Journal of Diabetes Mellitus
Vol.4 No.2(2014), Article ID:45054,11 pages
DOI:10.4236/jdm.2014.42015
Evaluation of Anti-Hyperglycemic and Anti-Hyperlipidemic Activities of Water Kefir as Probiotic on Streptozotocin-Induced Diabetic Wistar Rats
Muneer Alsayadi1,2*, Yaser Al Jawfi3, Meriem Belarbi2, Zoubida Soualem-Mami2, Hafida Merzouk4, Daoudi Chaban Sari2, Fatima Sabri2, Meriem Ghalim2
1Department of Food Science and Technology, Faculty of agriculture, Ibb University, Ibb, Yemen
2Laboratory of Natural Products, Department of Biology, Faculty of Natural and Life Sciences, Earth and Universe, University of Abou-Bekr Belkaïd, Tlemcen, Algeria
3Department of Food Safety, Station of Agriculture Researches, Sana’a, Yemen
4Laboratory of Physiology, Physiopathology and Biochemistry of Nutrition, Department of Biology, Faculty of Natural and Life Sciences, Earth and Universe, University of Abou-Bekr Belkaïd, Tlemcen, Algeria
Email: *Muneer782003@yahoo.com, smuneer006@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 December 2013; revised 5 January 2014; accepted 12 January 2014
Abstract
Diabetes mellitus is a predominant chronic disease which causes mortality of millions of people yearly. Its prevalence is on the rise worldwide. Water kefir is fermented food produced by a matrix of polysaccharides containing bacteria and yeasts, with therapeutic properties. Our study aimed to evaluate anti-hyperglycemic and anti-hyperlipidemic activities of water kefir on streptozotocin-induced diabetic Wistar rats. Adult Wistar rats were made diabetic by intraperitoneal injection of streptozotocin, and were given or not kefir in drinking water for 5 weeks. Body weight, glucose and lipid levels were measured. The results demonstrated evident improvement in body weight, glucose, and lipid profiles of treated rats comparing with diabetic or control rats. Water kefir is found to be less cost hypoglycemic and hypolipidimic treatment and less time consuming. Water kefir can potentially be useful food for diabetes to control glucose and lipid levels.
Keywords
Component, Formatting, Style, Styling, Anti-Hyperglycemic, Anti-Hyperlipidaemic, Water Kefir, Diabetes, Wistar Rat

1. Introduction
Diabetes mellitus is a chronic, hereditary disease characterized by an abnormally elevated level of blood glucose (hyperglycemia) and by the excretion of the excess of glucose in the urine (glycosuria). The basic defect appears to be an absolute or relative lack of insulin or decrease in insulin receptors on the membrane of the target cells, which lead to abnormalities in carbohydrate metabolism as well as in lipid and protein metabolism [1] .
Diabetes mellitus, a serious chronic metabolic disorder, is identified by hyperglycemia resulting from deficiency in insulin secretion and/or decreased reaction of the organs to insulin [2] [3] .
Diabetes mellitus has been and will probably continue to be classified as growth or juvenile onset and maturity or adult onset. The National Diabetes Data Group (NDDG) and the 1980, 1985 Expert Committees of WHO have proposed a classification without regard to age. The two primary types are insulin-dependent, ketosis prone diabetes mellitus (IDDM) or Type 1, and non-insulin-dependent, non-ketosis prone diabetes mellitus (NIDDM) or Type 2. In both the 1980 and 1985 reports, other classes of diabetes included other types [2] [4] . The cause for type 1 diabetes is an absolute deficiency of insulin secretion, whereas type 2 diabetes is a combination of resistance to insulin action and inadequate compensatory insulin secretory response.
The world prevalence of diabetes among adults (aged 20 - 79 years) was 6.4%, affecting 285 million adults, in 2010, and will increase to 7.7%, and 439 million adults by 2030 [5] .
Epidemiological studies have suggested that dyslipidaemia is a risk factor for diabetic neuropathy [6] . In diabetic subjects overproduction of FFA and impaired lipoprotein metabolism induces an increase in plasma lipid components [7] . Type 1 and Type 2 diabetes mellitus are associated with metabolic syndrome and a marked increase in the risk of coronary heart disease [8] . Adipose tissue is now recognized as an endocrine organ that contributes to the physiopathology of type 2 diabetes [9] . Diabetes mellitus has also been associated with an increased risk for developing premature atherosclerosis due to an increase in triglycerides (TG) and low-density lipoproteins (LDL), and decrease in high density lipoprotein levels (HDL) [10] .
Abnormalities of lipoprotein metabolism cause various hypoor hyperlipoproteinemias. The most common of these is diabetes mellitus, where insulin deficiency causes excessive mobilization of FFA and underutilization of chylomicrons and VLDL, leading to hypertriacylglycerolemia. Most other pathologic conditions affecting lipid transport are due primarily to inherited defects, some of which cause hypercholesterolemia, and premature atherosclerosis. Obesity particularly abdominal obesity is a risk factor for increased mortality, hypertension, type 2 diabetes mellitus, hyperlipidemia, hyperglycemia, and various endocrine dysfunctions [11] . In addition to the established major risk factors, atherosclerosis in type 2 diabetes is also related to alterations in lipid and lipoprotein profile [12] . Many epidemiological studies have demonstrated that type 2 diabetes mellitus is a wellknown risk factor for the development of cardiovascular disease, cerebrovascular disease, and peripheral vascular diseases [13] -[18] .
It is projected that emerging economies India and China alone will lose around US $0.5 trillion and $0.25 trillion, respectively, as a result of these chronic diseases in the next decade [19] .
