Advances in Molecular Imaging
Vol.3 No.3(2013), Article ID:33974,4 pages DOI:10.4236/ami.2013.33004
Visualization of Activated BAT in Mice, with FDG-PET and Its Relation to UCP1
1Service de Médecine Nucléaire, CHU d’Angers, Angers, France
2LISA ISTIA, Université d’Angers, Angers, France
3Service de Médecine Nucléaire, CHR d’Orleans, Orleans, France
4Université Paris Descartes, Institut Cochin, Paris, France
5Service Commun d’Animalerie Hospitalo, Universitaire (SCAHU), Angers, France
6Unité Inserm U646, Angers, France
7Service de Médecine Nucléaire, ICO, Paul Papin, Angers, France
Email: jeanguillaume@ieee.org
Copyright © 2013 Christian Jeanguillaume et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 30, 2013; revised May 30, 2013; accepted June 8, 2013
Keywords: FDG; PET; Brown Adipose Tissue; Uncoupling Protein 1; Mice
ABSTRACT
The visualization of symmetric structure by [18F]-FluoroDeoxyGlucose-Positron Emission Tomography (FDG-PET), corresponding to adipose density in computed tomography (CT), has led to the idea that Brown Adipose Tissue (BAT) could be present in adult human. This article studies the FDG uptake in a mice model deficient on Uncoupling Protein 1 (UCP1), in a simple thermal activation protocol. Methods: FDG were injected in mice, control and knock out (K.O.) for the UCP1. Before imaging mice were placed either in cold or warm environment. BAT uptake was evaluated by ratio named RISC. Results: In warm condition, mean value of the Ratio of Inter-Scapular uptake (RISC) was 1.34 +/− 0.27. After cold exposure, RISC increased 2 fold for control mice, male K.O. did not increase their RISC, female K.O. increased their RISC up to 2.45. Conclusion: Our study brought a further confirmation that FDG-PET visualised activated Brown Adipose Tissue. It gives a direct proof of the role of UCP1 in this process. The FDG uptake by cold female K.O. mice was unexpected.
1. Introduction
Knowledge in human physiology and medicine is rapidly changing. Recently our vision on the Brown Adipose Tissue (BAT) has dramatically changed. BAT was first described in marmot by Konrad Gessner in 1551 [1]. It is a tissue specialised in heat production, which does not involve shivering. In rodents and hibernating mammals, BAT is localized in the neck area, between the scapulae [2]. Its biological landmark is the Uncoupling Protein 1 (UCP1) which has been discovered in the late 70s [3]. Its complete structure was elucidated in the late 80s. This protein decreases the proton gradient generated in the mitochondrial membrane by the oxidative phosphorylation [4]. It thus allows heat generation without shivering. Although BAT was recognized in rodents and hibernating mammals for a long time, its role in the human body was largely underestimated. For example, just 10 years ago, it was assumed that adult human have essentially no BAT [5]. In 2002 Hany and co-workers hypothesized the presence of BAT to explain symmetrical [18F]-fluoroDeoxyGlucose FDG uptake in neck and upper chest regions in Positron Emission Tomography (PET) [6]. Recently, Cypess et al. gave the first histological correlation of the presence of BAT, UCP1 expression and FDG-PET uptake [7]. And Van Marken et al. demonstrated FDG uptake in healthy men [8].
This paper studied mice deficient in UCP1 to give a direct proof of the correlation between activated BAT, UCP1 and FDG-PET uptake. We compared normal and UCP1 deprived mice, in cold and warm atmosphere by studying their FDG-PET uptake.
2. Methods
2.1. Mice
This study followed the institutional guidelines for the manipulation of mice which are in accordance with the National Institutes of Health guidelines. All the mice were manipulated by a fully licensed staff (Pierre Legras) and the approval from the animal care committee had been obtained beforehand.
The strain used was C57BL aged 10 weeks and more, for a mean weight of 30 grams. UCP1 knock out mice were obtained by Pr D. Ricquier following a gift by Dr Leslie Kozak [9]. During the study mice were grown in our institution animal ward (SCAHU: service commun d’animalerie hospitalo-universitaire d’Angers). Each experiment involved 5 to 10 mice.
