Advances in Chemical Engineering and Science
Vol.06 No.04(2016), Article ID:69814,11 pages
10.4236/aces.2016.64031
Synthesis of High-Stability Acidic β/Al-MCM-41 and the Catalytic Performance for the Esterification of Oleic Acid
Zhiping Wang, Shitao Yu*
College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: May 9, 2016; Accepted: August 14, 2016; Published: August 17, 2016
ABSTRACT
β/Al-MCM-41 molecular sieve was synthesized and used to catalyze the esterification of oleic acid with short chain alcohols such as methanol, ethanol, isopropanol and isobutanol to obtain biodiesel. The results indicated that β/Al-MCM-41 exhibited the excellent catalytic activity and stability, which was obviously superior to traditional Al-MCM-41. The relationship between acidity and catalytic activity was in detail examined by NH3-TPD and Py-FTIR. Moreover, the kinetics of esterification of oleic acid with methanol showed that the average reaction order n was 1.97 and that activation energy was 50.01 kJ/mol.
Keywords:
Kinetics, Esterification, Molecular Sieve, β/Al-MCM-41

1. Introduction
With the increasingly serious in the energy shortage and environmental problems, biodiesel has been widely studied as a kind of non toxic fuel for renewable and easily biodegradable. Among all kinds of biodiesels, fatty acid ester, obtained from the esterification of oleic acid with alcohol, has received greater attention using as biofuel in recent years [1] [2] . This kind of biodiesel has the advantages of ready raw materials, simple production process and high calorific value [3] - [5] . In industry, liquid sulfuric acid was used as catalyst to produce fatty acid ester [6] . However, the traditional catalysts have shortcomings including serious corrosion of equipment, complicated separation procedures, serious environmental problems and byproducts because of strong oxidizing property. These greatly restricted the wide application of fatty acid ester. Therefore, choosing the reasonable catalyst is the key to promote the wide application of fatty acid ester. To meet the requirements of low carbon environmental protection and sustainable development, the catalyst should have the characteristics of high efficiency, recoverable and low corrosion. Therefore, solid acid is an important research direction used instead of the liquid acid to overcome the defects above mentioned [7] [8] .
For large molecular reaction is catalyzed by traditional microporous molecular sieves such as Y, Beta and ZSM-5, the large molecular reactant is not easy to enter the inner surface, while most reacts on the outer surfaces, so the activity and selectivity are affected [9] . Mesoporous molecular sieves such as MCM-41 possess a hexagonal arrangement of uniformly sized unidimensional mesoporous and both ends opened, 1.5 - 10 nm pore size distribution and large surface area so that it shows obvious superiority for reactions of large substrates [10] . Although MCM-41 has many merits, its weak acidity together with poor hydrothermal stability and other defects limits its application greatly [11] . To overcome the limitations of microporous and mesoporous molecular sieve, in recent years, the structure of microporous molecular sieve was introduced into the mesoporous molecular sieve. The mosoporous material was transformed into a crystalline structure. At the same time, the metal ions producing acid center was introduced into the skeleton. The high acidity and high hydrothermal stability of mesoporous material have been prepared [12] - [17] . The framework is highly crystalline and exhibits a remarkably catalytic activity and stability with environment friendly for many reactions [18] - [24] .
In present paper, we reported the preparation and characterization of the molecular sieves β/Al-MCM-41, and the application in the esterification of oleic acid with short chain alcohols. The activity, acidity and stability of the catalyst were investigated as well. A kinetic model for the esterification was established and the reaction kinetics was studied. To the best of our knowledge, this is the first report on the use of β/Al- MCM-41 molecular sieves as catalysts for the preparation of biodiesel.
2. Experimental
2.1. Regent and Equipment
All materials, including oleic acid, methanol, ethanol, isopropyl alcohol, isobutyl alcohol, sulfuric acid, tetraethyl ammonium hydroxide, sodium aluminate, hexadecyltrimethyl ammonium bromide (CTAMBr) and sodium hydroxide were purchased from Aldrich, and all materials were used directly after drying without further purification.