A major focus of current anti-diabetic research is the development of anti-hyperglycemic agents that are safe and free of negative side-effects.
Recently an increasing number of natural compounds are extracted from animal and plant sources with antidiabetic properties [20] [21] . Functional foods and nutraceuticals have become prescribed widely to prevent the occurrence of diabetes mellitus, and to attenuate the complications of hyperglycemia in diabetic patients, because of their effectiveness, limited side effects, and relatively low cost.
Fermented food, their components, and their microorganisms have been described to have hypoglycemic effects. Kefir can potentially be a useful food choice for patients with diabetes who are required to control their blood glucose levels [22] . The results of Maeda et al., [23] showed that kefiran had hypoglycemic effects in KKAy mice. The daily administration of viable Lactobacillus rhamnosus GG cells decreased blood glucose levels in a genetic type 2 diabetes model, KK-Ay mice [24] , Anti-diabetic effects of lactic acid bacteria on KK-Ay mice have been reported for Lactobacillus casei [25] , and dahi containing L. acidophilus NCDC14 and L. casei NCDC19 on high fructose induced diabetic rats [26] , and for dahi product on streptozotocin induced diabetes in rats [27] . Lactobacillus, Bifidobacterium and Streptococcus strains improve insulin resistance wild-type male C57BL6 mice [28] .
Lim et al. [29] concluded that the extract from solid fermented materials stimulates blood glucose absorption into muscle cells, and the PI3kinase/Akt protein pathway is involved in signal transmission. Consumption of Nono (Nigerian fermented milk) produced by wild lactic acid bacteria may be helpful in diabetes management [30] . Recent findings indicate that specific strains of lactic acid bacterium can be expected to be beneficial for the management of type 2 diabetes [31] .
Kefir defined as a refreshing fermented milk beverage, a viscous pourable liquid, with a smooth, slightly foamy body and whitish color. It is yeasty, acidic, mildly alcoholic, refreshing, slightly effervescent, and believed to contain many functional substances [32] -[34] . It is a beverage produced by the action of lactic acid bacteria (LAB) (lactobacilli, lactococci, leuconostocs), yeasts, and acetic acid bacteria (acetobacteria) on milk [35] [36] .
Recently kefir can be made from other medias of fermentation as Carbohydrate solutions [37] [38] , Cheese whey, and a lactose-rich waste of negligible cost [39] . This kefir which is based on carbohydrates is called sugary kefir or water kefir.
Water kefir is hazy and gluey beverage with a smooth, streamlined, fizzing texture and blond to yellowish color, it is acidic and yeasty, slightly alcoholic and refreshing taste with feeble sweetness. It is home-produced drink made by adding of kefir grain to sugar solution in water and incubating this mixture at 20˚C - 25˚C for at least 12 h, and then separation of kefir grain to other production. Pieces of fresh or dried fruit can be added for flavor and removed in the end of fermentation period.
Both Water kefir and milk kefir and their grains contain the same common groups of microorganisms (lactic acid, acetic acid bacteria, and yeasts) [40] -[48] .
Water kefir, because of its microorganisms (lactic acid, acetic acid and yeasts), and its important molecules such as polypeptide, polysaccharide, organic acid, and other compounds, can provide benefit microorganisms and bioactive molecules, Therefore, water kefir may play an important role in health improving and maintenance. But, to date, no study has investigated the bioactivities of the fermented metabolites of water kefir. The present study therefore, was designed to evaluate anti-hyperglycemic and anti-hyperlipidemic activities of water kefir on streptozotocin-induced diabetic wistar rats.
2. Materials and Methods
2.1. Chemicals
Streptozotocin (STZ) and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade, and deionized and distilled water was used throughout.
2.2. Water Kefir Preparation
Water kefir grains that obtained from laboratory of YALACTA (France), were washed with distilled water, and inoculated (5% (w/v)) in sugar solution (6.5% w/v) with mineral drinking water. 5 g/l of fresh apple pieces (purchased from the local market of Tlemcen city-Algeria) were added. The mixture was placed in glass bottle with plastic cover (not closed completely), and incubated at 21˚C for 24 h. It was stirred and mixed in intervals of 5 h. Apples pieces were deducted and kefir grains were sieving after fermentation period by filtration through a plastic sieve and washed to be used in other process. Water kefir drink was stored at 4˚C for 24 h, and different dilutions were prepared to be used in the same day.
2.3. Animals and Diets
All aspects of the experiment were conducted according to the guidelines provided by the ethical committee of the experimental animal care at Tlemcen University. Adult Wistar rats, weighing 200 to 250 g, were obtained from Pasteur institute (Algeria) and were housed at 20˚C individually in polypropylene cages and maintained on a 12:12-h light/dark cycle in the animal husbandry, Department of Biology, Faculty of life and Natural Sciences, ABBT University, ALGERIA. They were fed a commercial chow manufactured by the O.N.A.B (Food National office of Cattle, Remchi, Tlemcen). ONAB diet is composed of corn oil, cakes soya bean, a complex minerals and vitamins. The rats were allowed to free access to drinking water ad libitum. Before the study, rats were fed with AIN-93Standard diet [49] as presented in Table 1 for two weeks in order to adapt to diet.
2.4. Induction of Diabetes
STZ was dissolved in cold 0.01 M citrate buffer, pH 4.5 and always prepared freshly for immediate use within 5 minutes. Rats were fasted for overnight to induce diabetes by intraperitoneal (ip) injection of streptozotocin (STZ) at the dose of 60 mg/kg body weight. The normal control group was given citrate buffer without STZ. Animals were allowed to drink 5% glucose solution overnight to overcome the drug-induced hypoglycemia. The development of diabetes was confirmed after 48 hours of STZ injection. The animals with fasting blood glucose level more than 200 mg/dl were considered as diabetic and included in this study. Body weight and glycemia were measured weakly. For glucose assay, blood samples were collected from the tail-tip of the rats.
