M. (1996) Mechanisms of the Glycaemic Effects of Sulfonylureas. Hormone and Metabolic Research, 28, 456-463.
https://doi.org/10.1055/s-2007-979837 - 22. Panten, U., Schwanstecher, M. and Schwanstecher, C. (1996) Sulfonylurea Receptors and Mechanism of Sulfonylurea Action. Experimental and Clinical Endocrinology and Diabetes, 104, 1-9.
https://doi.org/10.1055/s-0029-1211414 - 23. Ogundeji, T., Ayo, J.O., Aluwong, T. and Mohammed, A. (2013) Behavioural Andhaematological Studies on Effects of Lycopene in Wistar Rats Subjected to Psychologicalstress. Journal of Neuroscience and Behavioral Health, 5, 30-35.
https://doi.org/10.5897/JNBH12.014 - 24. Faulkner, W.R. and King, J.W. (1970) Manual of Clinical Laboratory Procedures. Chemical Leather Company, 345.
- 25. Oyewale, J.O. (1992) Effects of Temperature and pH on the Osmotic Fragility of Erythrocytes of Domestic Fowl (Gallus domesticus) and Guinea Fowl (Numida maleagris). Research in Veterinary Science, 52, 1-4.
- 26. Draper, H.H. and Hadley, M. (1990) Malondialdehyde Determination as Index Oflipid Peroxidation. Methods in Enzymology, 186, 421-431.
- 27. Yavuz, T., Delibas, N., Yildirim, B., Altuntas, I., Candir, O., Cora, A., Karaman, N., Ibrisim, E. and Kutsal, A. (2004) Vascular Wall Damage in Rats Induced by Methidathion and Ameliorating Effect of Vitamin E and C. Archive of Toxicology, 78, 655-659.
https://doi.org/10.1007/s00204-004-0593-9 - 28. Dacie, J.V. and Lewis, S.M. (1991) Practical Haematology. 7th Edition, ELBS with Churchill Livingston, 37-85.
- 29. Mohammed, A., Tanko, Y., Okasha, M.A., Sadiq, Y. and Isa, A.I. (2008) Effect of Aqueous Methanolic Stem Bark of Maerua angolensis (Capparidaceae) Extract on Blood Glucose Levels of Streptozotocin-Induced Diabetic Wistar Rats. Research Journal of Pharmacology, 1, 75-78.
- 30. Krishna, D., Rao, S. and Satyanarayana, M.L. (2012) Serum Insulin Levels and Lipid Profiles of Streptozotocin Induced Diabetic Wistar Rats. Journal of Indian Veterinary Association, Kerala, 10, 22-26.
- 31. Daniel, E.E., Mohammed, A., Tanko, Y., Ahmed, A. and Rabiu, K.M. (2015) Hypoglycaemic Effect of Lycopene in Streptozotocin Induced Diabetic Wistar Rats. British Journal of Medicine and Medical Research, 7, 762-770.
https://doi.org/10.9734/BJMMR/2015/15908 - 32. Daniel, E.E., Mohammed, A., Tanko, Y. and Ahmed, A. (2015) Lycopene Ameliorates Atherogenic Cardiovascular Risk in Streptozotocin-Induced Diabetic Hyperlipidaemia in Wistar Rats. The Journal for Endocrinology and Metabolism, 105, 160-176.
- 33. Weir, G.C., Clore, E.T., Zmachinski, C.J. and Bonner-Weir, S. (1981) Islet Secretion in a New Experiment. Model for Non-Insulin Dependent Diabetes. Diabetes, 30, 590-595.
- 34. Szkudelski, T. (2001) The Mechanism of Alloxan and Streptozotocin Action in β-Cells of the Rat Pancreas. Physiological Research, 50, 537-546.
- 35. Duzguner, V., Kucukgul, A., Erdogan, S., Celik, S. and Sahin, K. (2008) Effect of Lycopene Administration on Plasma Glucose, Oxidative Stress and Body Weight in Streptozotocin Diabetic Rats. Journal of Applied Animal Research, 33, 17-20.
https://doi.org/10.1080/09712119.2008.9706888 - 36. Ali, M.M. and Agha, F.C. (2009) Amelioration of Streptozotocin-Induced Diabetes Mellitus, Oxidative Stress and Dyslipidemia in Rats by Tomato Extract Lycopene. Scandinavian Journal of Clinical and Laboratory Investigation, 69, 371-379.
https://doi.org/10.1080/00365510802658473 - 37. Aydin, M. and Celik, S. (2012) Effects of Lycopene on Plasma Glucose, Insulin Levels, Oxidative Stress, and Body Weights of Streptozotocin-Induced Diabetic Rats. Turkish Journal of Medical Sciences, 42, 1406-1413.