X-ray powder diffraction patterns of the samples were obtained on a XD-610 instrument using monochromatic Cu Kα radiation. It was operated at 30 kV and 20 mA with a step width of 0.02˚, diffraction region of 2θ = 1˚ - 10˚ and a scan speed of 2˚/min. All Py-FTIR spectra were recorded with a Nicolet NEXUS470 FTIR spectrometer in the range of 4000 - 500 cm−1. The samples were pretreated for 2 h at 400˚C and less than 10−3 Pa then reduced to room temperature. Pyridine was adsorbed onto the samples for 1 h and desorbed for 1 h under 2 Pa. The NH3-TPD was performed by a DLUT-1 automatic temperature programmed desorption apparatus. The sample is treated at 500˚C in nitrogen flow for 120 min. Then the temperature is reduced to 120˚C and keeps the sample in a flow of NH3 for 60 min. The amount of desorbed NH3 is determined after heating the sample up to 600˚C (heating rate of 10˚C min−1).
The aim products were analyzed by TRACE-GC-MS chromatographer with an FID and a DB-17MS phenyl methyl siloxanes capillary column (30 m × 0.25 mm). The temperature of injector and transference line were maintained constant at 270˚C. The temperature of the furnace was maintained at 180˚C for 30 min. The carrier gas was helium. The injection volume was 0.1 μL.
2.2. Catalyst Preparation
β/Al-MCM-41 was synthesized by stepwise crystallization method. First zeolite precursors solution with zeolite beta primary structure units were prepared according to a certain molar ratio of Al2O3:60 SiO2:2.5 Na2O:22 TEAOH:800 H2O, then transferred the mixture into an autoclave aging for 4 h at 140˚C to get a clear solution. The obtained zeolite precursors solution was added to CTAMBr solution according to a molar ratio of 1:5 Si:CTAMBr, mixed and agitated slowly for 30 min, regulated at pH = 8 - 9 using 5 mol/L sulfuric acid, stirred at 260 rpm for 1 h under the condition of normal temperature after adding alcohol, then the mixed solution was crystallized at 110˚C for 24 h, vacuum filtrated, washed, dried and calcined for 8 h at 550˚C to prepare β/Al-MCM-41.
2.3. Esterification of Oleic Acid
The catalyst was added into a mixture of oleic acid with either methanol, ethanol, isopropanol or isobutanol, and the reaction was carried out under autogenous pressure and intensive electronic stirring in an autoclave at the reaction temperature for 2 - 10 h. The results were calculated according to neutralization titration. The reaction has been repeated for three times and the results are of excellent stability. The esterification rate/% = (1-acid value of esterified product/acid value of reactant) × 100%.
Reaction equation:
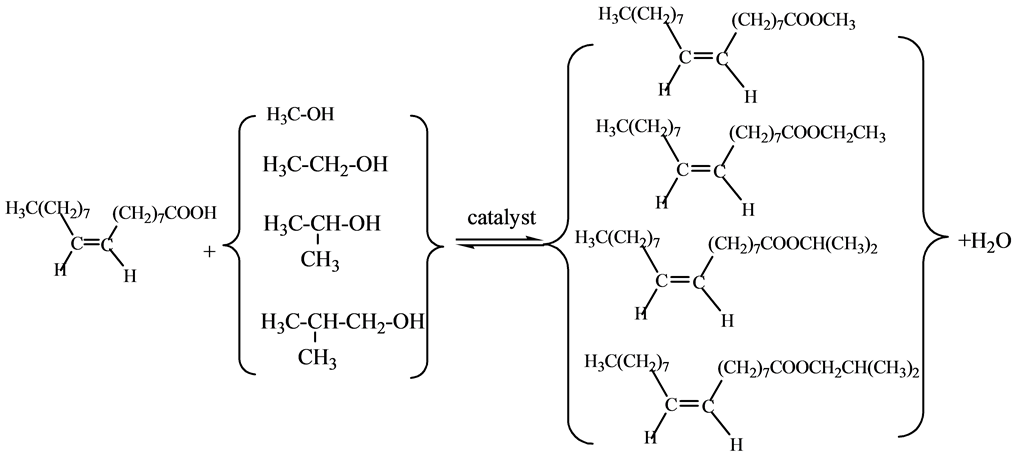
3. Results and Discussion
3.1. Choice of Catalysts