2.5. Experiment Design and Treatment
After one week of diabetes induction, rats were divided into four groups. Each group consisted of six rats. All groups feed with standard diet a long of experiment period and received water kefir with drinking water as follows: Group1 serve as Normal Control (NC) received standard diet and only drinking water. Group 2: Diabetics (D) received standard diet and drinking water, Group 3 - 5 (10% WK; 20% WK and 30% WK) received standard diet and 10%, 20%, and 30% of water kefir in drinking water respectively. The period of treatment was 35 days. Animals allowed to access to food and water or water kefir ad libitum.
At the end of the experiment and after overnight fasting, rats from each group were anaesthetized with intraperitoneal injection of sodium pentobarbital (60 mg/kg of body weight). The blood was drawn from the abdominal aorta, and serum was used for glucose and lipid profiles determinations.
2.6. Analytical Measurements
Blood glucose levels were measured by the glucose-oxidase method using an Accu-chek blood glucose meter. Total cholesterol (TC), Triglyceride (TG), high-density lipoprotein (HDL)-cholesterol levels were measured in serum samples by using enzymatic method kits (Roche Diagnostics). The lipoprotein cholesterol sub-fractions in the serum, HDL, LDL, VLDL were estimated by precipitation with sodium phosphotungstate-magnesium chloride and sodium dodecyle sulphate reagents.
2.7. Statistical Analysis
Statistical analysis of data was performed using SAS software version 9.1 [50] . Significance of differences were carried out by the analysis of variance (ANOVA) general linear models-Univariate method followed by Post Hoc Multiple Tukey Tests at p < 0.05. Results are represented as mean with standard errors of mean (mean ± SEM).
3. Results
3.1. The Effect of Water Kefir on the Body Weight of Rats
The effect of water kefir on the body weight of normal and STZ-induced diabetic Wistar rats, recorded for 35 days are shown in Figure 1. Body weight of normal control rats was increased during the period of experiment, on the contrary, with diabetic group which showed decreasing in body weight from the second week of experiment. The body weight of other groups treated with water kefir was ascended. The body weight of the rats treated with 10% of water kefir was ascending similarly with that of normal control, and 30% WK and 20% WK groups take the third and fourth order in body weight increasing. There were high significant differences between groups of treatment at (p ≤ 0.05), the multiple comparisons of treatment groups showed that diabetic control group was differed significantly at (p ≤ 0.05) from all of other groups. Although the changes of body weight of normal and diabetic rats during the time of experiment, there was no significant difference (p ≤ 0.05) between body weight of rats respects with the time of study. But by host hoc tests, a significant difference (p ≤ 0.05) appeared between body weight in the first day of study and that in sacrifice day.
3.2. Anti-Hyperglycemic Activity of Water Kefir
Anti-hyperglycemic effects of water kefir on normal control and STZ-induced diabetic rats which were measured
Table 1. Composition of the Standard diet.
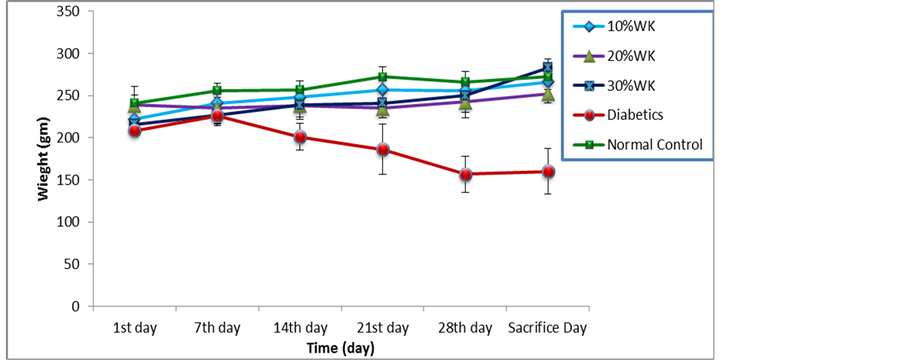
Figure 1. The effect of Water kefir on body weight of normal and STZ-induced Wistar Rats. Values are means ± SEM.
every week over the entire experimental time are reported in Figure 2. Fasting blood glucose levels of diabetic control (DC) group were increased after the induction by streptozotocin and remained over 400 g/dl up to the end of study. Blood glucose levels of diabetic rats groups which gave water kefir instead of drinking water were found to be decreased, the initial glucose concentration was 401 g/dl, 411 g/dl, and 405 g/dl in 10% WK, 20% WK, and 30% WK group respectively. After one week of treatment, blood glucose concentration was reduced by 12% in 10% WK group, 18.4% in 20% WK group, and 25% in 20% WK group, and at the end of experiment blood glucose concentration was diminished by 69 % in 10% WK group, 71% in 20% WK group, and 63% in 20% WK group from the initial concentrations.
Streptozotocin caused a significant increase in the glucose levels of experimental animals compared with normal control (p ≤ 0.05). The reduction of blood glucose was high significantly (p ≤ 0.05) in all water kefir received groups comparing with normal and diabetic control rats, but there were no significant differences between water kefir groups at (p ≤ 0.05). Through the time of experiment, a great significant reducing (p ≤ 0.05) were recorded in glucose levels of rats between the first day and each of the 14th, 21st, 28th, and sacrifice day; and between the 7th day and both of the 28th and sacrifice day.
