Soft Nanoscience Letters
Vol.1 No.4(2011), Article ID:7759,15 pages DOI:10.4236/snl.2011.14018
Poly(Ethylene-Co-Vinyl Acetate)/Clay Nanocomposites: Effect of Clay Nature and Compatibilising Agents on Morphological Thermal and Mechanical Properties
![]()
Department of Chemical Process Engineering, University of Padua, Padova, Italy.
Email: *elisabetta.ugel@unipd.it
Received June 7th, 2011; revised July 28th, 2011; accepted September 14th, 2011.
Keywords: EVA, Nanocomposites, Film Blowing, Hydrotalcite, Compatibiliser
ABSTRACT
A series of nanocomposites based on an ethylene vinyl acetate (EVA) copolymer with two types of inorganic clays was prepared by melt blending and film blowing.The tested clays were Hydrotalcite (a layered double hydroxide) and Dellite 72T (an organo-modified montmorillonite) in different percentage, the exfoliation degree of which has been evaluated in the presence of three types of compatibilisers. The nanocomposite morphology, thermal behaviour and mechanical properties were analysed as function of the nature and content of clays and in the presence of compatibilisers.
1. Introduction
Polymer/layered silicate nanocomposites (PLSN) have attracted both academic and industrial interest for more than 15 years, among to their particular properties. With small amounts of a clay (usually less than 5 wt%) an enhancement of mechanical properties, permeability, thermal stability and flame retardancy, can be obtained as function of the length/diameter ratio (aspect ratio) and dispersion of the inorganic phase in the polymer matrix [1-4]. The inorganic fillers used to produce PLSN are of two types: layered silicates with particle size in the nanometer range and double-layered metal hydroxides.
The typical layered silicates (e.g. mica, montmorillonite, vermiculite, hectorite, saponite, etc.) belong to the structural family known as the 2:1 phyllosilicates [5-8]. It is possible to produce clays with enhanced interlayer spaces by replacing the alkaline-earth metal cations with cationic surfactants such as N-alkylamonium salts [2,3,9, 10]. In the case of some polymers, the presence of just the clay is not enough to improve the nanocomposites performances, for instance the synthesis of well-exfoliated nanocomposites of the most used polyolefins (such as polypropylene and polyethylene) needs the addition of a compatibiliser do to their hydrophobicity and lack of suitable interactions with the polar surface of the clay [11]. At the present time, the most promising strategy with such polymers is the addition of a small amount of maleic anhydride-grafted polyolefin, which is miscible with the polymeric matrix. The maleated polyolefin works as a compatibiliser, increasing the affinity between the matrix and the filler thanks to the polar character of the anhydride [12]. This approach has been extensively studied for the polypropylene-based systems, including some commercial applications [13], while the systems based on polyethylene and its copolymers are by far less explored. The polymer blend nanocomposite, obtained in this way, provides a new class of materials which combines the properties of polymer nanocomposite and of polymer blends [14-16].
The clay-based fillers have recently been extended to the family of layered double hydroxides (LDH) [17] and different approaches to the preparation of LDH nanocomposites have been described in previous papers [18, 19]. The use of LDH particles as nanofiller represents an emerging field of application, which may present advantages in comparison to montmorillonite, due to its versatility in chemical composition and charge density and allowing multiple interactions with the polymer [20]. Besides, the structure of these particles presents hydroxide groups in great number, making them attractive to enhance thermal stability and to improve fire resistant properties in polymer systems [21,22].
The aim of this paper is to investigate the effect of the nature of the inorganic fillers on the morphological, thermal and mechanical properties of EVA nanocomposites. The family of ethylene vinyl acetate copolymers (EVA) have been extensively studied because of their use in a wide series of applications including telecommunication cable manufacturing, film for greenhouses. [1,4, 8,23-25]. We report the results concerning nanocomposites obtained from a defined grade of EVA (5 wt% vinyl acetate) and two different types of nanofillers: an organo-modified layered silicate (Dellite 72T) and the Hydrotalcite (Hycite 713).
In order to obtain a good interaction amongst the clay layers and the polymeric matrix we used three types of compatibilisers in the formulations of Dellite 72T nanocomposites: two different anhydride modified linear low density polyethylene (m-LLDPE) and a methyl acrylate modified linear low density polyethylene (ma-LLDPE).
The Wide Angle X-Ray Diffraction (WAXD), Transmission and Scanning Electron Microscopy (TEM and SEM), Thermogravimetric Analysis (TGA) were used to investigate the effect of the different clay typology and of the different compatibilisers on the morphological and thermal properties of the obtained nanocomposite materials, while tensile tests were performed to evaluate their physical-mechanical behaviour.
2. Experimental
2.1. Raw Materials
A commercial ethylene vinyl acetate copolymer (EVA), Polimeri Europa (Greenflex® FD 20), containing 5 wt% of vinyl acetate (VA); density 0.920 g/cm3 and with a melt flow index (190˚C/2.16 Kg) of 0.5 g/10min (ASTM D 1238) , was chosen as the matrix.
2.1.1. Commercial Clay Used Were:
• From Laviosa Chimica Mineraria S.p.A.: Dellite 72T®, a montmorillonite modified with dimethyldihydrogenated-tallow-ammonium ions (Figure 1).
• From CIBA Speciality Chemicals S.p.A.: Hycite 713, a natural hydrotalcite, aluminate (Al(OH)63) -(OC-6-11)-, magnesium carbonate hydroxide (2:6: 1:4) (9 Cl).
2.1.2. Commercial Compatibilisers Used Were:
• From DuPontTM: Fusabond® E MB-439D, an anhydride-modified linear low-density polyethylene polymer; density 0.920 g/cm3 and melt flow index (190˚C/2.16 Kg) of 2.7 g/10min (ASTM D 1238).
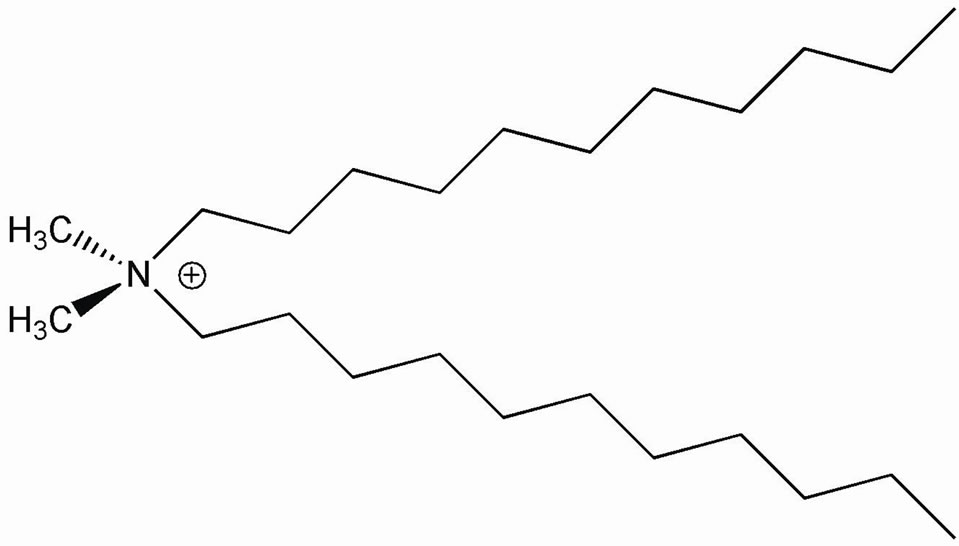
Figure 1. Organo-modification in Dellite 72T.
• From Auserpolimeri S.r.l.: Compoline CO/LL 05, a linear low density polyethylene modified with maleic anhydride; density 0.920 g/cm3 and melt flow index (190˚C/2.16 Kg) of 0.8 g/10min (ASTM D 1238).
• From DuPontTM: Elvaloy® 15024 EAC S, a copolymer of ethylene and methyl acrylate (24 wt%); density 0.920 g/cm3 and melt flow index (190˚C /2.16 Kg) of 50 g/10min (ASTM D 1238).
2.2. Preparation of Nanocomposites
EVA nanocomposites were prepared using melt blending technology in a co-rotating twin screw intermeshing extruder (D = 30 mm, L/D = 24). Before extrusion both inorganic fillers and compatibilisers were dehydrated in an oven at 80˚C for 8 - 10 hours.
In a first step, the masterbatches containing EVA filled with 25 wt% of inorganic clay and compatibilisers at 25 wt% with a working temperature of 170˚C and a twinscrew rate of 40 rpm have been prepared. The masterbatches were then diluted up to the desired percentage, using pristine EVA, by a second extrusion at the same operative condition but with a twin-screw rate of 120 rpm, nanocomposites were characterized by 1.5 wt% in Dellite 72T and 7.0 wt% in compatibiliser. In particular, we chose two maleic anhydride modified compatibilisers characterized by similar chemical structure but different MFI and the ethylene/methyl acrylate copolymer characterized by a different chemical structure and MFI.
The compatibilisers were used only with the modified montmorillonite (Dellite 72T) because there was not a good adhesion between Hydrotalcite and both the maleated or methyl acrylate LLDPE. Therefore, in this case, we produced a series of EVA nanocomposites with different content of Hydrotalcite (1, 2.5 and 4 wt% respectively) without addition of functionalized LLDPE in the formulations. As final step of EVA nanocomposites production, a blow extrusion in a pilot plant was made, obtaining films of 50 μm of thickness and a blow-up ratio (BUR) of 3:1.
In Tables 1 and 2, the compositions of the produced nanocomposites are summarised.
2.3. Nanocomposite Characterization
2.3.1 Wide Angle X-Ray Diffraction (WAXD)
WAXD patterns were recorded for all nanocomposite films on a Philips power diffractometer X’Pert MPD using PW 3123/00 curved Cu-filtered CuKα radiation at a generator voltage of 40 kV and generator current of 30 mA. The diffractograms were scanned in the 2θ range from 1.5˚ to 30˚ at 0.01˚/s rate in continuous scan, working in reflection geometry. The uncertainty in terms of dspacings was 0.05 nm (2σ).
2.3.2. Transmission and Scanning Electron Microscopy (TEM and SEM)
The level and degree of dispersion of the clay-platelets in nanocomposite films were investigated by high magnification transmission electron microscopy imaging, TEM, by Philips TEM mod. EM 208, using an acceleration voltage of 100 kV. Specimens were microtomed using a Laica Ultracut UCT.
Surface morphology and platelets dimensions of produced nanocomposite films were studied by E.S.E.M., Philips XL 30-XRF embedded, with an accelerating voltage set on 20 kV in order to avoid degradation of the samples.
2.3.3. Thermogravimetric Analysis (TGA)
The thermal stability of layered nanocomposite films was investigated by using a thermogravimetric analyzer, TA Instrument, Q600, with an heating rate of 20˚C /min with a temperature ranging from 40˚C to 900˚C and oxidizing atmosphere (air).
2.3.4. Tensile Tests
Young Modulus and Tensile Strength (elongation and stress at break) were measured on EVA nanocomposite films with a Galdabini SUN 2500, at room temperature, according to International Standard UNE-EN ISO 527-1. Mechanical properties were measured at a crosshead speed of 50 mm/min. Tensile property values reported represent the average from 5 to 8 samples.
3. Result and Discussion
3.1. Morphology
Morphological evaluation of samples was made on longitudinally stretched nanocomposite films.