The activities of different catalysts were investigated on the esterification of oleic acid. The detailed results are shown in Table 1. From No. 1 - 2, acidic catalysts are suitable for the esterification of oleic acid with methanol, the yield of methyl oleate was increased significantly. The catalytic effect was unsatisfactory catalyzed by pure β and pure Al-MCM-41 (No. 3 - 5). While sulfuric acid (H2SO4), as the higher catalytic activity catalyst for the esterification of oleic acid, has many disadvantages, such as too large amount of catalyst usage, serious corrosion of equipments, complicated separation pro- cedures, environmental problems, and by-products. It can be seen that β/Al-MCM-41 exhibited excellent catalytic activity, the conversion of oleic acid can reach about 70%, which obviously superior to Al-MCM-41. Use β/Al-MCM-41 to catalyze the esterification of oleic acid with ethanol, isopropanol and isobutanol, the yields of ester were higher (No. 6 - 11). The high catalytic activity and stability in the esterification of the catalyst was discussed.
The NH3-TPD profiles of β/Al-MCM-41 and Al-MCM-41 is shown in Figure 1. It can be seen that the both samples have a very strong peak at low temperature about 100˚C - 200˚C and a middle strong peak at high temperature about 700˚C - 800˚C, belonging to poor acid and strong acid, respectively. By quantitative analysis, the amount of the poor acid and strong acid for β/Al-MCM-41 are obviously more than Al-MCM-41. This is maybe the key reason why β/Al-MCM-41 is of high catalytic performance.
Table 1. Effect of different catalysts on reaction resultsa.
aReaction conditions: n(methanol):n(oleic acid) = 10:1, amounts of catalyst as 5% of the total mass of reactants, reaction temperature 120˚C, reaction time 8 h.
Figure 1. NH3-TPD of β/Al-MCM-41 and Al-MCM-41. (a) β/Al-MCM-41, (b) Al-MCM-41.
The Py-FTIR spectrum of β/Al-MCM-41 and Al-MCM-41 is shown in Figure 2. The presence of the band at 1596 cm−1 was associated with Bronsted acid sites, and the band at 1446 cm−1 was characteristic Lewis acid sites [23] . It was shown that Bronsted acid and Lewis acid sites co-exists on the surface of the β/Al-MCM-41 and Al-MCM-41. By comparison, the peak intensity of β/Al-MCM-41 is stronger than Al-MCM-41. The relative intensity of Brönsted acid/Lewis acid (B/L) over β/Al-MCM-41 and Al-MCM-41 was list, respectively. The results show that Brönsted acid and Lewis acid sites are basically equivalent and the co-action play the key effect on catalytic activity.
3.2. Catalysis Stability
The catalytic stability of β/Al-MCM-41 in the esterification of oleic acid was researched, and the results were shown in Figure 3. The catalysts were obtained by vacuum filtration and reused directly after drying without further purification. Relative to the initial conversion, when reuse in the fifth time, the conversion of oleic acid was above 60% still.
The reused β/Al-MCM-41 after 5 times was characterized by XRD and NH3-TPD. Figure 4 shows the XRD patterns of fresh and reused β/Al-MCM-41. Note that there are spectrum peaks at approximately 2θ = 2˚ and 4˚, which is characteristic for the hexagonally symmetric MCM-41 mesoporous structure [10] . The peaks of the fresh and reused samples are almost in the same position, which indicates that the catalyst structure was essentially unchanged; the better mesoporous structure still exists.