3.3. Anti-Hyperlipidemic Activity of Water Kefir
Figure 3 demonstrate the effect of water kefir in lipid profile in diabetics and normal wistar rats at the end of experiment. Total cholesterol (TC), Triglycerides (TG), low density lipoproteins, and very low density lipoproteins (VLDL) were found at high concentrations in diabetic rats, while the High density lipoproteins (HDL) in this group was the lowest concentration. In diabetic groups treated with 10%, 20%, and 30% of water kefir, all lipid profiles were outstandingly decreased in comparing with diabetics control group. Statistically, there were significant differences at (p ≤ 0.05) in all the results of lipid markers between the rat groups of experiment. From Post Hoc Tests results, the decreasing of TC in the normal control and treated groups are statistically
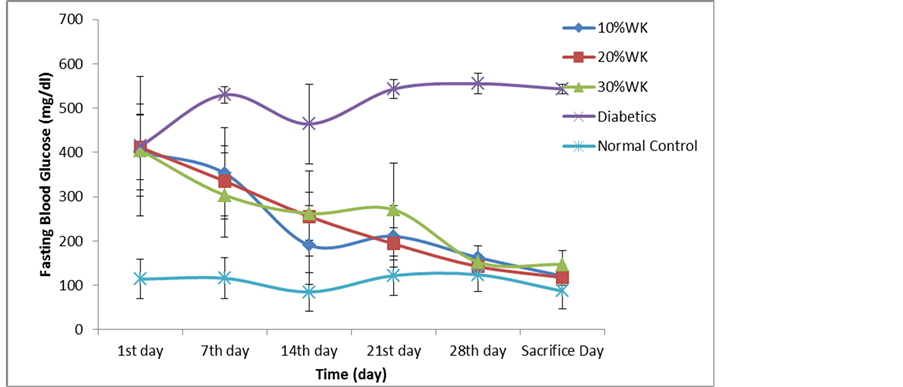
Figure 2. Anti-hyperglycemic effects of water kefir on STZ-induced diabetics rats. Values are means ± SEM.
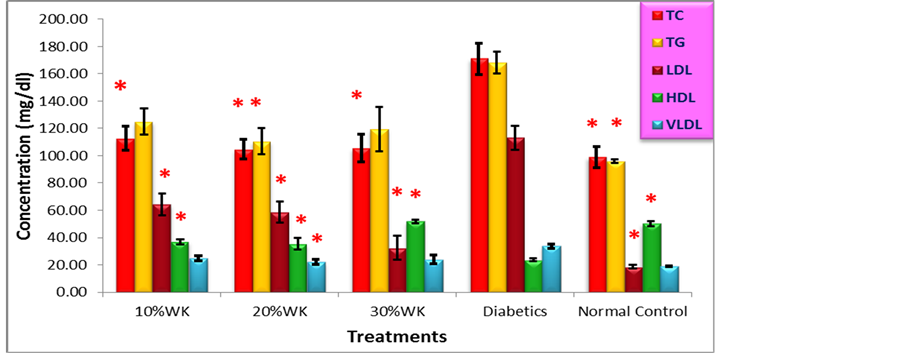
Figure 3. Anti-hyperlipidemic activities of Water kefir on Strepto-zotocin-induced diabetics Wistar Rats. Values are means ± SEM, * Statistically significant when compared to diabetics group at p ≤ 0.05.
significant, the group which consumed 20% Water Kefir have a higher significance at (p ≤ 0.05). The significant difference in TG presented only between diabetics and both of 20%WK and normal control groups. LDL were significantly reduced in all groups comparing with diabetics control with a preference of 30%WK and diabetics groups. On the other hand, HDL concentrations were significantly increased for all groups in opposing of diabetics group at (p ≤ 0.05), and only 20% WK group was differ significantly from diabetics in VLDL (p ≤ 0.05).
4. Discussion
The fundamental mechanism underlying hyperglycemia in diabetes mellitus involves the over production of glucose (excessive hepatic glycogenolysis and gluconeogenesis) and or decreased utilization of glucose by the tissues [51] .
Streptozotocin (STZ; N-nitro derivative of glucosamine) is a naturally occurring, broad spectrum antibiotic and cytotoxic chemical that is particularly toxic to the pancreatic, insulin producing beta cells in mammals [52] [53] STZ-induced diabetes is characterized by severe loss in body weight and this reduction is due to loss or degeneration of structural proteins, as the structural proteins are known a major contributor to body weight [54] [55] . Previous reports show that protein synthesis is decreased in all tissues due to decreased production of ATP and absolute or relative deficiency of insulin [56] .
This symptom was clear in our results which reveal significant reduction in body weight of diabetics rats comparing that of normal control. These results are in correspondence with the results of Judiono, et al., [57] which showed that the delta animal weight varied among the groups, except for the positive groups they gained with very small achievement bout 4.01 + 16.82 g. The result of the present study displayed improving in body weight by administration of water kefir, that may be referred to the presence of exopolysaccharides produced in kefir by its microorganisms, this effect of exopolysaccharides was reported in other studies [58] [59] .
Diseases in which prolonged elevated levels of VLDL, IDL, chylomicron remnants, or LDL occur in the blood (e.g., diabetes mellitus, lipid nephrosis, hypothyroidism, and other conditions of hyperlipidemia) are often accompanied by premature or more severe atherosclerosis. There is also an inverse relationship between HDL (HDL2) concentrations and coronary heart disease, and some consider that the most predictive relationship is the LDL: HDL cholesterol ratio [60] . Hypercholesterolemia and hypertriglyceridemia have been reported to occur in streptozotocin diabetics rats [61] [62] .