2.2. BAT Activation and Deactivation Protocol
To initiate BAT activation, the mice were placed in a cold environment. For this purpose, we placed the animals at 4˚C (fridge) during 4 hours before FDG injection. This model has been already validated in rats and mice [9].
Warm environment was realized by a heating bulb. For a total BAT deactivation a temperature over the thermal neutralization must be reached. The thermal neutralization temperature is the temperature that is sufficient to deactivate BAT. For mice it is 30˚C - 32˚C. In our experiment we raised the environment temperature to 39˚C and maintained it for 4 hours.
2.3. FDG Injection
Preliminary experiments were realized to compare images after FDG injection intravenously and intra-peritoneally. The experiments showed that vein puncture in black fur mice, is a difficult task, and it leads to the risk of vein rupture and inconsistent results. The easiness of the intra-peritoneally puncture, and its high reproducibility, were retained as the preferable way to inject FDG. Each mouse received 3 MBq of FDG in 0.05 ml saline buffer. After the FDG injection, mice were replaced in the same cold or warm environment as previously set, for one hour before imaging.
Each mouse was given 20 minutes before the images acquisition, 0.3 ml of sodique thiopental 1 g (final dilution 5 mg/ml in NaCl 0.9%) to keep quiet during the images acquisition.
2.4. TEP/TDM Acquisition Protocol
Images were obtained, in our clinical PET/CT (General Electric, Discovery ST). 3D mode acquisition was realized by one 15 cm step of 15 mn. Sleeping mice were placed on the examination table for data acquisition. Helical CT was performed (80 KV, 20 mAs) to provide data for attenuation correction of PET image and anatomical landmarks. PET images were reconstructed with 3.3 mm thick slices, with a field of view of 20 × 20 cm. Images were visualized in an ADW 4.2 image station (General Electric).
2.5. Uptake
The intensity of uptake was measured by the ratio of the number of true coincidences counted in an area (of 235 pixels) between the scapulae, over the number of counts obtained in a cerebellum area (of 175 pixels). This ratio is called RISC for Ratio of Inter-Scapular uptake scaled by cerebellum uptake.
2.6. Statistical Analysis
Data were computed in the STATVIEW software. Mean values between the different sets of measurements were compared by a standard Student t-test for the analysis of univariate variance (ANOVA) with a p < 0.05. A non parametric Kruskall-Wallis test with Dunns post test was also performed. Significance or not were found in accordance by both tests in our results.
3. Results
Visually, mice exposed to a cold environment present a strong hyper-metabolism of their BAT localized between the scapulae. A moderate hyper-metabolism is also seen around the aorta and in the para-vertebral area (Figure 1). Figures 2-4 illustrate and Table 1 specifies our results.
3.1. Control Mice
In a warm environment, which is for a temperature over the thermal neutrality, only faint activity is measured between the scapulae. RISC of 1.19 ± 0.33 for male mice and RISC of 1.27 ± 0.15 for female mice were measured.
At the opposite, mice exposed to a cold environment,
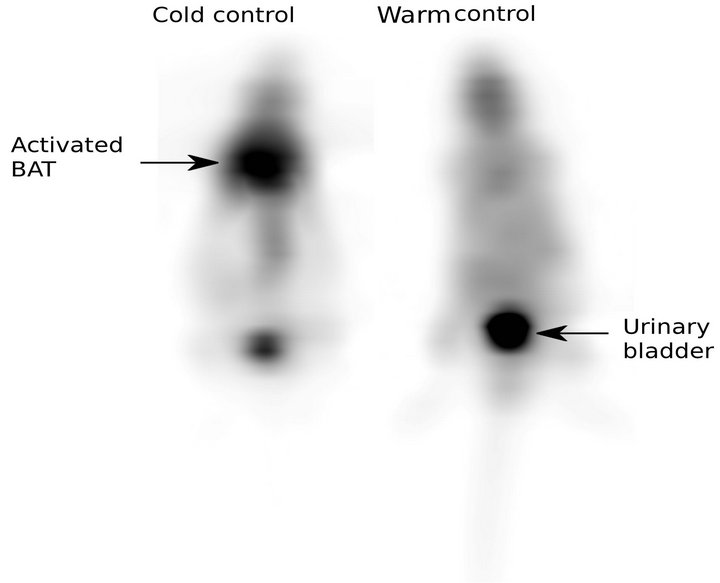
Figure 1. 18FDG-PET Images of control mice in warm and cold environments. Note the high Inter-Scapular uptake in cold environment.
exhibit an elevated RISC. A mean RISC of 2.41 +/− 0.5 for male mice and 2.92 +/− 0.89 for the female mice were measured.