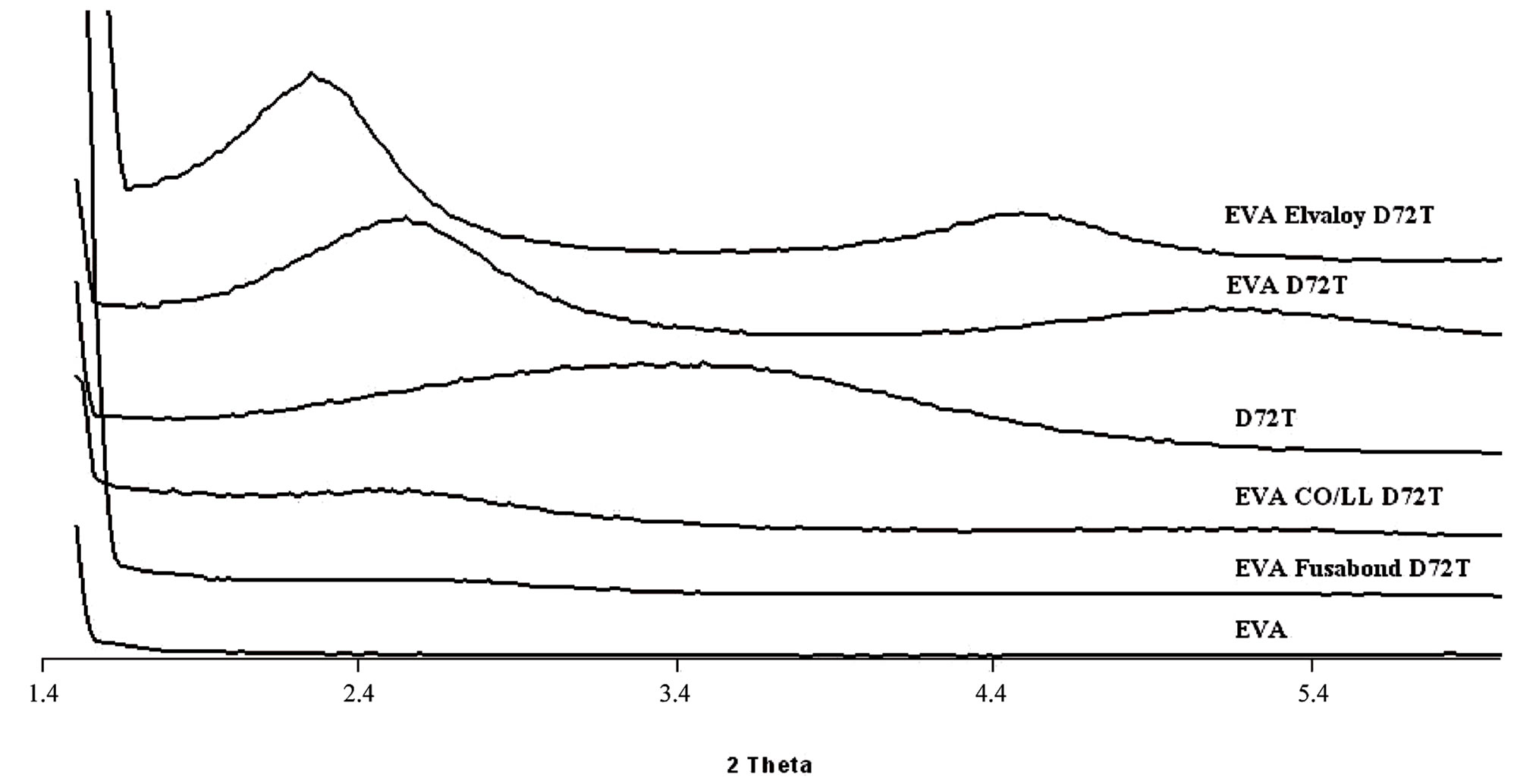
The XRD analysis is used as a very useful method to describe the extent of intercalation and exfoliation of the nanoclay having layered structure. The complete or high degree of exfoliation of the nanofiller in the polymer matrix certainly means disappearance of the corresponding peaks from the XRD spectra of the nanocomposites. However, the reverse may not be true always, because, the disappearance of peaks from the XRD spectrum could be caused by several reasons, for examples, extremely low concentration of the clay in the regions, where X-ray beams scan the material, or loss of symmetry in certain crystallographic direction [26]. X-ray diffractograms of all EVA-Dellite 72T based nanocomposites are reported in Figure 2(a), meanwhile the diffractograms of the EVA-Hydrotalcite based nanocomposites are reported in Figure 2(b).
Observing the XRD spectra of Figure 2(a) it is possible to see that the Dellite 72T presents a broad diffraction peak near 2θ = 3.5 deg. In the case of inclusions, inside the EVA matrix, the broad diffraction peak disappears, meanwhile two diffraction peaks centred on 2θ = 2.5 and 5.0 degrees appear. This experimental observation may be rationalised stating that the distance amongst Dellite 72T platelets has been increased and the clay has an in-
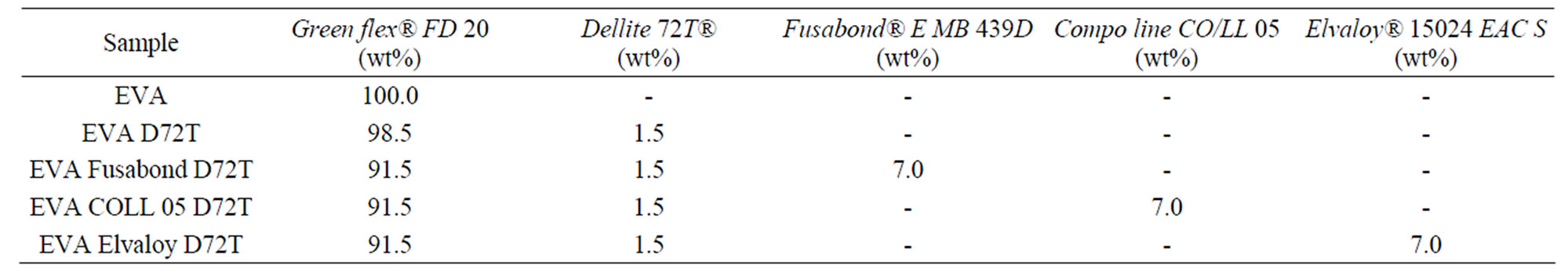
Table 1. Nanocomposites composition based on EVA, Dellite 72T and compatibilisers.
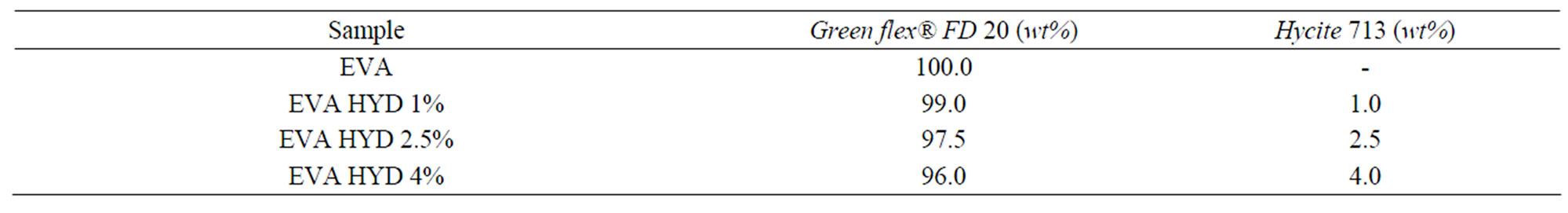
Table 2. Nanocomposites composition based on EVA and Hydrotalcite.
 (a)
(a) 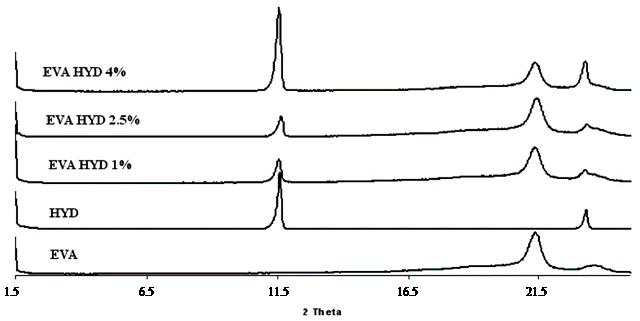 (b)
(b)
Figure 2. (a) XRD analyses of EVA, Dellite 72T (D72T) and nanocomposites in presence of the three types of compatibilisers; (b) XRD analyses of EVA and nanocomposites in presence of various contents of Hydrotalcite.
tercalated structure inside the EVA matrix. In the presence of compatibilisers, the Elvaloy 15024 EAC S one, there is a further increasing in platelets spacing as is noticeable by the presence of two diffraction peaks centred on 2θ = 2.2 and 4.4 degrees characteristic of an intercalated structure with spacing of d = 44 nm. In the case of Fusabond E MB-439D, as compatibiliser, it is possible to observe the presence of a weak diffraction peak centred on 2θ = 2.5 degrees and a weaker one on 2θ = 5.5 degrees. This experimental results may be rationalized by stating that the Dellite 72T has been partly exfoliated and partly intercalated during the mixing process, indicating a good affinity of the compatibilized EVA and the organomodified clay [27]. Moreover, the decreasing in the intensity of the clay diffraction peaks may be put in relation with a changing in the silicate layered structure, ascribed to the increasing in d-spacing as a consequence of the formation of intercalated-delaminated structures in the polymeric matrix [28,29]. The Figure 2(b) shows diffraction peaks of EVA-Hydrotalcite based nanocomposites. Comparing the diffraction spectra it is possible to observe that the diffraction patterns of the EVA-Hydrotalcite nanocompounds show, in the low 2θ region, the characteristic two Hydrotalcite diffraction peaks centred on the 2θ = 11.5 and 23.5 degrees. The XRD analysis of EVA-LDH composites show no significant change in the position of the basal peaks, but, all the signals become broader and surprisingly the higher order peaks either disappear or become weaker. Therefore, apparently the short range ordering of the LDH crystals is preserved and the intercalation process is not complete. The TEM analysis also supports this observation [30].
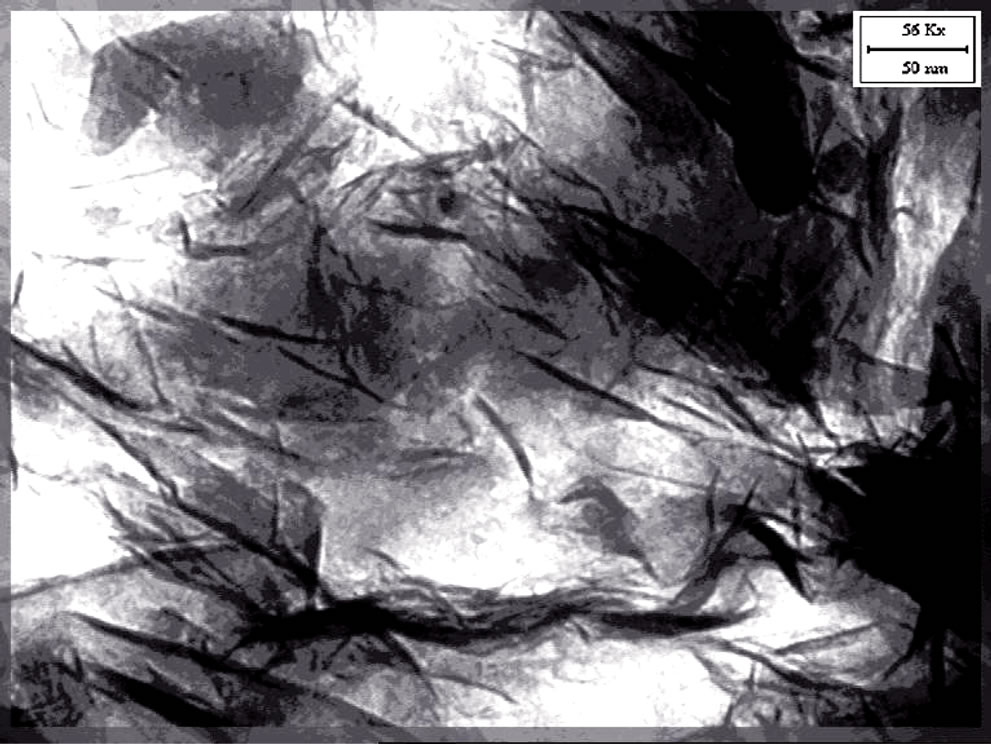
The TEM micrographs of the nanocomposites based on EVA, Dellite 72T and the three types of compatibilisers are reported in Figures 3(a)-(d).