Previous research suggests the reduction of inflammatory therapy on β-cells in pancreas contributed the synthesis of proinsulin to insulin by increased cell mass and insulin sensitivity [63] . Hariom et al. [64] indicated that, the probiotic dahi-supplemented diet significantly delayed the onset of glucose intolerance, hyperglycemia, hyperinsulinemia, dyslipidemia, and oxidative stress in high fructose-induced diabetics rats, indicating a lower risk of diabetes and its complications. Kefir consumption modulate significantly blood glucose, antioxidants (SOD, Catalase, GPx), peroxidation lipids (MDA), immune response (cytokines IL1, IL6, IL10) and pancreatic β-cell function [57] . Our investigate disclosed that the regular administration of water kefir can expressively lower blood glucose concentration, because of water kefir exopolysaccharides and water kefir contents of yeast and bacteria, that previously proven to have hypoglycemic activities [24] [65] . These results were in agreement with [26] who found that, Lactobacillus acidophilus and Lactobacillus casei significantly delayed the onset of glucose intolerance, hyperglycemia, hyperinsulinemia and dyslipidemia.
Previous study reported that, in STZ-induced diabetes, the increase in blood glucose levels is usually accompanied by an increase in plasma cholesterol, triglycerides, LDL and VLDL and decreases in HDL [66] . The results of El Khamisy [67] indicated that, supplementation with Bifidobacterium and Lactobacillus acidophilus alone and in combination significantly decreased the mean value of serum glucose, total cholesterol, triglycerides, LDL-C and VLDL-C and significantly increased HDL-C and insulin secretion as compared to control groups.
In this study, we have also observed an increasing in the concentration of TC, TG, LDL, and VLDL and in the same time decreasing of HDL concentration in streptozotocin induced diabetic rats. Total cholesterol, triglycerides, LDL, and VLDL of the streptozotocin induced diabetes rats treated with water kefir (10% - 30%) showed significant reduction, and improve the level of HDL cholesterol as compared to diabetics rats.
5. Conclusions
In conclusion, based on the results of the present study it can be suggested that water kefir has antidiabetic and antihyperlipidemic properties in animal model. Consumption of water kefir in (10% - 30%) concentrations for 5 weeks has shown beneficial effect not only on blood glucose but also on body weight and lipid profiles of streptozotocin-induced diabetic rats.
Water kefir was found to be inexpensive product with hypoglycemic and hypolipidemic effects even when consuming for short time. Therefore water kefir can potentially be a useful functional food choice for patients with diabetes who are required to control their blood glucose levels, and also for diminishing the risks of cardiovascular disease.
Acknowledgements
The authors wish to express their gratitude to the Laboratory of natural products—University Abou Bekr Belkaid—Tlemcen, Algeria, and the Ministries of Higher Education and scientific research of Yemen and Algeria, for their supporting, the facilities for this research work, and for technical assistance.
References
- Anderson, l., Dibble, M.V., Turkki, P.R., Mitchell, H.S. and Rynbergen, H.J. (1982) Nutrition in Health and Disease. 17th Edition, J.B. Lippincoott Company, East Washington, Philadelphia.
- WHO (1999) Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications Report of a WHO Consultation Part 1: Diagnosis and Classification of Diabetes Mellitus. World Health Organization, Department of Noncommunicable Disease Surveillance, Geneva.
- Singh, S.K., Rai, P.K., Jaiswal, D. and Watal, G. (2008) Evidence-Based Critical Evaluation of Glycemic Potential of Cynodon dactylon. Evidence-Based Complementary and Alternative Medicine, 5, 415-420. http://dx.doi.org/10.1093/ecam/nem044
- National Diabetes Data Group (1979) Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes, 28, 1039-1057.
- Shaw, J.E., Sicree, R.A. and Zimmet, P.Z. (2010) Global Estimates of the Prevalence of Diabetes for 2010 and 2030. Diabetes Research and Clinical Practice, 34, 4-14.
- Girach, A., Manner, D. and Porta, M. (2006) Diabetic Microvascular Complications: Can Patients at Risk Be Identified? A Review. International Journal of Clinical Practice, 60, 1471-1483. http://dx.doi.org/10.1111/j.1742-1241.2006.01175.x
- Aski Basavaraj, S., Devarnavadagi, B.B., Rudrappa, G. and Kashinath, R.T. (2012) Influence of Anti Diabetic Therapy on Plasma Lipid Profile and its Relation to Erythrocyte Membrane Lipid Levels in Type 2 Diabetic Subjects. Global Journal of Medical Research, 12, 1-9.
- Haffner, S.M., Lehto, S., Ronnemaa, T., Pyrala, K. and Laakso, M. (1998) Mortality from Coronary Heart Disease in Subjects with Type 2 Diabetes and in Nondiabetic Subjects with and without Prior Myocardial Infarction. The New England Journal of Medicine, 339, 229-234. http://dx.doi.org/10.1056/NEJM199807233390404
- De Ferranti, S. and Mozaffarian, D. (2008) The Perfect Storm: Obesity, Adipocyte Dysfunction, and Metabolic Consequences. Clinical Chemistry, 54, 945-955. http://dx.doi.org/10.1373/clinchem.2007.100156
- Betteridge, D.J. (1994) Diabetic Dyslipidemia. American Journal of Medicine, 96, S25-S31. http://dx.doi.org/10.1016/0002-9343(94)90228-3
- Mayes, P.A. and Botham, K.M. (2009) Lipid Transport and Storage. In: Murray, R.K., Bender, D.A, Botham, K.M., Kennelly, P.J., Rodwell, V.W. and Anthony, W.P., Eds., Harper’s Illustrated Biochemistry, 28th Edition, The McGraw-Hill Companies, Inc., New York, 205-218.