By comparing the NH3-TPD from Figure 5, the fresh and reused samples have the same profile. By quantitative analysis, the amount of the poor and strong acid were also slightly decreased, maybe this is the reason for the slightly decrease of catalytic activity. The reused catalyst was regenerated by calcinations and then used to catalyze the esterification. The conversion of oleic acid was 70%, which showed that the synthesized catalyst possesses good regeneration performance.
Figure 2. Py-FTIR of β/Al-MCM-41 and Al-MCM-41. (a) β/Al-MCM-41, (b) Al-MCM-41.
Figure 3. Results on reuse of the catalyst.
Figure 4. XRD patterns of fresh and reused β/Al-MCM-41. (a) fresh β/Al-MCM-41, (b) reused β/Al-MCM-41.
Figure 5. NH3-TPD of fresh and reused β/Al-MCM-41.
3.3. Reaction Kinetics of Esterification
In studies of esterification kinetics of oleic acid with methanol, methanol was excessive, the reaction rate can be expressed by Equation (1) [25] .
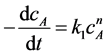 (1)
(1)
where cA represents the concentration of oleic acid at time t, k1 represents the rate constant of the reaction.
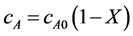 (2)
(2)
where cA0 is the initial concentration of oleic acid, X is the conversion of oleic acid. So Equation (1) could be written as follows:
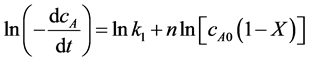 (3)
(3)
The effect of reaction temperature on conversion of oleic acid in the presence of β/Al-MCM-41was showed in Figure 6.
Experimental data were processed by means of computer software Origin 8.0. First
calculated instantaneous reaction rate corresponding to different concentration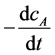 , then calculated
, then calculated 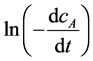 and lncA. Drawn the relationship between
and lncA. Drawn the relationship between 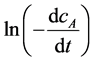 and
and
lncA according to the Equation (3), the slope of the line is the reaction order n and intercept is the reaction rate constant by linear regression.
The linear regression results of the data are showed in Table 2. All of the linear correlative coefficients were higher than 0.99. According this method, the reaction rate
Figure 6. Effect of reaction temperature on reaction rate in the presence of β/Al-MCM-41.
Table 2. Kinetic parameters of esterification at different temperature.
constant increased with the temperature arising. The average reaction order n was 1.97.
According to Arrhenius Equation,
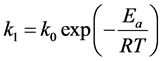 (4)
(4)
Using the rate constants above, the activation energy (Ea) could be obtained by Equation (5).
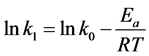 (5)
(5)
where k0 is pre-exponential factor, R is the gas constant and T is the temperature in Kelvin. The activation energy for this reaction calculated from the slope of Arrhenius plot shown in Figure 7 was 50.01 kJ/mol.
4. Conclusions
The molecular sieve β/Al-MCM-41 was synthesized successfully using β as silica- alumina source. The synthesized β/Al-MCM-41 was firstly used as catalyst in the esterification of oleic acid with short chain alcohols. The results indicated that β/Al-MCM-41 exhibited excellent catalytic activity and stability.
Figure 7. Arrhenius plot of reaction rate constant of esterification.
The kinetics of the reaction was also investigated. The results showed that the average reaction order n of the esterification of oleic acid with methanol was 1.97. The reaction rate is accelerated with the temperature arising. The relationship accorded with the Arrhenius Equation and the activation energy was 50.01 kJ/mol.
Acknowledgements
This research was supported by The Taishan Scholar Program of Shandong and Natural Science Foundation of China (31570573).
Cite this paper
Wang, Z.P. and Yu S.T. (2016) Synthesis of High-Stability Acidic β/Al-MCM-41 and the Catalytic Performance for the Esterification of Oleic Acid. Advances in Chemical Engineering and Science, 6, 305- 315. http://dx.doi.org/10.4236/aces.2016.64031
References
- 1. Zhang Y., Dube, M.A., Mclean, D.D. and Kates, M. (2003) Biodiesel Production from Waste Cooking Oil: 1. Process Design and Technological Assessment. Bioresource Technology, 89, 1-16.
http://dx.doi.org/10.1016/S0960-8524(03)00040-3 - 2. Bagly, S.T., Gratz, L.D., Johnson, J.H. and Mcdonald, J.F. (1998) Effect of an Oxidation Catalytic Converter and a Biodiesel on the Chemical, Mutagenic and Particle Size Characteristics of Emissions from a Diesel Engine. Environmental Science & Technology, 32, 1183- 1191.
http://dx.doi.org/10.1021/es970224q - 3. Jiang, J.C., Yang, K.H. and Nie, X.A. (2004) Research and Application of Biodiesel. Energy Research and Utilization, 5, 22-25.