TEM micrographs show a partial delamination of the single Dellite 72T in EVA matrix (Figure 3(a)).
In this case both intercalated structures and tactoids were observed, in agreement with XRD results.
Similar structures, but with higher degree in clay dispersion, were present in nanocomposites with Elvaloy 15024 EAC S and Compoline CO/LL05. Figures 3(c) and 3(d) show single platelets intercalated by EVA matrix and the wider interstitial space in Dellite 72T is
 (a)
(a)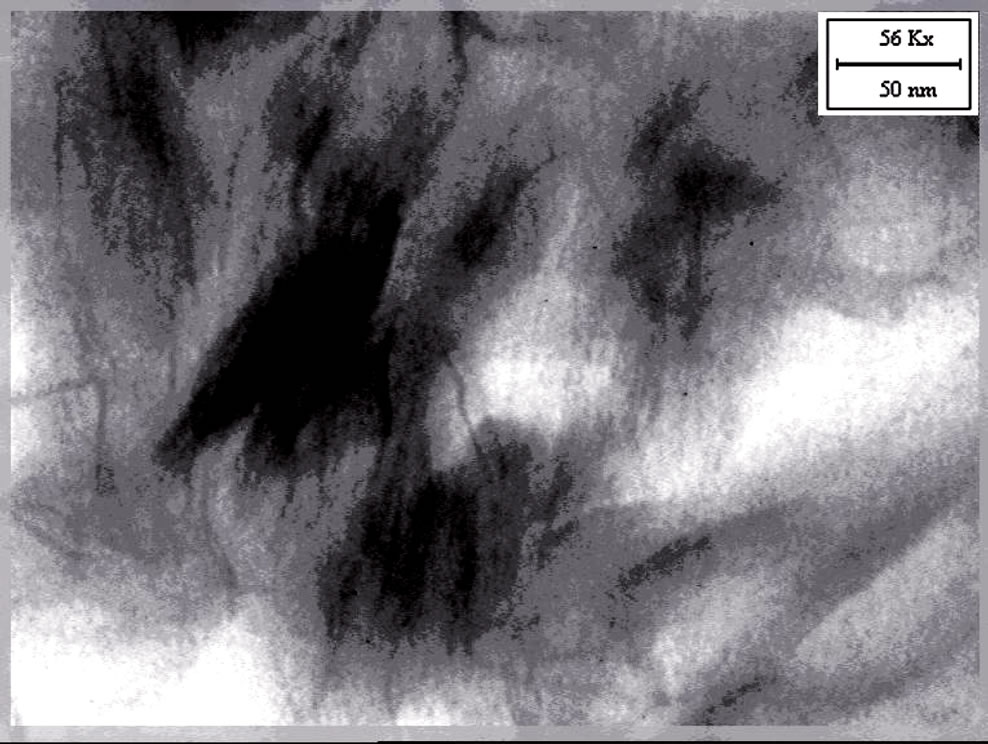 (b)
(b)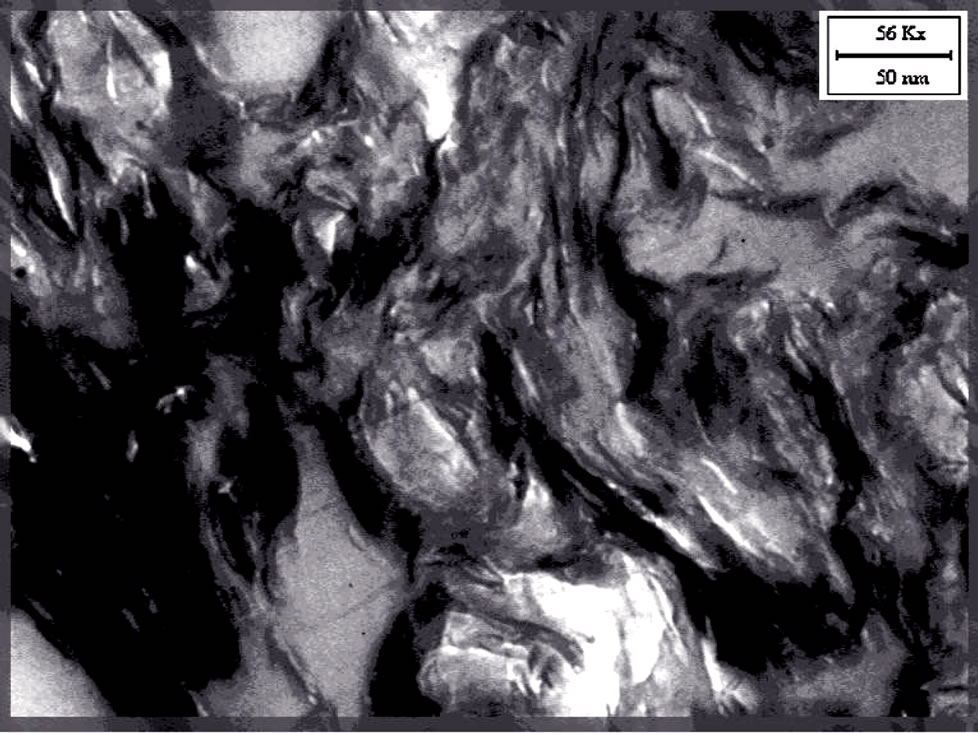 (c)
(c)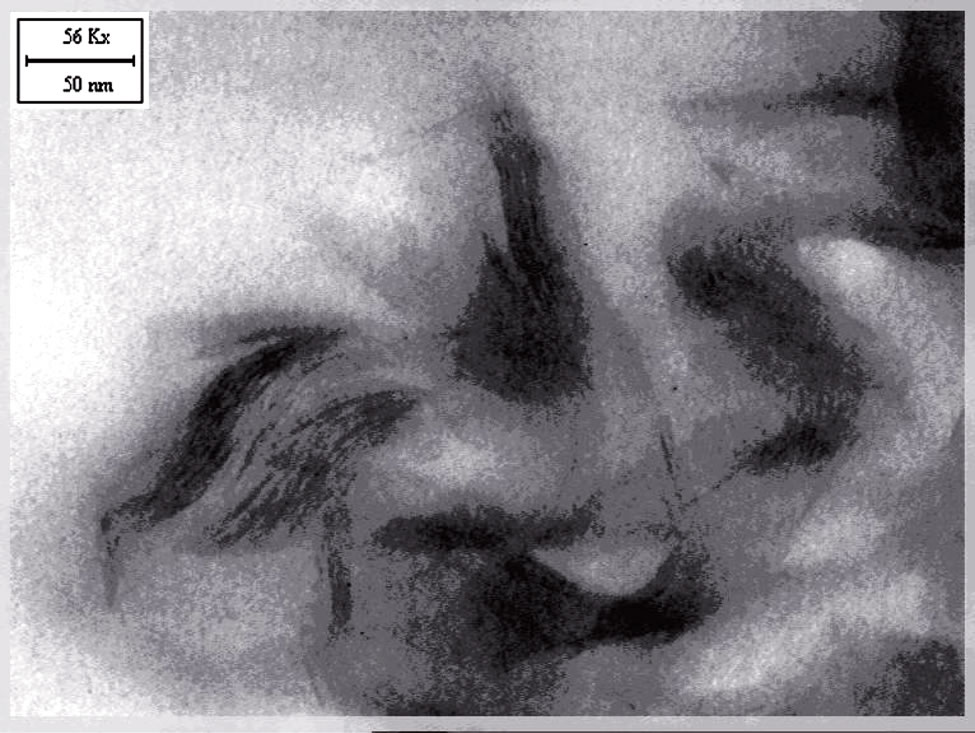 (d)
(d)
Figure 3. (a) TEM image of EVA D72T; (b) TEM image of EVA Fusabond D72T; (c) TEM image of EVA COLL 05 D72T; (d) TEM image of EVA Elvaloy D72T.
evident. The dark lines represent the thickness of individual clay layers or clay agglomerates. Thick darker lines display tactoids. In the obtained nanocomposites the Dellite 72T is partly intercalated and partly exfoliated, this indicates that a mixture of delaminated, intercalated layers and agglomerated tactoids may co-exist in the EVA compatibilized matrix.
It is known that significant effects of layered silicates on the mechanical properties, even in the absence of extensive exfoliation [31-34]. Intercalation, along with a reduction in thickness of the pristine clay tactoids containing hundreds of stacked layers, can be sufficient to increase the interfacial region, allowing the exploitation of the filler reinforcing effect [35,36].
We obtained the highest degree of exfoliation in nanocomposites based on EVA, Dellite 72T and Fusabond E MB-439D (Figure 3(b)). In this case there was an optimal dispersion of the inorganic filler in the polymer matrix and the arranged clay structure was almost completely lost [37,38].
In general, the presence of maleic anhydride or methyl acrylate in our samples gives rise to an higher degree of intercalation and exfoliation in some zones of nanocomposites, in agreement with XRD results.
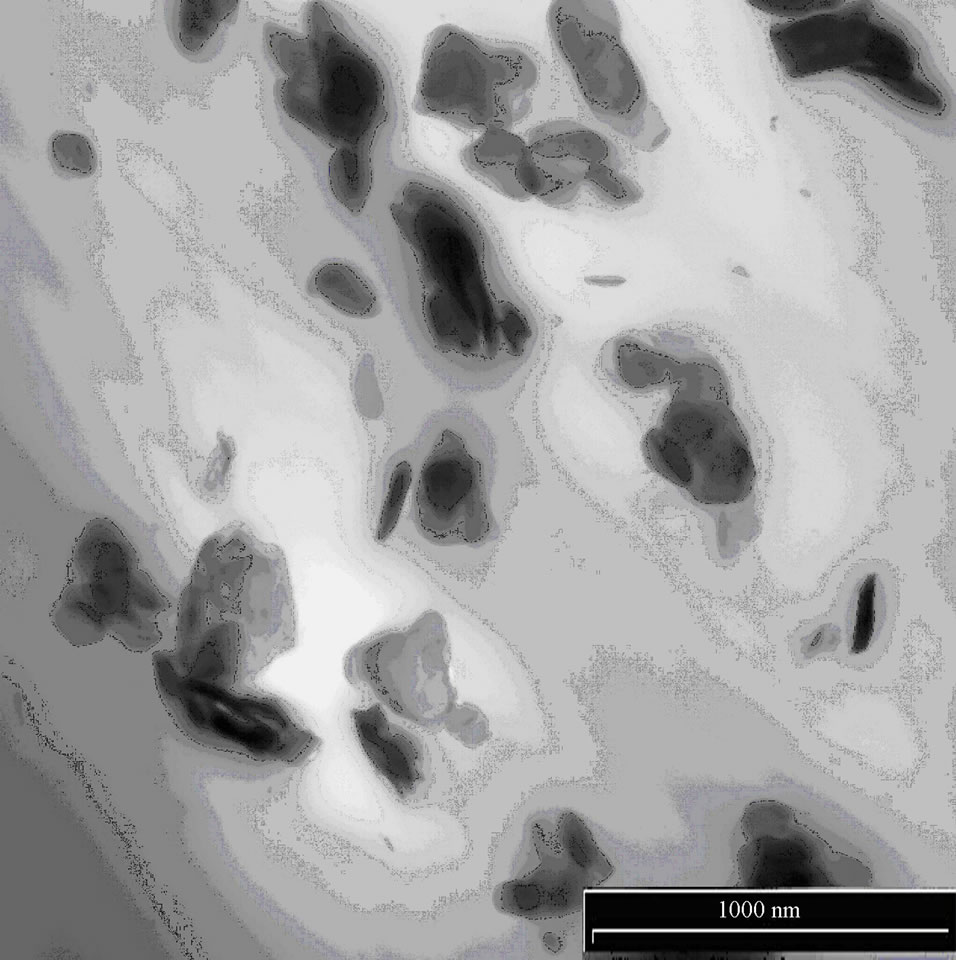
Figures 4(a,b) show TEM micrographs of EVA nanocomposites in the presence of Hydrotalcite (2.5 wt%). Hydrotalcite seems to straggle as micrometric agglomerates, being evident the absence of clay platelets. This was observed in the past by Camino et al. [39], who reported for Hydrotalcite a decrease in distance, from the basal spacing of d003 = 7.6033 Å, upon heating at 230˚C, the temperature at which the interstitial water is lost. The resulting partially degraded structure still remains layered and a significant structural change is evidenced only over 350˚C.