- Hayden, J.M. and Reaven, P.S. (2000) Cardiovascular Disease in Diabetes Mellitus Type 2: A Potential Role for Novel Cardio-Vascular Risk Factors. Current Opinion in Lipidology, 11, 519-528. http://dx.doi.org/10.1097/00041433-200010000-00010
- Bener, A., Zirie, M. and Al-Rikkabi, R. (2005) Genetics, Obesity and Environmental Risk Factors Associated with Type 2 Diabetes. Croatian Medical Journal, 46, 302-307.
- Maher, V.M. and Brown, B.G. (2000) Lipoprotein(a) and Coronary Heart Disease. Current Opinion in Lipidology, 6, 226-235.
- Smaoui, M., Hammami, S., Chaaba, R., Attia, N., Ben Hamda, K., Masmoudi, A.S., Mahjoub, S., Bousslama, A., Ben Ferhat, M. and Hammami, M. (2004) Lipids and Lipoprotein(a) Concentrations in Tunisian Type 2 Diabetic Patients Relationship to Glycemic Control and Coronary Heart Disease. Journal of Diabetes and its Complications, 18, 258-263. http://dx.doi.org/10.1016/S1056-8727(03)00075-8
- Schlitt, A., Blankenberg, S., Bickel, C., Meyer, J., Hafner, G., Jiang, X.C. and Rupprecht, H.J. (2005) Prognostic Value of Lipo-Proteins and Their Relation to Inflammatory Markers among Patients with Coronary Artery Disease. International Journal of Cardiology, 102, 477-485. http://dx.doi.org/10.1016/j.ijcard.2004.05.056
- Tseng, C.H. (2004) Lipoprotein(a) Is an Independent Risk Factor for Peripheral Arterial Disease in Chinese Type 2 Diabetic Patients in Taiwan. Diabetes Care, 27, 517-521. http://dx.doi.org/10.2337/diacare.27.2.517
- Bazzano, L.A., Serdula, M.K. and Liu, S. (2003) Dietary Intake of Fruits and Vegetables and Risk of Cardiovascular Disease. Current Atherosclerosis Reports, 5, 492-499. http://dx.doi.org/10.1007/s11883-003-0040-z
- Daar, A.S., Singer, P.A., Persad, D.L., Pramming, S.K., Matthews, D.R., Beaglehole, R., et al. (2007) Grand Challenges in Chronic Non-Communicable Diseases. Nature, 450, 494-496. http://dx.doi.org/10.1038/450494a
- Apostolidis, E., Kwon, Y.I. and Shetty, K. (2007) Inhibitory Potential of Herb, Fruit, and Fungal-Enriched Cheese against Key Enzymes Linked to Type 2 Diabetes and Hypertension. Innovative Food Science and Emerging Technologies, 8, 46-54. http://dx.doi.org/10.1016/j.ifset.2006.06.001
- Dae, Y.K., Daily, J.W., Hyun, J.K. and Park, S. (2010) Antidiabetic Effects of Fermented Soybean Products on Type 2 Diabetes. Nutrition Research, 30, 1-13. http://dx.doi.org/10.1016/j.nutres.2009.11.004
- Kong, K. (2009) Effects of Kefirs on Glycemic, Insulinemic and Satiety Responses. Graduate Theses and Dissertations, Paper 10310, Iowa State University, Ames.
- Maeda, H., Zhu, X., and Mitsuoka, T. (2004) Effects of an Exopolysaccharide (Kefiran) from Lactobacillus Kefiranfaciens on Blood Glucose inKKAy Mice and Constipation in SD Rats Induced by a Low-Fiber Diet. Bioscience and Microflora, 23, 149-153.
- Honda, K., Moto, M., Uchida, N., He, F. and Hashizume, N. (2011) Anti-Diabetic of Lactic Acid Bacteriain Normal and Type 2 Diabetic Mice. Journal of Clinical Biochemistry and Nutrition, 51, 96-101.
- Matsuzaki, T., Yamazaki, R., Hashimoto, S. and Yokokura, T. (1997) Antidiabetic Effects of an Oral Administration of Lactobacillus casei in a Non-Insulin-Dependent Diabetes Mellitus (NIDDM) Model Using KK-Ay Mice. Endocrine Journal, 44, 357-365. http://dx.doi.org/10.1507/endocrj.44.357
- Yadav, H., Jain, S. and Sinhá, P.R. (2007) Antidiabetic Effect of Probiotic Dahi Containing Lactobacillus acidophilus and Lactobacillus casei in High Fructose Fed Rats. Nutrition, 23, 62-68. http://dx.doi.org/10.1016/j.nut.2006.09.002
- Yadav, H., Jain, S. and Sinha, P.R. (2008) Oral Administration of Dahi Containing Probiotic Lactobacillus acidophilus and Lactobacillus casei Delayed the Progression of Streptozotocin-Induced Diabetes in Rats. The Journal of Dairy Research, 75, 189-195. http://dx.doi.org/10.1017/S0022029908003129
- Ma, X., Hua, J. and Li, Z. (2008) Probiotics Improve High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance by Increasing Hepatic NKT Cells. Journal of Hepatology, 49, 821-830. http://dx.doi.org/10.1016/j.jhep.2008.05.025
- Lim, S.-I. and Lee, B.-Y. (2010) Anti-Diabetic Effect of Material Fermented Using Rice Bran and Soybean as the Main Ingredient by Bacillus sp. Journal of the Korean Society for Applied Biological Chemistry, 53, 222-229. http://dx.doi.org/10.3839/jksabc.2010.035
- laleye, S.A., igbakin, A.P. and Akinyanju, J.A. (2008) Antidiabetic Effects of Nono (A Nigerian Fermented Milk) on Alloxan-Induced Diabetic Rats. American Journal of Food Technology, 3, 394-398.