- 4. Zhou, S., Chen, Y. and Wang, Y.H. (2015) Research and Application of Biodiesel. China Chemical Trade, 5, 47-49.
- 5. An, W.J., Xu, D.P. and Wang, H.J. (2005) Chemical Production Methods of the Biodiesel Fuel. Cereals and Oils, 7, 3-6.
- 6. Vieville, C., Mouloungui, Z. and Gaset, A. (1993) Esterificaton of Oleic Acid by Methanol Catalyzed by p-Toluenesulfoic Acid and the Cation-Exchange Resins K241 and K1481 in Supercritical Carbon Dioxide. Industrial & Engineering Chemistry Research, 32, 2065-2068.
http://dx.doi.org/10.1021/ie00021a031 - 7. Harmer, M.A., Sun, Q. and Farneth, W.E. (1996) High-Surface-Area Nafion Resin/Silica Nanocomposites—A New Class of Solid Acidcatalyst. Journal of the American Oil Chemists’ Society, 118, 7708-7715.
http://dx.doi.org/10.1021/ja9541950 - 8. Wim, D.B., Dirk, E.D. and Wim, M.V. (1999) Mesoporous Sulfonic Acids as Selective Heterogeneous Catalysts for the Synthesis of Monoglycerides. Journal of Catalysis, 182, 156- 164.
http://dx.doi.org/10.1006/jcat.1998.2353 - 9. Rohan, D., Canaff, C. and Guisnet, M. (1998) Acetylation of Anisole by Acetic Anhydride over a HBEA Zeolites-Origin of Deactivation of the Catalyst. Journal of Catalysis, 177, 296- 305.
http://dx.doi.org/10.1006/jcat.1998.2108 - 10. Beck, J.C., Vartuli, J.C., Roth, W.J., Leonowicz, M.E., Kresge, C.T., Schmitt, K.D., Chu, C.T.W., Olson, D.H. and Sheppard, E.W. (1992) A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. Journal of the American Chemical Society, 114, 10834-10843.
http://dx.doi.org/10.1021/ja00053a020 - 11. Kresge, C.T., Leonowicz, M.E., Roth, W.J., Vartuli, J.C. and Beck, J.S. (1992) Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystaltemplate Mechanism. Nature, 359, 710-712.
http://dx.doi.org/10.1038/359710a0 - 12. Karlsson, A., Stocker, M. and Schmidt, R. (1999) Composites of Micro- and Mesoporous Materials: Simultaneous Syntheses of MFI/MCM-41 Like Phases by a Mixed Template Approach. Microporous and Mesoporous Materials, 27, 181-192.
http://dx.doi.org/10.1016/S1387-1811(98)00252-2 - 13. Huang, L.M., Guo, W.P., Deng, P., Xue, Z.Y. and Li, Q.Z. (2000) Investigation of Synthesizing MCM-41/ZSM-5 Composites. The Journal of Physical Chemistry B, 104, 2817-2823.
http://dx.doi.org/10.1021/jp990861y - 14. Zhang, H.J. and Li, Y.D. (2008) Preparation and Characterization of Beta/MCM-41 Composite Zeolite with a Stepwise-Distributed Pore Structure. Powder Technology, 183, 73-78.
http://dx.doi.org/10.1016/j.powtec.2007.11.013 - 15. Jiang, T.S., Qi, L.W., Ji, M.R., Ding, H.H., Li, Y.H., Tao, Z.F. and Zhao, Q. (2012) Characterization of Y/MCM-41 Composite Molecular Sieve with High Stability from Kaolin and Its Catalytic Property. Applied Clay Science, 7, 32-40.