We extruded nanocomposites at 170˚C, so Hydrotalcite does not change its basal spacing. TEM images reported below show the presence of Hydrotalcite agglomerates and no intercalation was observed in our materials; this fact is confirmed also by XRD diffractograms, where peaks are not shifted towards lower diffraction angles [22, 30,40].
The SEM images were used to study the nanocomposite film surfaces and to calculate the sizes of inorganic agglomerates on films surfaces. The SEM images reported in the Figures 5(a) and 5(b) point out the film surfaces of EVA-Fusabond-Dellite 72T 1.5 wt% and EVA-Hydrotalcite 2.5 wt% nanocomposites having the best results in terms of filler dispersion in EVA matrix (as shown by XRD spectra and TEM images).
Images are quite out-of-focus because of the low conductivity of the polymer matrices.
 (a)
(a)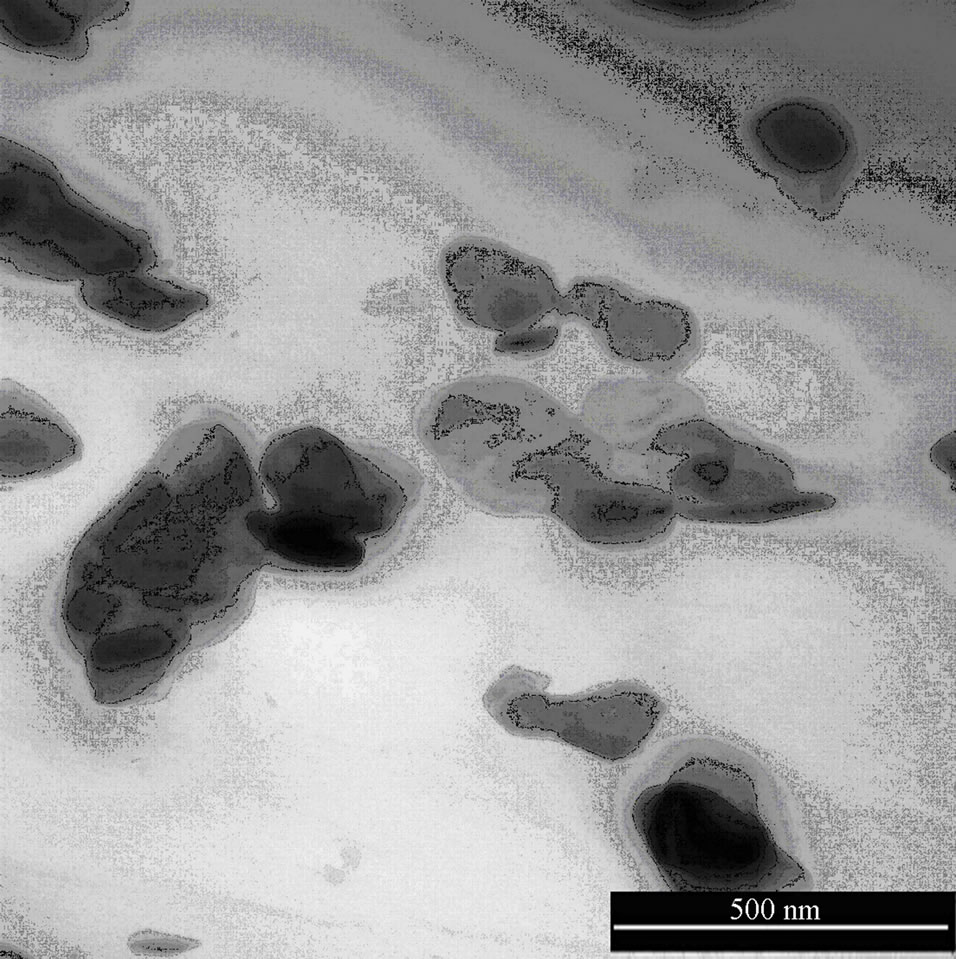 (b)
(b)
Figure 4. (a) TEM image of EVA HYD 2.5%, scale of enlargement 1000 nm; (b) TEM image of EVA HYD 2.5%, scale of enlargement 500 nm.
The SEM studies, on the above mentioned films, revealed the presence of a significant amount of cavities around the clay inclusions for the nanocomposites based on both Dellite 72T and Hydrotalcite. The used fillers are present on the EVA film surfaces as agglomerates of about 200 - 300 nm in length. As far the case of EVAFusabond-Dellite 72T nanomaterials, the presence of
 (a)
(a)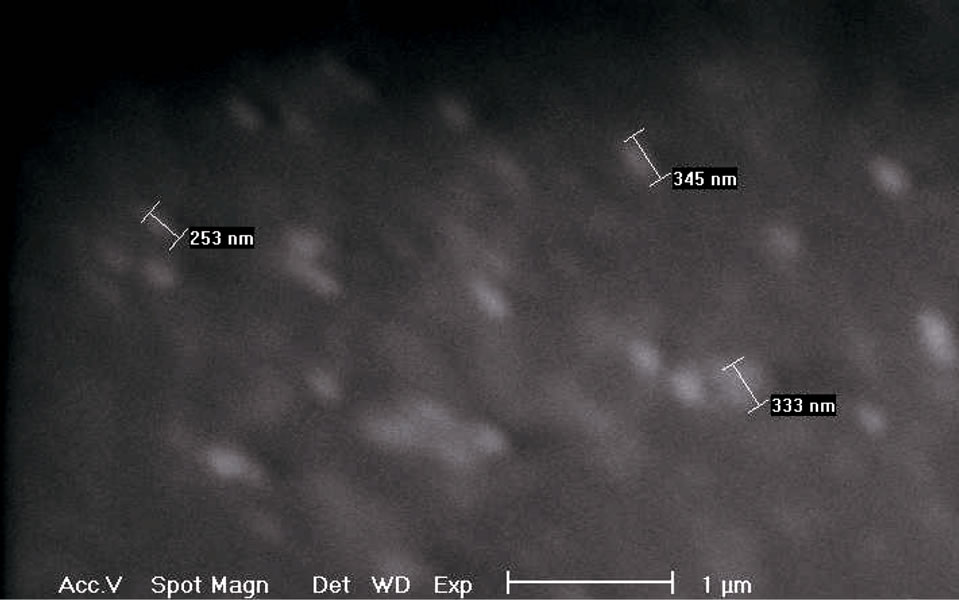 (b)
(b)
Figure 5. (a) SEM image of EVA Fusabond D72T and dimensions of clay agglomerates on the nanocomposites surface; (b) SEM image of EVA HYD 2.5% and dimensions of clay agglomerates on the nanocomposite surface.
these agglomerates may explain the premature breakingup of the samples during the tensile tests, as it will be shown later.
In the case of EVA-Hydrotalcite, Figure 5(b), the filler agglomerates seem to be more homogeneous by distributed on the film surface and the cavities are less evident. The better dispersion of Hydrotalcite and its spherical form (as TEM images show) seem to promote the slip of the EVA macromolecules and so best mechanical properties are expected [41].
3.2. Thermogravimetric Analysis
In order to study the influence of the clay nature and amount, and of the different typology of compatibilisers on the thermal degradation of the produced nanocomposite films, thermogravimetric analyses under air-flow, were carried on.
The thermal analyses elucidated that the thermo-oxidation of ethylene vinyl acetate copolymer (EVA) takes place in two steps in the range of 300˚C - 400˚C and 400˚C - 550˚C, respectively. A de-acetylation process, with production of gaseous acetic acid and the formation of carbon-carbon double bonds along the polymer backbone, takes place during the first step, thereabouts an oxidation and a volatilization of the unsatured chains, through a statistical chain braking, occurs in the second step, in agreement with the data reported in the literature. [26,42] Between 550˚C and 600˚C, all organic residues were volatilized.
Figures 6(a)-(b) show the TGA and DTG curves of the neat clays while Figures 7(a)-(d) indicate the effect of the different types of clay and the different nature of compatibilisers on the thermal stability of the produced nanocomposites.
In Table 3, both the temperatures of the maximum mass loss rate (calculated from derivative curves (DTG), for the two steps of degradation of EVA nanocomposites), and the inorganic residue percentages calculated at 650˚C (which confirmed the accuracy of the dilution process) are reported.
There is a clear dependence of the nature of filler on both the first and the second degradation step of the EVA matrix. The maximum rate of weight loss, for the first degradation event of pure EVA, occurs at 345.73˚C while it occurs at higher temperature for all the nanocomposites. The same trend is observed for the second degradation step, with the maximum of weight loss rate located at 440.09˚C for pure EVA.
Analyzing the curves, reported in Figure 7(a) and (c), it is observed that, under air flow, three main phenomena occur between the polymer (EVA) matrix and the filler: initially, at low temperature, the polymer polarity decreases due to the deacetylation process, then (above 160˚C) the presence of acidic sites, of clay mineral, catalyzed the degradation of the organic modifier. The
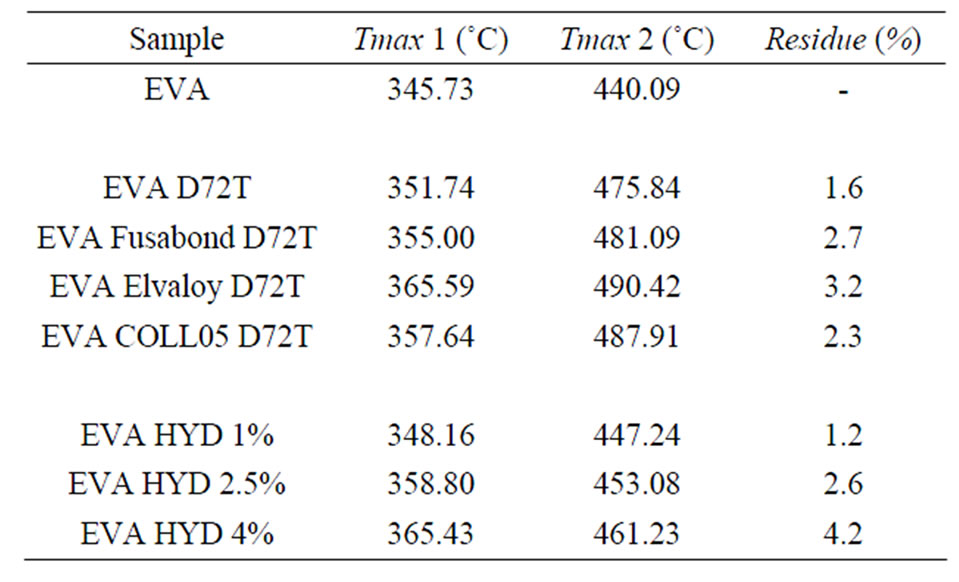
Table 3. Temperatures of the maximum mass loss rate calculated from derivative curves (Tmax 1 and Tmax 2 related to the first and second degradation step respectively) and residues percentage calculated at 650˚C.