- Honda, K., Moto, M., Uchida, N., He, F. and Hashizume, N. (2012) Anti-Diabetic Effects of Lactic Acid Bacteria in Normal and Type 2 Diabetic Mice. Journal of Clinical Biochemistry and Nutrition, 2, 96-101.
- Vikram, M.V. (2004) Fermented Liquid Milk Products. In: Hui, Y.H., Meunier-Goddik, L., Josephsen, J., Nip, W.-K. and Stanfield, P.S., Eds., Handbook of Food and Beverage Fermentation Technology, Marcel Dekker, Inc., New York, 1-16.
- Gilliland, S.E. (1990) Health and Nutritional Benefits from Lactic Acid Bacteria. FEMS Microbiology Reviews, 87, 175-188. http://dx.doi.org/10.1111/j.1574-6968.1990.tb04887.x
- Farnworth, E.R. (2005) Kefir—A Complex Probiotic. Food Science and Technology Bulletin: Functional Foods, 2, 1- 17.
- Farnworth, E.R. and Mainville, I. (2008) Kefir—A Femented Milk Product. In: Farnworth, E.R., Ed., Handbook of Fermented Functional Foods. 2nd Edition, Taylor & Francis Group, LLC, New York, 89-127.
- Hallé, C., Leroi, F., Dousset, X. and Pidoux, M. (1994) Les kéfirs: Des associations bactéries lactiques-levures. In: Bactéries Lactiques: Aspects Fondamentaux et Technologiques, Lorica, Uriage, 169-182.
- Harta, O., Iconomopoulou, M., Bekatorou, A., Nigam, P., Kontominas, M. and Koutinas, A. (2004) Effect of Various Carbohydrate Substrates on the Production of Kefir Grains for Use as a Novel Baking Starter. Food Chemistry, 88, 237-242. http://dx.doi.org/10.1016/j.foodchem.2003.12.043
- Bergmann, R.S., Pereira, M.A. and Veiga, S.M. (2010) Microbial Profile of a Kefir Sample Preparations: Grains in Natural and Lyophilized and Fermented Suspension. Food Science and Technology (Campinas), 30, 1022-1026.
- Papapostolou, H., Bosnea, L.A., Koutinas, A.A. and Kanellaki, M. (2008) Fermentation Efficiency of Thermally Dried Kefir. Bioresource Technology, 15, 6949-6956. http://dx.doi.org/10.1016/j.biortech.2008.01.026
- Chen, T.-H., Wang, S.-Y., Chen, K.-N., Liu, J.-R. and Chen, M.J. (2009) Microbiological and Chemical Properties of Kefir Manufactured by Entrapped Microorganisms Isolated from Kefir Grains. Journal of Dairy Science, 92, 3002- 3013. http://dx.doi.org/10.3168/jds.2008-1669
- Magalhães, K.T. and Pereira, G.D. (2010) Microbial Communities and Chemical Changes during Fermentation of Sugary Brazilian Kefir. World Journal of Microbiology and Biotechnology, 26, 1241-1250.
- Pidoux, M. (1989) The Microbial Flora of Sugary Kefir Grain (The Gingerbeer Plant): Biosynthesis of the Grain from Lactobacillus hilgardii Producing a Polysaccharide Gel. MIRCEN Journal of Applied Microbiology and Biotechnology, 5, 223-238. http://dx.doi.org/10.1007/BF01741847
- Waldherr, F.W., Doll, V.M., Meißner, D. and Vogel, R.F. (2010) Identification and Characterization of a Glucan-Producing Enzyme from Lactobacillus hilgardii TMW 1.828 Involved in Granule Formation of Water Kefir. Food Microbiology, 27, 672-678.
- Gulitz, A., Stadie, J., Wenning, M., Ehrmann, M.A. and Vogel, R.F. (2011) The Microbial Diversity of Water Kefir. International Journal of Food Microbiology, 151, 284-288. http://dx.doi.org/10.1016/j.ijfoodmicro.2011.09.016
- Miguel, M.G. da C.P., Cardoso, P.G., Magalhães, K.T. and Schwan, R.F. (2011) Profile of Microbial Communities Present in Tibico (Sugary Kefir) Grains from Different Brazilian States. World Journal of Microbiology and Biotechnology, 27, 1875-1884.
- Galli, A., Fiori, E., Pagani, M.A. and Ottogalli, G. (1995) Composizionemicrobiologica e chimicadeigranuli di Kefir “di frutta”. Annali di Microbiologia ed Enzimologia, 45, 85-95.
- Garrote, G.L., Abraham, A.G. and De Antoni, G.L. (2001) Chemical and Microbiological Characterization of Kefir Grains. Journal of Dairy Research, 68, 639-652. http://dx.doi.org/10.1017/S0022029901005210
- Franzetti, L., Galli, A., Pagani, M.A. and de Noni, I. (1998) Microbiological and Chemical Investigations on “Sugar Kefir” Drink. Annali di Microbiologia ed Enzimologia, 48, 67-80.