- 38. Sevim, C.Y., Fatmagül, Y. and Ebubekir, C. (2013) Effect of Lycopene Application in Rats with Experimental Diabetes Using Lipoprotein, Paraoxonase and Cytokines. Journal of Membrane Biology, 246, 621-626.
https://doi.org/10.1007/s00232-013-9575-2 - 39. José, A.M., José, G., Liliana, G., María del Carmen, C., Eduardo, M., Jaime, E., César, E. and Manuel García-Luna, G. (2010) Effect of Sodium Fluoride Ingestion on Malondialdehyde Concentration and the Activity of Antioxidant Enzymes in Rat Erythrocytes. International Journal of Molecular Science, 11, 2443-2452.
https://doi.org/10.3390/ijms11062443 - 40. Kolanjiappan, K., Manoharan, S. and Kayalvizhi, M. (2002) Measurement of Erythrocyte Lipids, Lipid Peroxidation, Antioxidants and Osmotic Fragility in Cervical Cancer Patients. Clinica Chimica Acta, 326, 143-149.
- 41. Doreeviae, N.Z., Barbiae, G.M., Markoviae, S.D., Ognjanoviae, B.K., Stajn, A.S., Zikiae, R.V. and Saièiae, Z.S. (2008) Oxidative Stress and Changes in Antioxidative Defense System in Erythrocytes of Preeclampsia in Women. Reproductive Toxicology, 25, 213-218.
- 42. Hussein, J., Mostafa, E., El-Waseef, M., El-Khayat, Z., Badawy, E. and Medhat, D. (2011) Effect of Omega-3 Fatty Acids on Erythrocyte Membrane in Diabetic Rats. Macedonian Journal of Medical Sciences, 4, 234-239.
https://doi.org/10.3889/MJMS.1857-5773.2011.0180 - 43. Yang, W., Fu, J., Yu, M., Huang, Q., Wang, D., Xu, J., Deng, Q., Yao, P., Huang, F. and Liu, L. (2012) Effects of Flaxseed Oil on Anti-Oxidative System and Membrane Deformation of Human Peripheral Blood Erythrocytes in High Glucose Level. Lipids in Health and Disease, 11, 28.
https://doi.org/10.1186/1476-511X-11-88 - 44. Mohammed, R.K., Ibrahim, S., Atawodi, S.E., Eze, E.D., Suleiman, J.B., Ugwu, M.N. and Malgwi, I.S. (2013) Anti-Diabetic and Haematological Effects of N-Butanol Fraction of Alchornea cordifolia Leaf Extract in Streptozotocin-Induced Diabetic Wistar Rats. Scientific Journal of Biological Sciences, 2, 45-53.
- 45. Bryszewska, M., Zavodnik I.B., Niekurzale, A. and Szosland, K. (1995) Oxidative Processes in Red Blood Cells from Normal and Diabetic Individuals. Biochemistry and Molecular Biology International, 37, 345-354.
- 46. Andallu, B. and Varadacharyulu, N.C. (2003) Antioxidant Role of Mulberry (Morus indica L. cv. Anantha) Leaves in Streptozotocin-Diabetic Rats. Clinica Chimica Acta, 338, 3-10.
- 47. Stahl, W. and Sies, H. (2003) Antioxidant Activity of Carotenoids. Molecular Aspects of Medicine, 24, 345-351.
- 48. Shin, S., Ku, Y., Babu, N. and Singh, M. (2007) Erythrocyte Deformability and Its Variation in Diabetes Mellitus. Indian Journal of Experimental Biology, 45, 121-128.
- 49. Megha, S. and Sehyun, S. (2009) Changes in Erythrocyte Aggregation and Deformability in Diabetes Mellitus: A Brief Review. Indian Journal of Experimental Biology, 47, 7-15.
- 50. Arun, P.M. (2013) Erythrocyte Fragility Increases with the Duration of Diabetes in Indian Population. International Journal of Basic and Applied Medical Sciences, 3, 172-177.