 (a)
(a)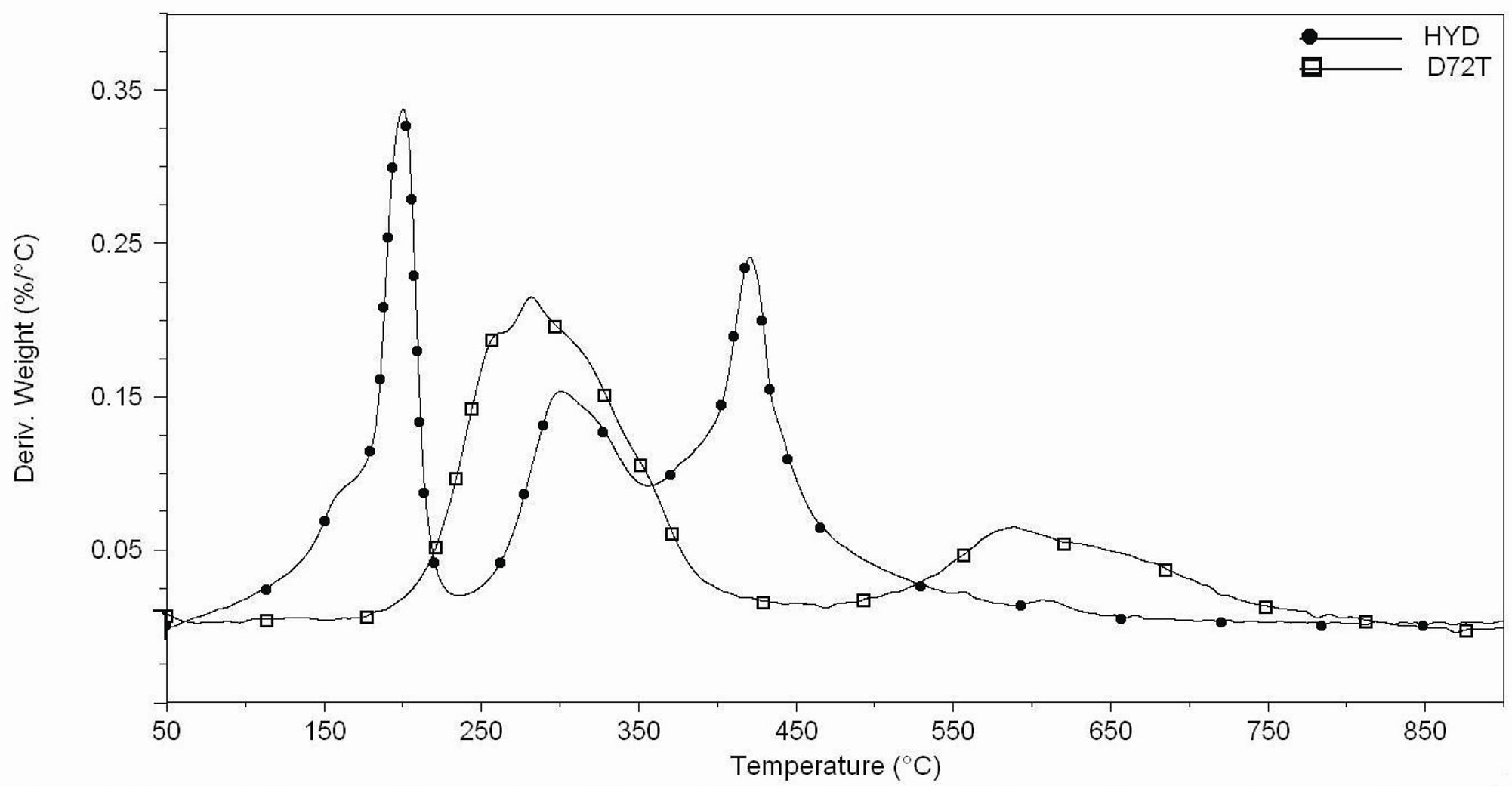 (b)
(b)
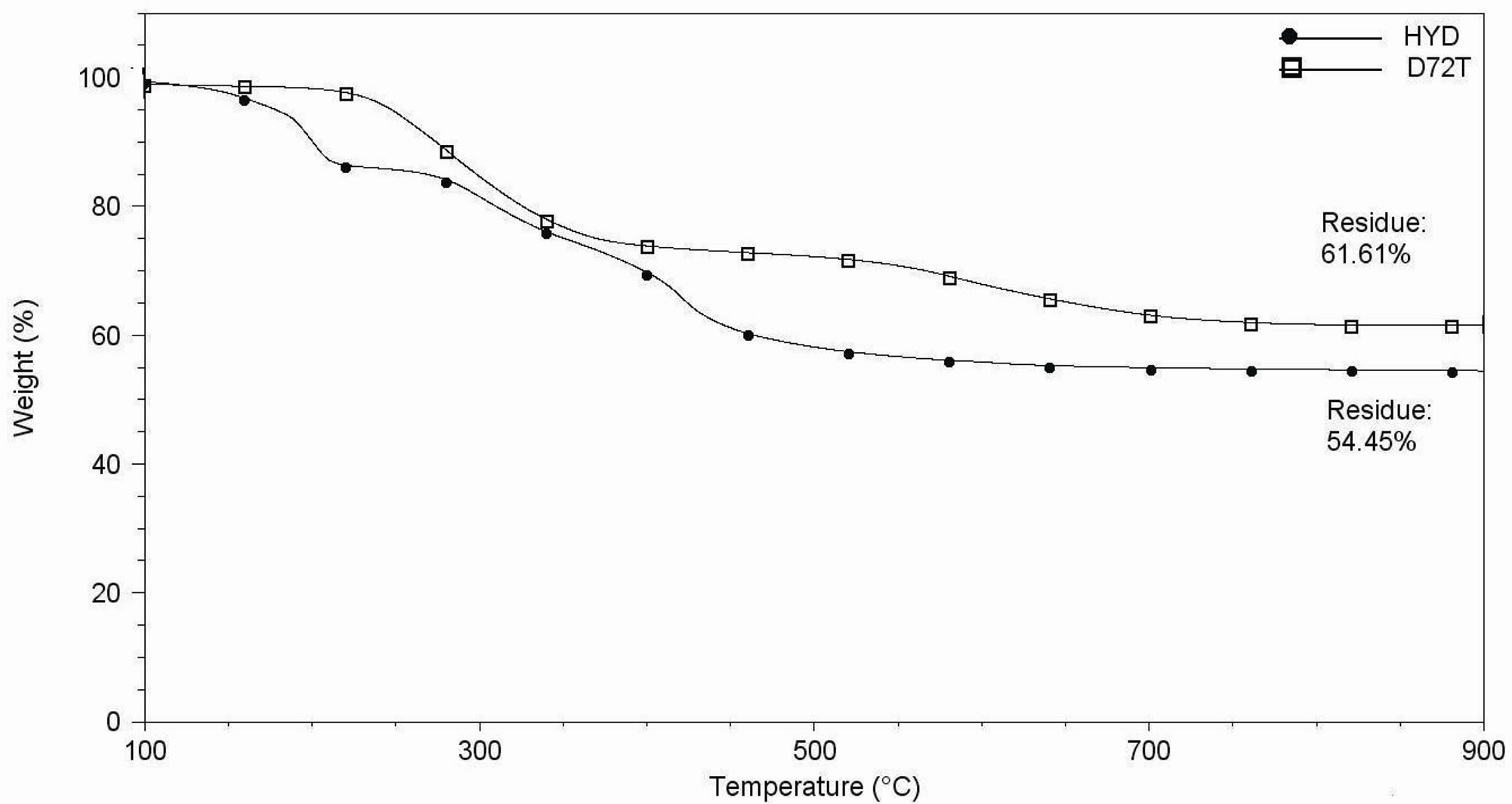
Figure 6. (a) Thermogravimetric analyses under air flow of Hydrotalcite (HYD) and Dellite 72T (D72T); (b) DTG curves under air flow of Hydrotalcite (HYD) and Dellite 72T (D72T).
degradation products were mainly water, aldehydes, carboxylic acids, various aliphatic compounds and carbon dioxide [43]. For neat Dellite 72T, the peaks at 260˚C and 580˚C, in Figure 6(b), can be attributed to these phenomena. However, the partial oxidation of the EVA increases the polarity of the polymer matrix thanks to the formation of polar groups such as ketones and lactones. Thus, at high temperature, an intercalated structure can
 (a)
(a)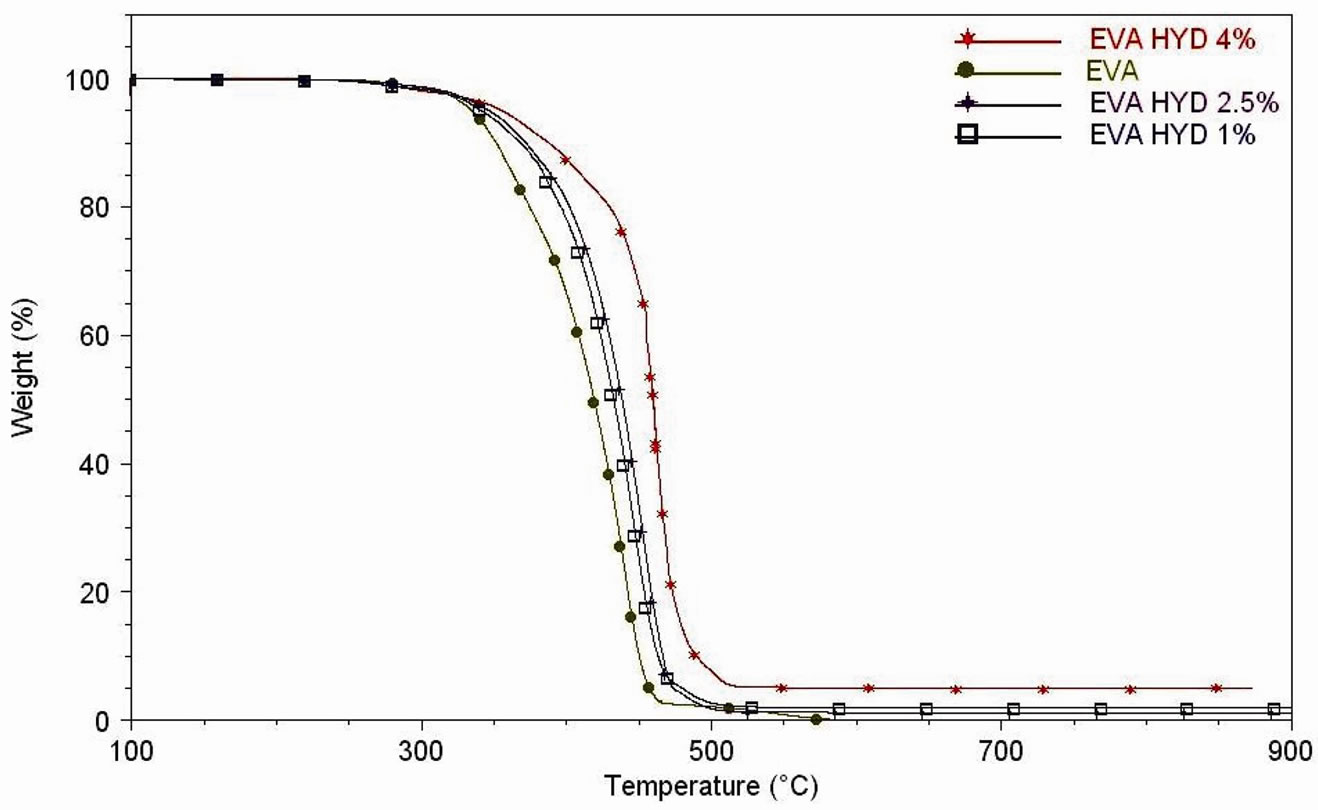 (b)
(b)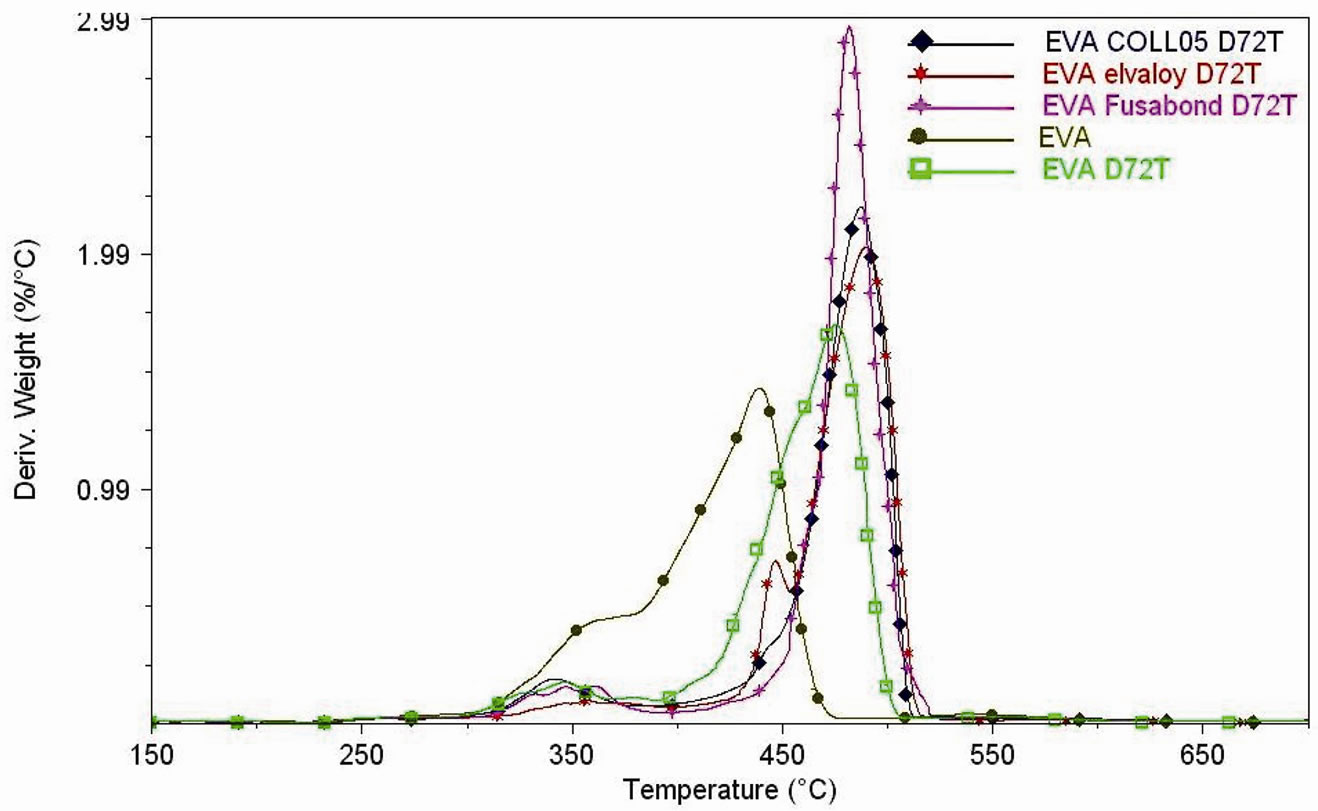 (c)
(c) (d)
(d)
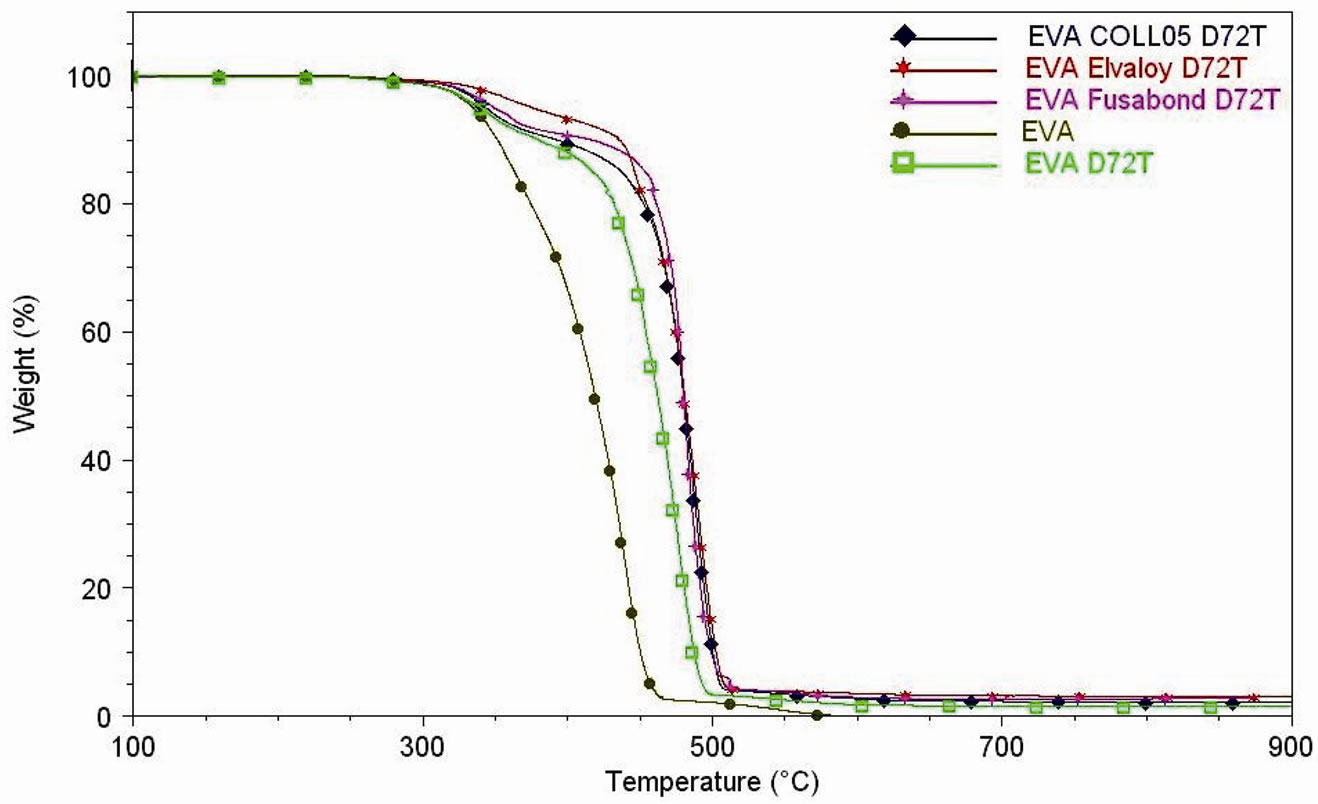
Figure 7. (a) Thermogravimetric analyses under air flow of EVA, EVA-Dellite 72T (1.5 wt%) nanocomposite films and EVADellite 72T nanocomposite films with three types of compatibilisers; (b) Thermogravimetric analyses under air flow of EVA and EVA nanocomposite films (1, 2.5 and 4 wt% of Hydrotalcite); (c) DTG curves under air flow of EVA, EVA-Dellite 72T (1.5 wt%) nanocomposite films and EVA-Dellite 72T nanocomposite films with three types of compatibilisers; (d) DTG curves under air flow of EVA and EVA nanocomposite films (1, 2.5 and 4 wt% of Hydrotalcite).
be obtained together with the increase of both mobility and polarity of the polymer chains and the clay [44]. Depending on the degradation conditions, it is possible to observe an increase or a decrease of the basal spacing of the filler. The first situation occurred when the volatile products are trapped into the galleries of the clay (thus expanding the particles), the second one is observed when the particles collapsed due to the degradation products elimination [43].
Camino et al. studied the thermal behaviour of Hydrotalcite under air flow, They demonstrated that this material undergoes a first loss of water around 200˚C and significant structural changes are evident at higher temperature (between 250˚C and 500˚C) due to condensation of hydroxyls bounded to different metals present between the sheet of the filler itself [39]; Figures 6(a) and 6(b) confirm this behaviour.
Analyzing the curves reported in Figure 7(a)-(d), it is observed that the nanocomposite films do not start the degradation step at the same temperature.
So far, the thermal shift concerning with the maximum of weight loss rate may be ascribable to a slow increasing in the thermal degradation rate on increasing of temperature. Therefore, the thermal degradation delay may be rationalised in terms of decreasing in evolution rate of volatile products produced by the thermal degradative process inside EVA matrix [45]. The group of Zanetti faced the question of the thermal-degradation delay of the EVA matrices and they proposed that the significant delay of weight loss, in air, is ascribable to the barrier effect promoted by the collapsing of the exfoliated structure of well dispersed clay platelets as a consequence of matrix degradation and formation an insulating layer [46].