- Reeves, P.G., Nielsen, F.H. and Fahey, G.C. (1993) AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. The Journal of Nutrition, 123, 1939-1951.
- SAS (2001) SAS 9.1 Software Package. SAS Institute, Cary.
- Latner, A. (1958) Clinical Biochemistry. Saunders, Philadelphia.
- Takeshita, F., Kodama, M., Yamamoto, H., Ikarashi, Y., Ueda, S., Teratani, T., Yamamoto, Y., Tamatani, T., Kanegasaki, S., Ochiya, T. and Quinn, G. (2006) Streptozotocin-Induced Partial Beta Cell Depletion in Nude Mice without Hyperglycaemia Induces Pancreatic Morphogenesis in Transplanted Embryonic Stem Cells. Diabetologia, 49, 2948- 2958. http://dx.doi.org/10.1007/s00125-006-0432-z
- Hayashi, K., Kojima, R. and Ito, M. (2006) Strain Differences in the Diabetogenic Activity of Streptozotocin in Mice. Biological & Pharmaceutical Bulletin, 29, 1110-1119. http://dx.doi.org/10.1248/bpb.29.1110
- Chen, V. and Ianuzzo, C.D. (1982) Doasge Effect of Streptozotocin on Rat Tissue Enzyme Activities and Glycogen Concentration. Canadian Journal of Physiology and Pharmacology, 60, 1251-1256. http://dx.doi.org/10.1139/y82-183
- Rajkumar, L., Srinivasan, N., Balasubramanian, K. and Govindarajulu, P. (1991) Increased Degradation of Dermal Collagen in Diabetic Rats. Indian Journal of Experimental Biology, 29, 1081-1083.
- Murray, R.K., Bender, D.A., Botham, K.M., Kennelly, P.J., Rodwell, V.W. and Anthony, W.P. (2009) Harper’s Illustrated Biochemistry. 28th Edition, The McGraw-Hill Companies, Inc., China.
- Judiono, Djokomoeljanto, R. and Hadisaputro, S. (2012) Biomolecular Aspects of Plain Kefir Antidiabetic Potentials. International Journal of Food, Nutrition & Public Health, 5, 7-23.
- Kim, D.H., Yang, B.K., Jeong, S.C., Hur, N.J., Das, S., Yun, J., et al. (2001) A Preliminary Study on the Hypoglycemic Effect of the Exo-Polymers Produced by Five Different Medicinal Mushrooms. Journal of Microbiology and Biotechnology, 11, 167-171.
- Hwang, H.-J., Kim, S.-W., Lim, J.-M., Joo, J.-H., Kim, H.-O., Kim, H.-M., et al. (2005) Hypoglycemic Effect of Crude Exopolysaccharides Produced by a Medicinal Mushroom Phellinus baumii in Streptozotocin-Induced Diabetic Rats. Life Sciences, 76, 3069-3080. http://dx.doi.org/10.1016/j.lfs.2004.12.019
- Peter, M.A. and Kathleen, B.M. (2009) Cholesterol Synthesis, Transport, & Excretion. In: Murray, R.K., Bender, D.A, Botham, K.M., Kennelly, P.J., Rodwell, V.W. and Anthony, W.P., Eds., Harper’s Illustrated Biochemistry, 28th Edition, The McGraw-Hill Companies, Inc., New York, 219-230.
- Sharma, S.R., Dwivedi, S.K. and Swarup, D. (1996) Hypoglycemic and Hypolipidaemic Effects of Cinnamomum tomala nees Leaves. Indian Journal of Experimental Biology, 34, 372-374.
- Pushparaj, P., Tan, C.H. and Tan, B.K. (2000) Effects of Averrhoa bilimli Leaf Extract on Blood Glucose and Lipids in Streptozotocin Diabetic Rats. Journal of Ethnopharmacolgy, 72, 69-76. http://dx.doi.org/10.1016/S0378-8741(00)00200-2
- Donath, M.Y., Marianne B.-S., Helga, E. and Jan, A.E. (2009) Islet Inflammation Impairs the Pancreatic Beta-Cell in Type 2 Diabetes. Physiology, 24, 325-331. http://dx.doi.org/10.1152/physiol.00032.2009
- Hariom, Y., Shalini, J. and Sinha, P.R. (2007) Antidiabetic Effect of Probiotic Dahi Containing Lactobacillus acidophilus and Lactobacillus casei in High Fructose Fed Rats. Nutrition, 23, 62-68. http://dx.doi.org/10.1016/j.nut.2006.09.002
- Wu, C., lin, H., Wu, G., Wang, S. and Tsai, G. (2011) In Vitro Investigation of the Hypoglycemic Activity of Yeasts Using Models of Rat Epididymal Adipocyte and Differentiated Mouse 3T3-L1 Adipocyte. African Journal of Biotechnology, 35, 6773-6783.
- Mitra, S.K., Gopumadhavan, S., Muralidhar, T.S., Anturlika, S. and Sujatha, M.B. (1995) Effect of D-400, a Herbomineral Preparation on Lipid Profile, Glycated Hemoglobin and Glucose Tolerance in Streptozotocin Induced Diabetes in Rats. Indian Journal of Experimental Biology, 33, 798-800.
- El khamisy, A.E. (2010) Effect of Bifidobacterium and Lactobacillus acidophilus in Diabetic Rats. Proceedings of the 5th Arab and 2nd International Annual Scientific Conference on Recent Trends of Developing Institutional and Academic Performance in Higher Specific Education Institutions in Egypt and Arab World, Mansoura, 14-15 April 2010, 2426-2439.
NOTES

*Corresponding author.