3.2. UCP1 Deficient Mice
In warm condition, only a faint Inter-Scapular metabolism was measured. RISC of 1.58 ± 0.3 for male mice and of 1.35 ± 0.06 for female mice was measured.
In cold environment, as expected, male K.O. mice presented a faint Inter-Scapular metabolism with mean RISC values measured to 1.57 +/− 0.3.
Female K.O. mice presented an elevated RISC value measured to 3.58 +/− 1.59. The mean increase between warm and cold environment was 2.65 (p < 0.01) for the female K.O.
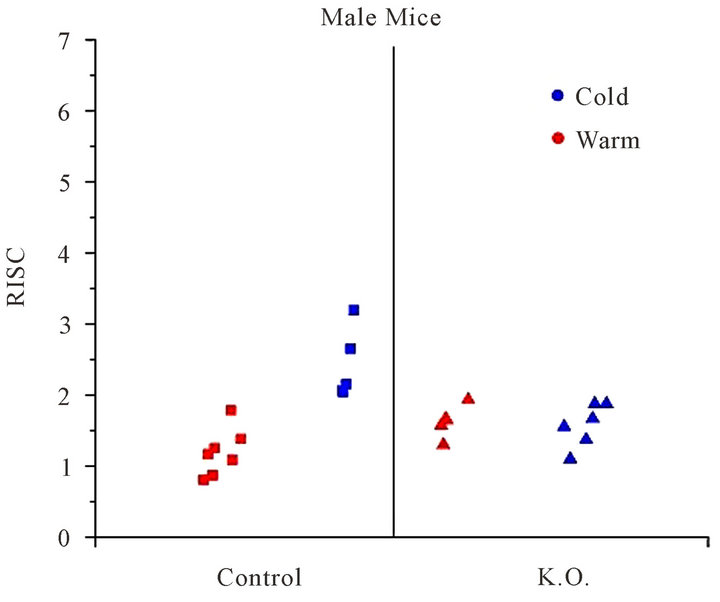
Figure 2. RISC: Ratio of Inter-Scapular FDG uptake, for male mice, in cold (blue) and warm (red) environment.
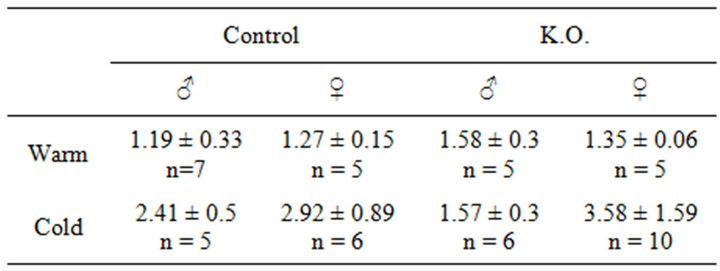
Table 1. RISC Ratio of Inter-Scapular uptake.
3.3. Comparison Control versus Deficient
In a warm environment no difference were found between control mice and deficient mice, either male or female.
No difference was noted between male deficient mice placed in cold or warm environment.
A small RISC increase (22%) was seen between female control mice and female deficient mice, but this increase, is not statistically significant.