In more recent years, an alternative hypothesis has been proposed from Lewin et al. [47,48]. According to this hypothesis, a migration process of the clay has to be assumed occurring at temperatures below those typical for the pyrolysis. The clay particles migrate to the surface before combustion take place and so the charred barrier accumulates before the gasification of the sample [47]. Many factors, inducing the migration of the filler particles, were suggested: the temperature and the viscosity gradient, the gases formed during the decomposition of the surfactant and the polymer. The observed increase in the distance between the layers of Dellite 72T (as shown by XRD data) may be due to the pressure of the accumulated decomposition gases which were trapped in the OLS structure [48,49]. The decrease in the concentration of the surfactant of the filler interlayer creates disorder between the polymeric chains and the clay platelets: in fact the decomposition gases of the surfactant (confined in the interlayer spaces of the clay) push the two external layers far from each other and help the creation of an intercalated-exfoliated structure [48].
Here we noticed that the presence of both Dellite 72T and Hydrotalcite increases thermal stability of EVA matrix (as reported in Table 3) both in terms of the first and the second step of degradation. It was reported that the decomposition reactions of the OLS alone and in the presence of the polymer are similar, but the rate and the mechanism are strongly influenced by the presence of the polymer itself, in particular there is a catalytic effect of the aluminosilicate layers in the MMT in the acceleration of the decomposition of the nanocomposites [50]. In our cases this effect is much less evident and this fact may be attributed to the presence of the polymeric chains intercalated in the inner layer of the Dellite 72T which are responsible to lesser contact with catalytic surfaces; the same hypothesis can also be made in the case of Hydrotalcite [47,48]. The presence of compatibilisers further improves the thermal stability of the materials increasing the dispersion of the silicate layers (as XRD spectra show) which work as barrier for heat diffusion [51] (Elvaloy® 15024 EAC S seems to give the better effect).
In the case of Hydrotalcite based nanocomposites the scenarios is quite different: the filler shows loss of water and condensation of hydroxyl groups. It seems that a coalescence of clay particles occurred. The nanostructure is thus destroyed, its colloidal nature is lost and a sort of microcomposite is obtained [22,48] as XRD data and TEM images have demonstrated. TGA and DTG curves (see Figures 7(b)-(d)), show a profile with three main decomposition steps. The first one occurs between 250˚C - 380˚C and it can be associated with water loss of Hydrotalcite and the deacetylation of EVA matrix, the second one occurs between 380˚C - 500˚C and it is related with the thermal decomposition of the aliphatic chains and the condensation of hydroxyl groups of the filler. Finally, above 500˚C the dehydroxylation of the LDH layers takes place.
3.3 Mechanical properties
Mechanical properties were evaluated on films and the results of the mechanical tests in terms of tensile strength at break (σr), elongation at break (EB) and elastic modulus (E), were reported in Table 4.
The stress-strain diagrams, performed at a crosshead speed of 50 mm/min, show plastic deformation with necking in all materials. The tensile modulus was computed as the tangent of the initial part of the stress-strain curve, less than 1% strain. The tensile yield point, although not so clear in some cases, was uniformly, but arbitrarily, determined as the point at which the slope of the curve changes from positive to negative.