- 51. Qujeq, D., Habibinudeh, M., Daylmkatoli, H. and Rezvani, T. (2005) Malondialdehyde and Carbonyl Contents in the Erythrocytes of Streptozotocin-Induced Diabetic rats. International Journal of Diabetes and Metabolism, 13, 96-98.
- 52. Ahmed, F.N., Naqvi, F.N. and Shafiq, F. (2006) Lipid Peroxidation and Serum Antioxidant Enzymes in Patients with Type 2 Diabetes Mellitus. Annals of the New York Academy of Sciences, 1084, 481-489.
https://doi.org/10.1196/annals.1372.022 - 53. Gauri, S.V. and Vijaya, A.H. (2008) RBC Membrane Composition in Insulin Dependent Diabetes Mellitus in Context of Oxidative Stress. Indian Journal of Clinical Biochemistry, 23, 223-226.
https://doi.org/10.1007/s12291-008-0050-2 - 54. Sharma, R.K., Jaiswal, S.K., Siddiqi, N.J. and Sharma, B. (2012) Effect of Carbofuran on Some Biochemical Indices of Human Erythrocytes in Vitro. Cell and Molecular Biology, 58, 103-109.
- 55. Tavazzi, B., Di Pierro, D., Amorini, A.M., Fazzina, G., Tuttobene, M., Giardina, B. and Lazzarino, G. (2000) Energy Metabolism and Lipid Peroxidation of Human Erythrocytes as a Function of Increased Oxidative Stress. European Journal of Biochemistry, 267, 684-689.
https://doi.org/10.1046/j.1432-1327.2000.01042.x - 56. Mansour, S.A., Mossa, A.T. and Heikal, T.M. (2009) Effects of Methomyl on Lipid Peroxidation and Antioxidant Enzymes in Rat Erythrocytes: In Vitro Studies. Toxicology and Industrial Health, 25, 557-563.
https://doi.org/10.1177/0748233709349829 - 57. Akande, M.G., Aliu, Y.O., Ambali, S.F. and Ayo, J.O. (2014) Protective Effect of Taurine in Chlorpyrifos and Lead-Induced Haematological Alterations in Wistar Rats. Toxicological and Environmental Chemistry, 96, 171-182.
- 58. Aldrich, K.J., Saunders, D.K., Sievert, L.M. and Sievert, G. (2006) Transaction of the Kansans. Academy of Sciences, 109, 149-158.
- 59. Adenkola, A.Y., Ayo, J.O., Sackey, A.K.B. and Adelaiye, A.B. (2010) Erythrocyte Osmotic Fragility of Pigs Administerd Ascorbic Acid and Transported by Road for Short Term Duration during the Harmattan Season. African Journal of Biotechnology, 9, 226-233.
- 60. Saba, A.B., Oyagbemi, A.A. and Azeez, O.I. (2010) Antidiabetic and Haematinic Effects of Parquetina nigrescens on Alloxan Induced Type-1 Diabetes and Normocytic Normochromic Anaemia in Wistar Rats. African Health Sciences, 10, 276-283.
- 61. El-Komy, M.M. and Hassan, H.A. (2005) The Role of Tomato-Juice as a Protective Agent against Thioacetamide Hepatotoxicity in Male Rats. Comparative Physiology, 46, 217-234.
- 62. Moreira, E.A.M., Fagundes, R.L.M., Filho, D.W., Neves, D., Sell, F., Bellisle, F. and Kupek, E. (2005) Effects of Diet Energy Level and Tomato Powder Consumption on Antioxidant Status in Rats. Clinical Nutrition, 24, 1038-1046.
- 63. Atessahin, A., Yilmaz, S., Karahan, I., Ceribas, A.O. and Karauglu, A. (2005) Effects of Lycopene against Cisplant-Induced Nephrotoxicity and Oxidative Stress in Rats. Toxicology, 212, 116-123.
- 64. Yilmaz, S., Atessahin, A., Sahna, E., Karahan, I. And Ozer, S. (2006) Protective Effect of Lycopene on Adriamycin-Induced Cardiotoxicity and Nephrotoxicity. Toxicology, 218, 164-171.
- 65. Kumar, P. and Kumar, A. (2009) Effects of Lycopene and Epigallocatechin-3-Gallate against 3-Nitropropionic Acid Induced Cognitive Dysfunction and Gluthathione Depletion in Rats: A Novel Nitric Oxide Mechanism. Food and Chemical Toxicology, 47, 2522-2530.