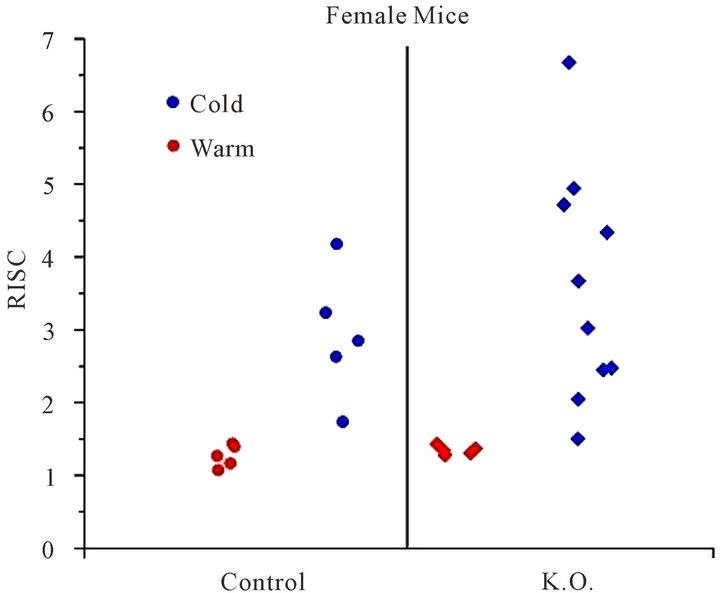
Figure 3. RISC: Ratio of Inter-Scapular FDG uptake, for female mice, in cold (blue) and warm (red) environment.
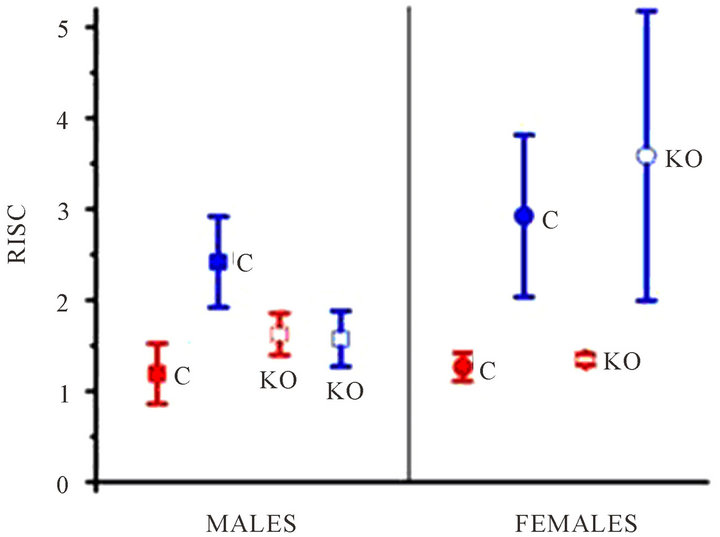
Figure 4. RISC: Ratio of Inter-Scapular FDG uptake, mean and standard deviation. C: control; KO: UCP1 deficient; blue: cold environment; red: warm environment.
4. Discussion
The discussion will be cut in two parts, the results observed in mice, and the repercussion that one might extrapolate to humans.
4.1. For the C57BL Mice
Our study adds another confirmation that FDG-PET really visualizes activated BAT. The known anatomy of the BAT in mice, and the predictable behaviour of the control mice when exposed to warm or cold environment, is a strong argument in favour of this hypothesis. To our knowledge, the comparison involving UCP1 K.O. mice was not realised before. The low uptake observed in UCP1-deficient male mice after cold exposure, confirm the link between FDG-PET uptake and activation of BAT.
The fact that female UCP1 deficient mice activate their BAT when placed in a cold environment was unexpected. Enerbäck et al. [9] observed a partial deficiency on thermoregulation in 41 of the same strand homozygous mice. Among this set 85% percent were cold sensitive the proportion of male was 56%. These authors also observed that these mice were not obese, even after high-fat diet. These observation and ours suggest that another protein that the UCP1 is involved in the heat production in mice. The participation of an UCP type protein, different from the UCP1 may be suggested to explain the strong hyper-metabolism observed among deficient female mice in cold environment. Enerbäck and al have shown that in the same homozygous mice, UCP2 mRNA from BAT is increased compared to heterozygous mice. In Enerbäck study no gender or age difference was observed in the thermoregulation deficiency. More works will be necessary to explain the difference, between FDG-PET uptake in cold environment and thermoregulation efficiency.
4.2. Consequence for Humans
Although a straight extrapolation to human of results observed in mice may expose to errors, it is a known fact that mammals share a lot of common traits. The proof that BAT is present in the human adult and its relation to FDG-PET uptake, has recently emerged with histological proof [7]. What our results confirm is that FDG-PET visualizes activated BAT and does not visualize non activated BAT.