The experimental results show a trend in the mechanical properties of nanocomposites, as a function of type
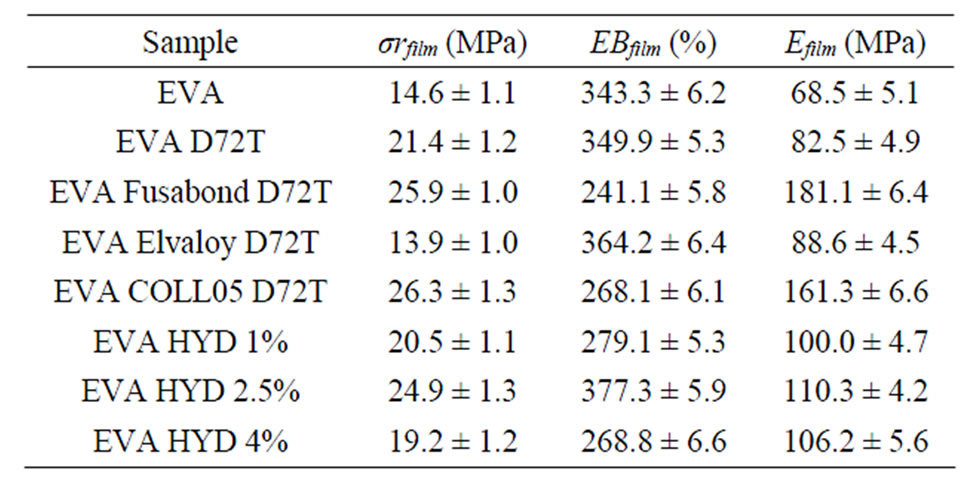
Table 4. Tensile properties data of EVA nanocomposite films: tensile strength at break (σr), elongation at break (EB) and elastic modulus (E).
and content of nanoclay and type of compatibiliser.
The mechanical behaviour of the nanocomposites is another indirect confirmation that both Dellite 72T and Hydrotalcite are partly intercalated, somewhat exfoliated and in part present as collapsed tactoids in EVA matrix. In fact in the optimal exfoliated nanocomposites the percolation threshold connected with the increase of elastic modulus is reasonably lower than 10% [52]; all our nanocomposites show a moderate increase in the elastic modulus which can be ascribed to the formation of filler platelets/tactoids [3]. Generally, as a consequence of the higher stiffness of the inorganic clay (due to its high aspect ratio and platelet structure) both elastic modulus and strength at break are slightly higher in nanocomposites than in neat polymer [29,53], this trend is confirmed in all our samples. The simultaneous incorporation of Dellite 72T and various types of compatibilisers seems to influence the mechanical properties of our nanocomposites. It was not possible to obtain films of the three neat compatibilisers owing the inability to extrude them; in fact they are very pitchy materials that stick to the internal walls and the screw of the extruder, so they are workable only as dispersed additives in other polymer matrices.
The enhancement in the E-modulus of nanocomposites became more significant with the incorporation of the compatibiliser agents, this is believed to be associated with the functionality of the maleic anhydride and the methyl acrylate, which promotes the interaction between the clay and the polymeric phase [34,36]. The two types of m-LLDPE significantly increase both elastic modulus and strength at break (increments are of +164% and +135% in the elastic modulus and of +77% and +80% in the strength at break respectively for EVA Fusabond D72T and EVA CO/LL05 D72T), these improvements may be attributed to the better dispersion of the clay guaranteed by the enhanced interfacial adhesion between the clay and the EVA matrix, as the XRD spectra and TEM images have shown. At the same, the presence of the methyl acrylate based compatibilisers seems not to produce the same trend (the EVA Elvaloy D72T is not characterized by a clear improvement in elastic modulus), this fact can be explained considering that methyl acrylate does not form rigid chemical bonds with the polymer matrix, while maleic anhydride is more reactive and is able to give stronger bond with the EVA matrix [29].
As far the elongation at break (EB), the best result was obtained in the case of EVA Elvaloy D72T with an increase of 29% compared to pristine EVA, while the presence of the two m-LLDPE based compatibilisers seem to mildly compromise the elongation behaviour of the films. This fact could be explained considering that Elvaloy has the highest melt flow index (50 g/10min against 2.7 and 0.8 g/10min for Fusabond E MB-439D and Compoline COLL/05 respectively), so the higher the value of MFI, the higher is the mobility of macromolecules and it is possible to obtain best results in terms of EB. Also, m-LLDPE based compatibilisers greatly enhance the hardness and stiffness of the nanocomposite material but they affect the elongation behavior [34].
In the case of nanocomposites obtained using Hydrotalcite, previous papers reported that higher content of inorganic filler gives more elevated elastic modulus and stress at break [30,36]. This is a consequence both of the higher stiffness of the HYD particles compared to the EVA matrix and of the HYD platelets, which are built of only one polyhedral-made layer, often corrugated (as seen by TEM images). At the same time, the greater the amount of inorganic charge, the lower the tensile strength is (as the EVA HYD 4% sample shows), this may be due to the presence of large tactoids of nanoclay which act as a stress concentrators and lead to easy crack initiation and propagation points, thus, premature failure with low energy absorption triggers (as σr and EB values show in the case of the nanocomposites with the highest percentage of Hydrotalcite).
The nanocomposites based on EVA and Hydrotalcite show an unexpected and interesting trend: in fact, the sample with 2.5 wt% of inorganic filler shows the highest mechanical properties: +70% in stress at break, +10% in elongation at break and +61% in elastic modulus.
This fact can be related to the hypothesis that there is an optimal and maximum percentage of the Hydrotalcite in EVA matrix beyond which a decrease in mechanical behaviour of the material is observed. A deeper investigation about this hypothesis is needed but is beyond the aim of the present paper.
4. Conclusions
In this paper, we studied the morphological, thermal and mechanical properties of EVA-based nanocomposites obtained using two types of inorganic fillers (Dellite 72T and Hydrotalcite). These properties are the result also of the clay content and the presence of different types of compatibilisers.
All EVA based nanocomposites were synthesized by melt blending technique using a co-rotating twin screw intermeshing extruder and by film blowing technique in a pilot plant, obtaining films with thickness of 50 μm and a blow-up ratio (BUR) of 3:1.
Morphological characterization was carried out by using WAXD, TEM and SEM analyses; XRD spectra of EVA-Dellite nanocomposites show an intercalation effect which may be improved in the presence of compatibilisers since to obtain a partly exfoliated, partly intercalated structure.
In the nanocomposites based on Hydrotalcite, the clay is dispersed in form of agglomerates; an homogeneous dispersion of the filler depends on the clay content and the nanocomposite EVA HYD 2.5% showed the better result. Hydrotalcite seems to be present in EVA matrix as tactoids and no intercalation-delamination effect was observed.
TEM analyses confirm these results and show the intercalated-exfoliated platelets of Dellite 72T in the presence of compatibilisers and the agglomerates of Hydrotalcite, respectively.
The SEM studies reveal the presence of a significant amount of cavities around the clay surfacial inclusions for the nanocomposites based above all on the Dellite 72T.
The presence of both Dellite 72T and Hydrotalcite increases thermal stability (estimated by thermogravimetric analysis) both in terms of the first and the second step of degradation of EVA matrix.
The mechanical tests performed on EVA-Dellite nanocomposites reveal a trend depending on the presence and the typology of the compatibiliser because their contribute to the material stiffness by toughen the polymer matrix. The elastic modulus and strength at break were strongly increased, depending on the degree of intercalation-delamination shown in the nanomaterials.
For EVA-Hydrotalcite based nanocomposites, the mechanical analyses reveal that the greater the amount of inorganic charge, the lower the tensile strength is (as the EVA HYD 4% sample shows), reasonably due to the presence of large tactoids of nanoclay which act as a stress concentrators and lead to easy crack initiation and propagation points.
5. Acknowledgements
E. Ugel thanks Prof. Roberta Bertani and Dr. Paolo Sgarbossa for their collaboration in all the test and analysis.
REFERENCES
- S. S. Ray and M. Okamoto, “Polymer/Layered Silicate Nanocomposites: A Review from Preparation to Processing,” Progress in Polymer Science, Vol. 28, August 2003, pp. 1539-1641.
- J. Jancar, J. F. Douglas, F. W. Starr, S. K. Kumar, P. Cassagnau, A. J. Lesser, S. S. Sternstein and M. J. Buehler, “Current Issues in Research on Structure-Property Relationships in Polymer Nanocomposites,” Polymer, Vol. 51, No. 15, April 2010, pp. 3321-3343. doi:10.1016/j.polymer.2010.04.074
- R. Scaffaro, L. Botta, M. Ceraulo and F. P. La Mantia, “Effect of Kind and Content of Organo-Modified Clay on Properties of PET Nanocomposites,” Journal of Applied Science, 2011, Published Online.
- W. Gianelli, G. Camino, N. T. Dintcheva, S. Lo Verso and F. P. La Mantia, “EVA-Montmorillonite Nanocomposites: Effect of Processing Conditions,” Macromolecular Materials and Engineering, Vol. 289, March 2004, pp. 238- 244. doi:10.1002/mame.200300267
- E. Passglia, M. Bertoldo, F. Ciardelli, D. Prevosto and M. Lucchesi, “Evidences of Macromolecular Chains Confinement of Ethylene-Propylene Copolymer in Organophilic Montmorillonite Nanocomposites,” European Polymer Journal, Vol. 44, No. 5, February 2008, pp. 1296-1308. doi:10.1016/j.eurpolymj.2008.02.011
- F. Bergaya, B. K. G. Theng and G. Lagaly, “Handbook of Clay Science,” Elsevier Science, 2006.
- H. S. Katz and J. W. Milewski, “Handbook of Fillers for Plastics,” Springer, Berlin, 1987.
- W. Clegg, A. J. Blake and R. O. Gould, “Crystal Structure Analysis: Principles and Practice (International Union of Crystallography Texts on Crystallography),” Oxford Science Publications, Oxford, 2002.
- M. Zanetti, G. Camino, R. Thomann and R. Mulhaupt, “Synthesis and Thermal Behaviour of Layered SilicateEVA Nanocomposites,” Polymer, Vol. 42, February 2002, pp. 4501-4507.
- A. Okada, Y. Fukoshima, S. Inagaki, A. Usuki, S. Sugiyama, T. Kurashi and O. Kamigaito, US Patent 4, 739,007, 1988.
- M. Modesti, A. Lorenzetti, D. Bon and S. Besco, “Thermal Behaviour of Compatibilised Polypropylene Nanocomposites: Effect of Processing Conditions,” Polymer Degradation and Stability, Vol. 91, No. 4, April 2006, pp. 672-680. doi:10.1016/j.polymdegradstab.2005.05.018
- M. Modesti, A. Lorenzetti, D. Bon and S. Besco, “Effect of Processing Conditions on Morphology and Mechanical Properties of Compatibilised Polypropylene NanocompoSites,” Polymer, Vol. 46, No. 23, August 2005, pp. 10237-10245. doi:10.1016/j.polymer.2005.08.035
- S. Hotta and D. R. Paul, “Nanocomposites Formed from Linear Low Density Polyethylene and Organoclay,” Polymer, Vol. 45, No. 22, October 2004, pp. 7639-7654. doi:10.1016/j.polymer.2004.08.059
- J. S. Borah, N. Karak and T. K. Chaki, “Effect of Organoclay Platelets on Morphology and Properties of LLDPE/EMA Blends,” Materials Science and Engineering A, Vol. 528, No. 6, December 2010, pp. 2820-2830.