http://dx.doi.org/10.1016/j.clay.2012.04.016 - 16. Shen, Y. and Lu, A.C. (2012) Preparation and Characterization of Mixed Matrix Membranes Based on PVDF and Three Inorganic Fillers (Fumed Nonporous Silica, Zeolite 4A and Mesoporous MCM-41) for Gas Separation. Chemical Engineering Journal, 192, 201-210.
http://dx.doi.org/10.1016/j.cej.2012.03.066 - 17. Guo, W.P., Huang, L.M., Deng, P., Xue, Z.Y. and Li, Q.Z. (2001) Characterization of Beta/MCM-41 Composite Molecular Sieve Compared with the Mechanical Mixture. Microporous and Mesoporous Materials, 4, 427-434.
http://dx.doi.org/10.1016/S1387-1811(01)00217-7 - 18. Ooi, Y.S., Zakaria, R., Mohamed, A.R. and Bhatia, S. (2004) Synthesis of Composite Material MCM-41/Beta and Its Catalytic Performance in Waste Used Palm Oil Cracking. Applied Catalysis A: General, 274, 15-23.
http://dx.doi.org/10.1016/j.apcata.2004.05.011 - 19. Chang, Y.G., Zhang, Y., Wang, X.L. and Lian, P.Y. (2006) Synthesis of β/Al-MCM-41 and Its Catalytic Performance in Alkylation. Industrial Catalysis, 14, 13-17.
- 20. Ji, X.F., Qin, Z.F., Dong, M., Wang, G.F. and Wang, J.G. (2008) Acylation of Anisole with Acetic Anhydride over Hβ/ MCM-41 Composite Molecular Sieves. Journal of Fuel Chemistry and Technology, 36, 621-624.
- 21. Javadian, H. and Taghavi, M. (2014) Application of Novel Polypyrrole/Thiol-Functionalized Zeolite Beta/MCM-41 Type Mesoporous Silica Nanocomposite for Adsorption of Hg2+ from Aqueous Solution and Industrial Wastewater: Kinetic, Isotherm and Thermodynamic Studies. Applied Surface Science, 15, 487-494.
http://dx.doi.org/10.1016/j.apsusc.2013.11.020 - 22. Kumar, N., Arvela, P.M., Ylasalmi, T., Villegas, J., Heikkila, T., Leino, A.R., Kordas, K., Salmi, T. and Murzin, D. (2012) Dimerization of 1-Butene in Liquid Phase Reaction: Influence of Structure, Pore Size and Acidity of Beta Zeolite and MCM-41 Mesoporous Material. Microporous and Mesoporous Materials, 1, 127-134.
http://dx.doi.org/10.1016/j.micromeso.2011.05.032 - 23. Cativiela, C., Figueras, F., Fraile, J.M., Garcia, J.I., Mayoral, J.A., Menorval, L.C. and Pires, E. (1993) Comparison of the Catalytic Properties of Protonic Zeolites and Exchanged Clays for Diels-Alder Synthesis. Applied Catalysis A: General, 101, 253-267.
http://dx.doi.org/10.1016/0926-860X(93)80273-S - 24. Dimitrova, R., Gunduz, G. and Spassova, M. (2006) A Comparative Study on the Structural and Catalytic Properties of Zeolites Type ZSM-5, Mordenite, Beta and MCM-41. Journal of Molecular Catalysis A: Chemical, 243, 17-23.
http://dx.doi.org/10.1016/j.molcata.2005.08.015 - 25. Song, C.C., Qi, Y.Q., Deng, T.S., Hou, X.L. and Qin, Z.F. (2010) Kinetic Model for the Esterification of Oleic Acid Catalyzed by Zinc Acetate in Subcritical Methanol. Renewable Energy, 35, 625-628.
http://dx.doi.org/10.1016/j.renene.2009.08.004.