This fact implies that the presence of BAT in adult is largely underestimated by the sole percentage of FDGPET positive scan. Cypess [7] found a prevalence of activated BAT of 3.1% in men and 7.5 % in women. They also show a slight but convincing inverse correlation between prevalence and outdoor temperature. Recently Ouellet et al. [10] visualized BAT in all the six healthy men studied, placed in cold environment.
All this results emphasizes on the high prevalence of BAT in human adult.
To avoid image of activated BAT which may lead to interpretation difficulties in FDG-PET examination, a simple protocol with heating blankets may be discussed. Although beta blockers have been proposed and have shown there efficacy, placing the patient in a warm environment is simpler and may present less side-effects.
Finally the role of UCPs type protein, in energy balance and thermoregulation, can be linked with several pathological states. To name but a few, obesity, diabetes, cachexia and fever have been told to be linked with UCP family. FDG-PET may then play an important role by visualizing activated Brown Adipose Tissue in patient or experimental subject.
5. Conclusion
This study gives another proof of the correlation between UCP1, BAT and FDG-PET uptake. It underlines the fact that only activated BAT can be visualized by FDG-PET uptake. Finally it discovers an unexpected gender difference in BAT thermoregulation and raises the participation of others protein than the UCP1 in female mammals.
REFERENCES
- K. Gessner, “Conradi Gesneri Medici Tigurine Historiae Animalium: Lib. I De Quadrupedibus Viviparis,” 1551.
- Afzelius, “Brown Adipose Tissue: Its Gross Anatomy, Histology and Cytology,” In: O. Lindberg, Ed., Brown Adipose Tissue, Elsevier, New York, 1970, pp. 1-28.
- D. G. Nicholls and E. Rial, “A History of the First Uncoupling Protein, UCP1,” Journal of Bioenergetics and Biomembranes, Vol. 31, No. 5, 1999, pp. 399-406.
- D. Ricquier and F. Bouillaud, “Mitochondrial Uncoupling Proteins: From Mitochondria to the Regulation of Energy Balance,” The Journal of Physiology, Vol. 529, No. 1, 2000, pp. 3-10. doi:10.1111/j.1469-7793.2000.00003.x
- B. Canon and J. Nedergaard, “Brown Adipose Tissue: Function and Physiological Significance,” Physiological Reviews, Vol. 84, No. 1, 2003, pp. 277-359. doi:10.1152/physrev.00015.2003
- T. F. Hany, E. Gharehpapagh, E. M. Kamel, A. Buck, J. Himms-Hagen and G. K. von Schulthess, “Brown Adipose Tissue: A Factor to Consider in Symmetrical Tracer Uptake in the Neck and Upper Chest Region,” European Journal of Nuclear Medicine and Molecular Imaging, Vol. 29, No. 10, 2002, pp. 1393-1401. doi:10.1007/s00259-002-0902-6
- A. M. Cypess, M. M. Sanaz Lehman, M. B. Gethin Williams, et al. “Identification and Importance of Brown Adipose Tissue in Adult Humans,” The New England Journal of Medicine, Vol. 360, No. 15, 2009, pp. 1509- 1517. doi:10.1056/NEJMoa0810780
- D. W. Van Marken Lichtenbelt, J. W. Vanhommerig, N. M. Smulders, et al. “Cold-Activated Brown Adipose Tissue in Healthy Men,” The New England Journal of Medicine, Vol. 360, No. 15, 2009, pp. 1500-1508. doi:10.1056/NEJMoa0808718
- S. Enerback, A. Jacobsson, E. M. Simpson, C. Guerra, H. Yamashita, M. E. Harper, et al. “Mice Lacking Mitochondrial Uncoupling Protein Are Cold-Sensitive But Not Obese,” Nature, Vol. 387, No. 6628, 1997, p. 90. doi:10.1038/387090a0
- V. Ouellet, S. M. Labbé, D. P. Blondin, S. Phoenix, B. Guérin, F. Haman, E. E. Turcotte, D. Richard and A. C. Carpentier, “Brown Adipose Tissue Oxidative Metabolism Contributes to Energy Expenditure during Acute Cold Exposure in Humans,” Journal of Clinical Investigation, Vol. 122, No. 2, 2012, pp. 545-552.