- R. Scaffaro, L. Botta, M. C. Ristretta and F. P. La Mantia, “Preparation and Characterization of Polyamide 6/PolyEthylene Blend-Clay Nanocomposites in the Presence of Compatibilisers and Stabilizing System,” Polymer Degradation and Stability, Vol. 95, August 2010, pp. 2547- 2554. doi:10.1016/j.polymdegradstab.2010.07.029
- R. Scaffaro, M. C. Mistretta and F. P. La Mantia, “Compatibilized Polyamide 6/Polyethylene Blend-Clay Nanocomposites: Effect on the Degradation and Stabilization of the Clay Modifier,” Polymer Degradation and Stability, Vol. 93, No. 7, April 2008, pp. 1267-1274. doi:10.1016/j.polymdegradstab.2008.04.008
- F. Leroux and J. P. Besse, “Polymer Interleaved Layered Double Hydroxide: A New Emerging of Nanocomposites,” Chemisty of Materials, Vol. 13, September 2001, pp. 3507-3515. doi:10.1021/cm0110268
- C. Vaysse, L. Guerlou-Demourgues, C. Delmas and E. Duguet, “Tentative Mechanism for Acylate Intercalation and in Situ Polymerization in Nickel-Based Layered Double Hydroxides,” Macromolecules, Vol. 37, No. 1, December 2004, pp. 45-51. doi:10.1021/ma025882w
- W. Chen, L. Feng and B. Qu, “Preparation of Nanocomposites by Exfoliation of ZnAl Layered Double Hydroxides in No-Polar LLDPE Solution,” Chemistry of Materials, Vol. 16, January 2004, pp. 368-370. doi:10.1021/cm0303484
- M. Ardanuy and J. I. Velasco, “Mg-Al Layered Double Hydroxide Nanoparticles. Evaluation of the Thermal Stability in Polypropylene Matrix,” Applied Clay Science, Vol. 51, No. 3, December 2010, pp. 341-347.
- T. Nogueira, R. Botan, F. Wypych and L. Lona, “Study of Thermal and Mechanical Properties of PMMA/LDHs Nanocomposites Obtained by in Situ Bulk Polymerization,” Composites: Part A, Vol. 42, April 2009, pp. 1025- 1030.
- X. Wang, R. Rathore, P. Songtipya, M. Jimenez-Gasco, E. Manias and C. A. Wilkie, “EVA-Layered Double Hydroxide (Nano)Composites: Mechanism of Fire Retardancy,” Polymer Degradation and Stability, Vol. 96, March 2011, pp. 301-313. doi:10.1016/j.polymdegradstab.2010.03.014
- A. Riva, M. Zanetti, M. Braglia, G. Camino and L. Falqui, “Thermal Degradation and Rheological Behaviour of EVA/Montmorillonite Nanocomposites,” Polymer Degradation and Stability, Vol. 77, February 2002, pp. 299- 304. doi:10.1016/S0141-3910(02)00065-4
- M. Zanetti, S. Lomakin and G. Camino, “Polymer Layered Silicate Nanocomposites,” Macromolecular Materials and Engineering, Vol. 279, July 2000, pp. 1-9. doi:10.1002/1439-2054(20000601)279:1<1::AID-MAME1>3.0.CO;2-Q
- S. Duquesne, C. Jama, M. Le Bras, R. Delobel, P. Recourt and J. M. Gloaguen, “Elaboration of EVA-Nanoclay Systems-Characterization, Thermal Behaviour and Fire Performance,” Composites Science and Technology, Vol. 63, No. 8, June 2003, pp. 1141-1148. doi:10.1016/S0266-3538(03)00035-6
- S. Peeterbroeck, M. Alexendre, R. Jèrome and Ph. Dubois, “Poly(Ethylene-co-vinyl Acetate)/Clay Nanocomposites: Effect of Clay Nature and Organic Modifiers on Morphology, Mechanical and Thermal Properties,” Polymer Degradation and Stability, Vol. 90, November 2005, pp. 288-294. doi:10.1016/j.polymdegradstab.2005.03.023
- K. A. Moly, H. J. Radusch, R. Androsh, S. S. Bhagawan and S. Thomas, “Nonisothermal Crystallisation, Melting Behaviour and Wide Angle X-Ray Scattering Investigations on Linear Low Density Polyethylene (LLDPE)/ Ethylene Vinyl Acetate (EVA) Blends: Effects of Compatibilisation and Dynamic Crosslinking,” European Polymer Journal, Vol. 41, No. 6, June 2005, pp. 1410- 1419. doi:10.1016/j.eurpolymj.2004.10.016
- T. Kurauchi, A. Okada, T. Nomura, T. Nishio, S. Segua and R. Deguchi, SAE Technical Paper Series 910584, 1991
- W. Zhang, D. Chen, Q. Zhao and Y. Fang, “Effect of Different Kinds of Clay and Different Vinyl Acetate Content on the Morphology and Properties of EVA/clay Nanocomposites,” Polymer, Vol. 44, No. 26, December 2003, pp. 7953-7961. doi:10.1016/j.polymer.2003.10.046
- F. R. Costa, M. Abdel-Goad, U. Wagenknecht and G. Heinrich, “Nanocomposites Based on Polyethylene and Mg-Al Layered Double Hydroxide. I. Synthesis and Characterization,” Polymer, Vol. 46, No. 12, March 2005, pp. 4447-4453. doi:10.1016/j.polymer.2005.02.027
- S. Mehta, F. M. Mirabella, K. Rufener and A. Bafna, “Thermoplastic Olefin/Clay Nanocomposites: Morphology and Mechanical Properties,” Journal of Applied Polymer Science, Vol. 92, February 2004, pp. 928-934. doi:10.1002/app.13693
- C. Y. Lew, W. R. Murphy and G. M. McNally, “Preparation and Properties of Polyolefin-Clay Nanocomposites,” Polymer Engineering & Science, Vol. 44, July 2004, pp. 1027-1035. doi:10.1002/pen.20096
- V. Causin, C. Marega, A. Marigo, G. Ferrara, G. Idiyatullina and F. Fantinel, “Morphology, Structure and Properties of a Poly(1-Butena)/Montmorillonite Nanocomposite,” Polymer, Vol. 47, June 2006, pp. 1036-1045.
- W. S. Chow, A. Abu Bakar, Z. A. Mohd Ishak, J. KargerKocsis and U. S. Ishiaku, “Effect of Maleic AnhydrideGrafted Ethylene-Propylene Rubber on the Mechanical, Rheological and Morphological Properties of Organoclay Reinforced Polyamide 6/Polypropylene Nanocomposites,” European Polymer Journal, Vol. 41, No. 4, January 2005, pp. 687-696. doi:10.1016/j.eurpolymj.2004.10.041
- R. A. Vaia and H. D. Wagner, “Framework for Nanocomposites,” Materials Today, Vol. 7, November 2004, pp. 32-37. doi:10.1016/S1369-7021(04)00506-1
- E. Eastwood, S. Viswanathan, C. P. O’Brien, D. Kumar and M. D. Dadmun, “Methods to Improve the Properties of Polymer Mixtures: Optimizing Intermolecular Interacttions and Compatibilization,” Polymer, Vol. 46, May 2005, pp. 3957-3963. doi:10.1016/j.polymer.2005.02.073
- S. Sànchez-Valdes, C. J. Picazo-Rada and M. LopezQuintanilla, “Polyethylene Grafted Maleic Anhydride to Improve Wettability of Liquid on Polyethylene Films,” Journal of Applied Polymer Science, Vol. 79, No. 10, January 2001, pp. 1802-1808. doi:10.1002/1097-4628(20010307)79:10<1802::AID-APP80>3.0.CO;2-T
- H. Yang, Y. Song, B. Xu and Q. Zheng, “Preparation of Exfoliated Low-Density Polyethylene/Montmorillonite Nanocomposites through Melt Extrusion,” Chemical Research in Chinese Universities, Vol. 22, No. 3, June 2006, pp. 383-387. doi:10.1016/S1005-9040(06)60122-0
- G. Camino, A. Maffezzoli, M. Braglia, M. De Lazzaro and M. Zammarano, “Effect of Hydroxydes and Hydroxycarbonate Structure on Fire Retardant Effectiveness and Mechaniccal Properties in Ethylene-Vinyl Acetate Copolymer,” Polymer Degradation and Stability, Vol. 74, March 2001, pp. 457-464. doi:10.1016/S0141-3910(01)00167-7
- A. B. Morgan and J. W. Gilman, “Characterization of Polymer-Layered Silicate (Clay) Nanocomposites by Transmission Electron Microscopy and X-Ray Diffraction: A Comparative Study,” Journal of Applied Polymer Journal, Vol. 87, February 2002, pp. 1329-1338.
- J. Morawiec, A. Pawlak, M. Slouf, A. Galeski, E. Piorkowska and N. Krasnikowa, “Preparation and Properties of Compatibilized LDPE/Organo-Modified Montmorillonite Nanocomposites,” European Polymer Journal, Vol. 41, No. 5, May 2005, pp. 1115-1122. doi:10.1016/j.eurpolymj.2004.11.011
- P. Kiliaris and C. D. Papaspyrides, “Polymer/Layered Delicate (Clay) Nanocomposites: An Overview of Flame Retardancy,” Progress in Polymer Science, Vol. 35, No. 7, March 2010, pp. 902-958. doi:10.1016/j.progpolymsci.2010.03.001
- R. Scaffaro, M. C. Mistretta, F. P. La Mnatia and A. Frache, “Effect of Heating of Organo-Montmorillonites under Different Atmospheres,” Applied Clay Science, Vol. 45, No. 4, June 2009, pp. 185-193. doi:10.1016/j.clay.2009.06.002
- H. O. Pastore, A. Frache, E. Boccaleri, L. Marchese and G. Camino, “Heat Induced Structure Modifications in Polymer-Layered Silicate Nanocomposites,” Macromolecular Materials and Engineering, Vol. 289, June 2004, pp. 783-786. doi:10.1002/mame.200400109
- A. Riva, M. Zanetti, M. Braglia, G. Camino and L. Falqui, “Thermal Degradation and Rheological Behaviour of EVA/Montmorillonite Nanocomposites,” Polymer Degradation and Stability, Vol. 77, February 2002, pp. 299- 304. doi:10.1016/S0141-3910(02)00065-4
- M. Zanetti, T. Kashiwagi, L. Falqui and G. Camino, “Cone Calorimeter Combustion and Gasification Studies of Polymer Layered Silicate Nanocomposites,” Chemistry of Materials, Vol. 14, 2, January 2002, pp. 881-887. doi:10.1021/cm011236k
- M. Lewin, “Reflections on Migration of Clay and Structural Changes in Nanocomposites,” Polymer for Advanced Technologies, Vol. 17, February 2006, pp. 758- 763. doi:10.1002/pat.762
- M. Lewin, E. M. Pearce, K. Levon, A. Mey-Marom, M. Zammarano, C. A. Wilkie and B. N. Jang, “Nanocomposites at Elevated Temperatures: Migration and Structural Changes,” Polymer for Advanced Technologies, Vol. 17, August 2006, pp. 226-234. doi:10.1002/pat.684
- Monticelli, Z. Musina, A. Frache, F. Bellocci, G. Camino and S. Russo, “Influence of Compatibiliser Degradation on Formation and Properties of PA6/Organoclay Nanocomposites,” Polymer Degradation and Stability, Vol. 92, No. 3, December 2006, pp. 370-378.
- W. Xie, Z. Gao, W. P. Pan, D. Hunter, A. Singh and R. Vaia, “Thermal Degradation Chemistry of Alkyl Quaternary Ammonium Montmorillonite,” Chemistry of Materials, Vol. 13, March 2001, pp. 2979-2990.
- M. Valera-Zaragoza, E. Ramirez-Vargas, F. J. MedellinRodriguez and B. M. Huerta-Martinez, “Thermal Stability and Flammability Properties of Heterophasic PP-EP/EVA /Organoclay Nanocomposites,” Polymer Degradation and Stability, Vol. 91, No. 6, October 2006, pp. 1319-1325. doi:10.1016/j.polymdegradstab.2005.08.011
- K. Wang, S. Liang, J. Deng, H. Yang, Q. Zhang, Q. Fu, X. Dong, D. Wang and C. C. Han, “The Role of Clay Network on Macromolecular Chain Mobility and Relaxation in Isotactic Polypropylene/Organoclay Nanocomposites,” Polymer, Vol. 47, No. 20, August 2006, pp. 7131-7144. doi:10.1016/j.polymer.2006.07.067
- C. H. Jeon, S. H. Ryu and Y. W. Chang, “Preparation and Characterization of Ethylene Vinyl Acetate Copolymer/ Montmorillonite Nanocomposite,” Polymer International, Vol. 52, January 2003, pp. 153-157. doi:10.1002/pi.1066

